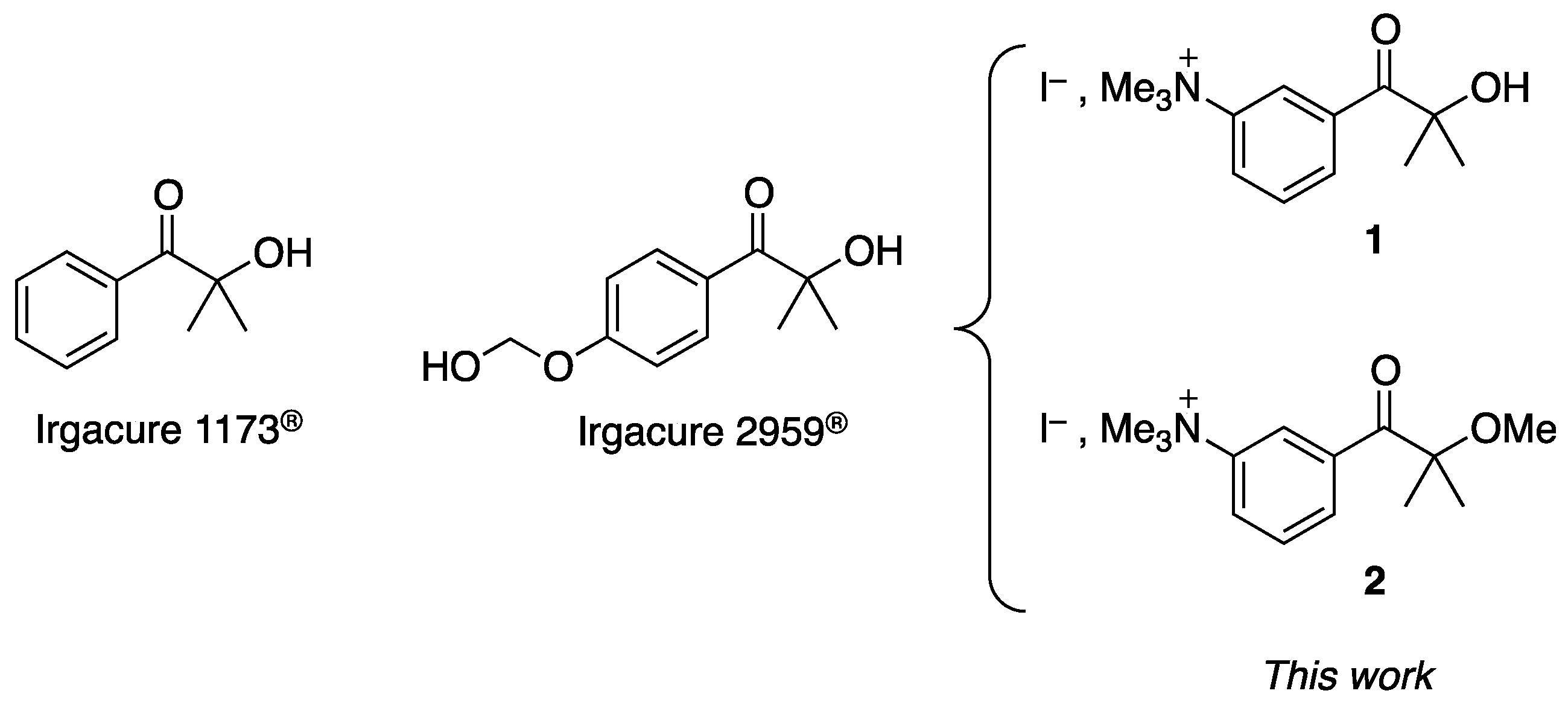
Development of Water-Soluble Type I Photoinitiators for Hydrogel Synthesis
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials/Synthesis of the Water-Soluble Dyes
2.1.1. Synthesis of 2-Hydroxy-2-methyl-1-(3-aminophenyl)propan-1-one (3)
2.1.2. Synthesis of 3-(2-Methoxy-2-methylpropanoyl)-N,N,N-trimethylbenzenaminium Iodide (2)
2.1.3. Synthesis of 3-(2-Hydroxy-2-methylpropanoyl)-N,N,N-trimethylbenzenaminium Iodide (1)
2.2. Photopolymerization Reactions
2.3. Hydrogels Synthesis and Characterization
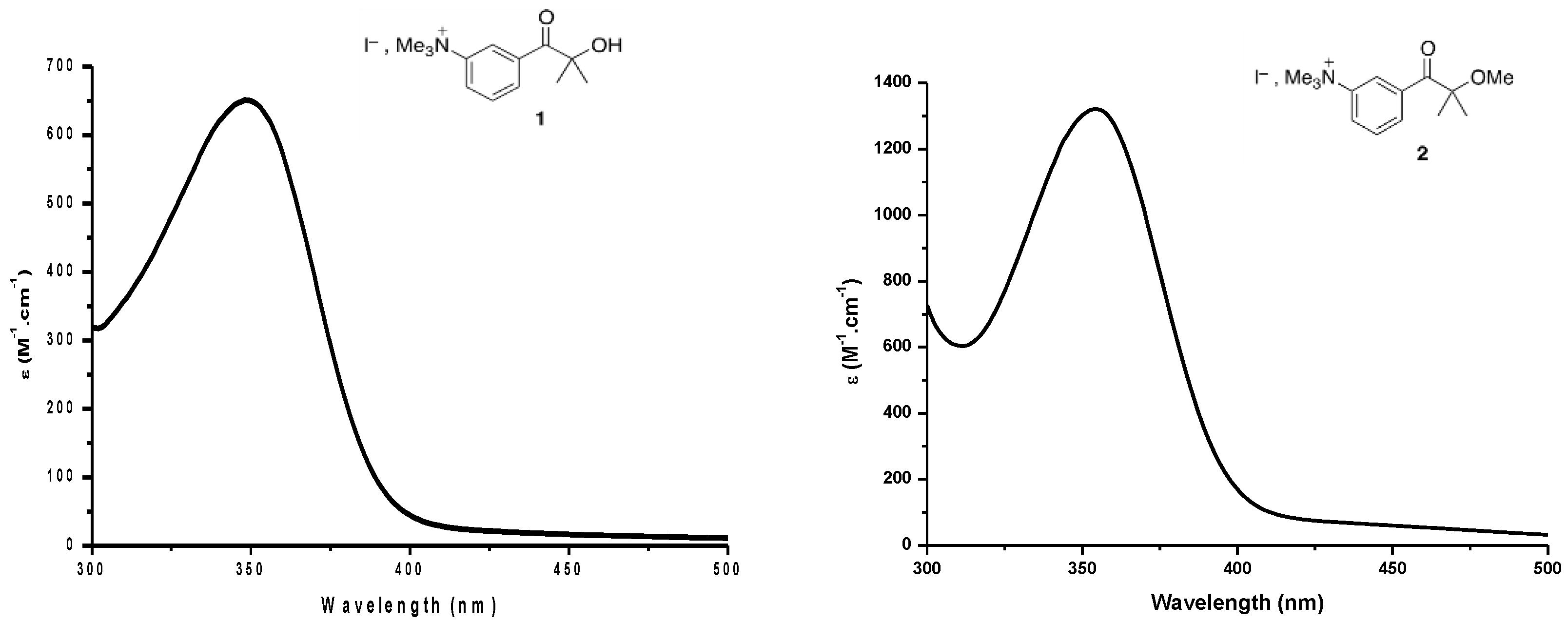
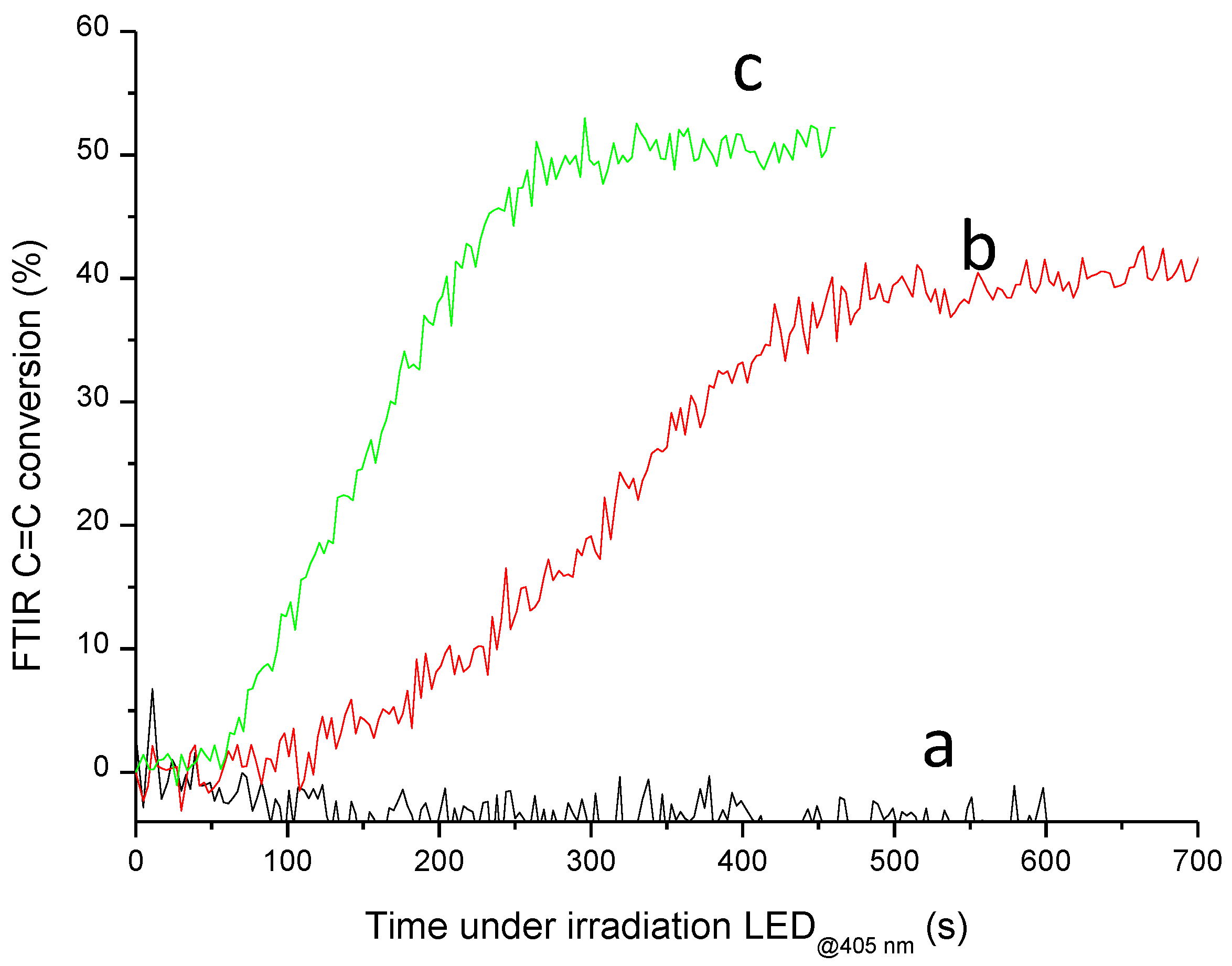
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, P.; Guthrie, J.T.; Davis, M.J.; Godfrey, A.A.; Green, P.N. Sensitized photoinitiated grafting of N-vinyl-2-prrolidone (NVP) to woolen substrates. J. Appl. Polym. Sci. 1981, 26, 521–527. [Google Scholar] [CrossRef]
- Bonamy, A.; Fouassier, J.P.; Lougnot, D.J.; Green, P.N. Novel and efficient water-soluble photoinitiators for polymerization. J. Polym. Sci. Polym. Letter. Ed. 1982, 20, 315–320. [Google Scholar] [CrossRef]
- Dietliker, K. A Compilation of Photoinitiators Commercially Available for UV Today; SITA: Edinburgh, UK, 2002. [Google Scholar]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Lalevée, J.; Fouassier, J.-P. Photoinitiators: Structures, Reactivity and Applications in Polymerization; Wiley: Weinheim, Germany, 2021. [Google Scholar]
- Ullrich, G.; Ganster, B.; Salz, U.; Moszner, N.; Liska, R. Photoinitiators with functional groups. IX. Hydrophilic bisacylphosphine oxides for acidic aqueous formulations. J. Polym. Sci. A Polym. Chem. 2006, 44, 1686–1700. [Google Scholar] [CrossRef]
- Leppard, D.; Eichenberger, E. Process for Preparing Acylphosphines and Derivatives. WO 2000032612A1, 8 June 2000. [Google Scholar]
- Murer, P.; Wolf, J.-P.; Burkhardt, S.; Grützmacher, H.; Stein, D.; Dietliker, K. Process for Preparing Acylphosphanes and Derivatives Thereof. U.S. Patent 7687657B2, 30 March 2010. [Google Scholar]
- Rouillard, A.; Berglund, C.M.; Lee, J.Y.; Polacheck, W.; Tsui, Y.; Bonassar, L.J.; Kirby, B. Methods for Photocrosslinking Alginate Hydrogel Scaffolds with High Cell Viability. Tissue Eng. Part C Methods 2011, 17, 173–179. [Google Scholar] [CrossRef]
- Billiet, T.; Geveert, E.; De Schryver, T.; Cornalissen, M.; Dubruel, P. The 3D Printing of Gelatin Methacrylamide Cell-Laden Tissue-Engineered Constructs with High Cell Viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Gou, M.; Qu, X.; Zhu, W.; Xiang, M.; Yang, J.; Zhang, K.; Wei, Y.; Chen, S. Bio-inspired detoxification using 3D-printed hydrogel nanocomposites. Nat. Commun. 2014, 5, 3774–3783. [Google Scholar] [CrossRef] [Green Version]
- Wenz, A.; Borchers, K.; Tovar, G.E.; Kluger, P.J. Bone Matrix Production in Hydroxyapatite-Modified Hydrogels Suitable for Bone Bioprinting. Biofabrication 2017, 9, 044103. [Google Scholar] [CrossRef]
- Colderon, G.; Thai, P.; Hsu, C.; Grigoryan, B.; Gibson, S.M.; Dickinson, M.E.; Miller, J.S. Tubulogenesis of Co-Cultured Human iPS-derived Endothelial Cells and Human Mesenchymal Stem Cells in Fibrin and Gelatin Methacrylate Gels. Biomater. Sci. 2017, 5, 1652–1660. [Google Scholar] [CrossRef]
- Benedikt, S.; Wang, J.; Markovic, M.; Moszner, N.; Dietliker, K.; Ovsianikov, A.; Grützmacher, H.; Liska, R. Highly efficient water-soluble visible light photoinitiators. J. Polym. Sci. A Polym. Chem. 2016, 54, 473–479. [Google Scholar] [CrossRef]
- Occhetta, P.; Sadr, N.; Piraino, F.; Redaelli, A.; Moretti, M.; Rasponi, M. Fabrication of 3D cell-laden hydrogel microstructures through photo-mold patterning. Biofabrication 2013, 5, 035002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lougnot, D.; Fouassier, J. Comparative reactivity of water soluble photoinitiators as viewed in terms of excited states processes. J. Polym. Sci. A Polym. Chem. 1988, 26, 1021–1033. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Burr, D.; Wieder, F. Water-soluble photoinitiators: Primary processes in hydroxy alkyl phenyl ketones. J. Polym. Sci. A Polym. Chem. 1991, 29, 1319–1327. [Google Scholar] [CrossRef]
- Knaus, S.; Gruber, H.J. Photoinitiators With Functional Groups. III. Water-Soluble Photoinitiators Containing Carbohydrate Residues. J. Polym. Sci. A Polym. Chem. 1995, 33, 929–939. [Google Scholar] [CrossRef]
- Kojima, K.; Ito, M.; Morishita, H.; Hayashi, N. A novel water-soluble photoinitiator for the acrylic photopolymerization type resist system. Chem. Mater. 1998, 10, 3429–3433. [Google Scholar] [CrossRef]
- Pawar, A.A.; Saada, G.; Cooperstein, I.; Larush, L.; Jackman, J.A. High-performance 3D printing of hydrogels by water-dispersible photoinitiator nanoparticles. Sci. Adv. 2016, 2, e150138. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent Advances in Photo-Crosslinkable Hydrogels for Biomedical Applications. BioTechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M.; Aggor, F.S.; Awad, A.M.; El-Aref, A.T. An innovative method for preparation of nanometal hydroxide superabsorbent hydrogel. Carbohydr. Polym. 2013, 91, 693–698. [Google Scholar] [CrossRef]
- Buchholz, F.L.; Graham, A.T. Modern Superabsorbent Polymer Technology; Chapter 1–7; Wiley VCH: New York, NY, USA, 1998. [Google Scholar]
- Brannon-Peppas, L.; Harland, R.S. Absorbent polymer technology. J. Control. Release 1991, 17, 297–298. [Google Scholar]
- Yuhui, L.; Guoyou, H.; Xiaohui, Z.; Baoqiang, B.; Yongmei, C.; Tingli, L.; Jian, L.T.; Feng, X. Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar]
- Stampfl, J.; Liska, R.; Ovsianikov, A. Multiphoton Lithography: Techniques, Materials, and Applications; Wiley: Weinheim, Germany, 2016. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A Review. J. Adv. Res. 2013, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tehfe, M.-A.; Monot, J.; Malacria, M.; Fensterbank, L.; Fouassier, J.-P.; Curran, D.P.; Lacôte, E.; Lalevée, J. A Water-Compatible NHC-Borane: Photopolymerizations in Water and Rate Constants for Elementary Radical Reactions. ACS Macro Lett. 2012, 1, 92–95. [Google Scholar] [CrossRef]
- Lalevée, J.; Telitel, S.; Tehfe, M.-A.; Fouassier, J.-P.; Curran, D.P.; Lacôte, E. N-Heterocyclic Carbene Boranes Accelerate Type I Radical Photopolymerizations and Overcome Oxygen Inhibition. Ang. Chem. 2012, 51, 5958–5961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Song, L.; Niu, G. Controllable properties and microstructure of hydrogels based on crosslinked poly(ethylene glycol) diacrylates with different molecular weights. J. Appl. Polym. Sci. 2011, 121, 531–540. [Google Scholar] [CrossRef]
- Duncan, E.J.S.; Brousseau, P. Comparison of the Uniaxial Tensile Modulus and Dynamic Shear Storage Modulus of a Filled Hydroxy-Terminated Polybutadiene and GAP Propellant. J. Mater. Sci. 1996, 31, 1275–1284. [Google Scholar] [CrossRef]
- Gäbler, S.; Stampfi, J.; Koch, T.; Seidler, S.; Schüller, G.; Redl, H.; Juras, V.; Trattnig, S.; Weidisch, R. Determination of the viscoelastic properties of hydrogels based on polyethylene glycol diarylate (PEGDA) and human articular cartilage. Int. J. Mater. Eng. Innov. 2009, 1, 3–20. [Google Scholar] [CrossRef]
- Chen, H.; Vahdati, M.; Xiao, P.; Dumur, F.; Lalevée, J. Water-Soluble Visible Light Sensitive Photoinitiating System Based on Charge Transfer Complexes for the 3D Printing of Hydrogels. Polymers 2021, 13, 3195. [Google Scholar] [CrossRef]



| Irgacure 2959 + 4 | 1 + 4 | 2 + 4 | |
|---|---|---|---|
| Swelling ratio (%) | 80 | 83 | 77 |
| Storage Modulus (G’) (DMA shear stress, MPa) | 1 | 3.1 | 2.3 |
| Young Modulus (tensile tests, MPa) | - | 11.7 | 9.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubry, B.; Dumur, F.; Lansalot, M.; Bourgeat-Lami, E.; Lacôte, E.; Lalevée, J. Development of Water-Soluble Type I Photoinitiators for Hydrogel Synthesis. Macromol 2022, 2, 131-140. https://doi.org/10.3390/macromol2010008
Aubry B, Dumur F, Lansalot M, Bourgeat-Lami E, Lacôte E, Lalevée J. Development of Water-Soluble Type I Photoinitiators for Hydrogel Synthesis. Macromol. 2022; 2(1):131-140. https://doi.org/10.3390/macromol2010008
Chicago/Turabian StyleAubry, Bérengère, Frédéric Dumur, Muriel Lansalot, Elodie Bourgeat-Lami, Emmanuel Lacôte, and Jacques Lalevée. 2022. "Development of Water-Soluble Type I Photoinitiators for Hydrogel Synthesis" Macromol 2, no. 1: 131-140. https://doi.org/10.3390/macromol2010008
APA StyleAubry, B., Dumur, F., Lansalot, M., Bourgeat-Lami, E., Lacôte, E., & Lalevée, J. (2022). Development of Water-Soluble Type I Photoinitiators for Hydrogel Synthesis. Macromol, 2(1), 131-140. https://doi.org/10.3390/macromol2010008









