Effect of Physical Properties and Chemical Substitution of Excipient on Compaction and Disintegration Behavior of Tablet: A Case Study of Low-Substituted Hydroxypropyl Cellulose (L-HPC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Physical Characterization of Powder Materials
2.2.2. Compression of Powders and Evaluation of Tablet Properties
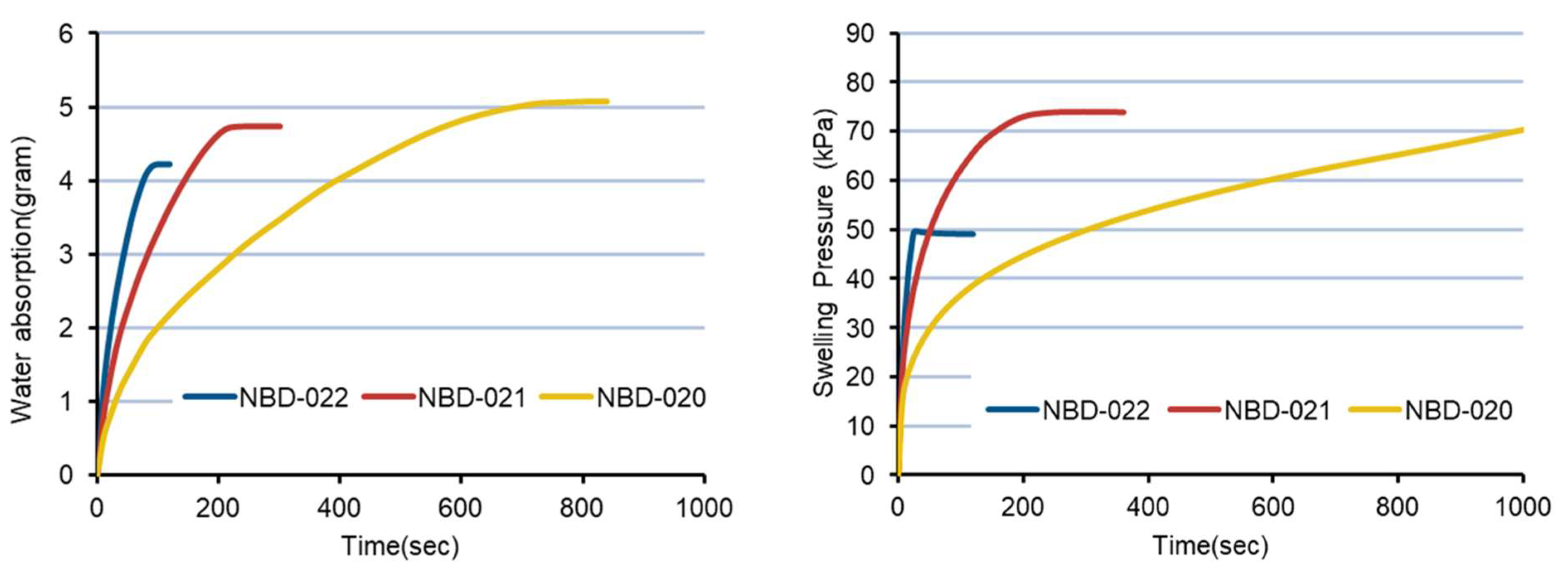
2.2.3. Evaluation of Swelling Pressure and Water Absorption of L-HPC Powder
2.2.4. Estimation of Compressibility and Compactibility Parameters
Compressibility of L-HPC Grades Powder Materials
Compactibility of L-HPC Grades Powder Materials
2.2.5. Non-Linear Regression Analysis and Statistical Evaluation
2.2.6. Effect of Different L-HPC Grades on Porosity and Disintegration Time Using 32 Full-Factorial Design
3. Results and Discussion
3.1. Physicochemical and Morphological Properties of L-HPC Grades
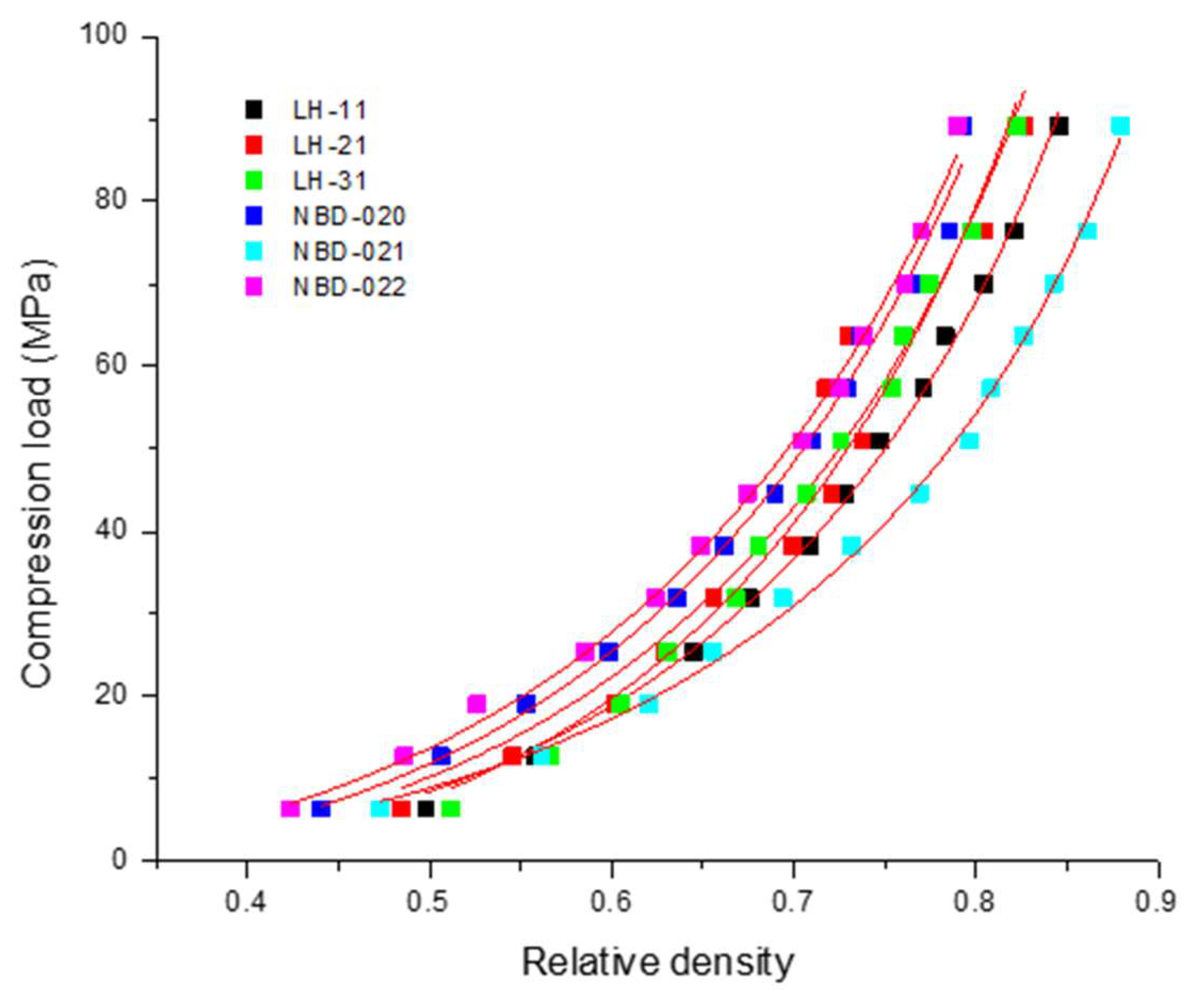
3.2. Compressibility of Different Grades of L-HPC
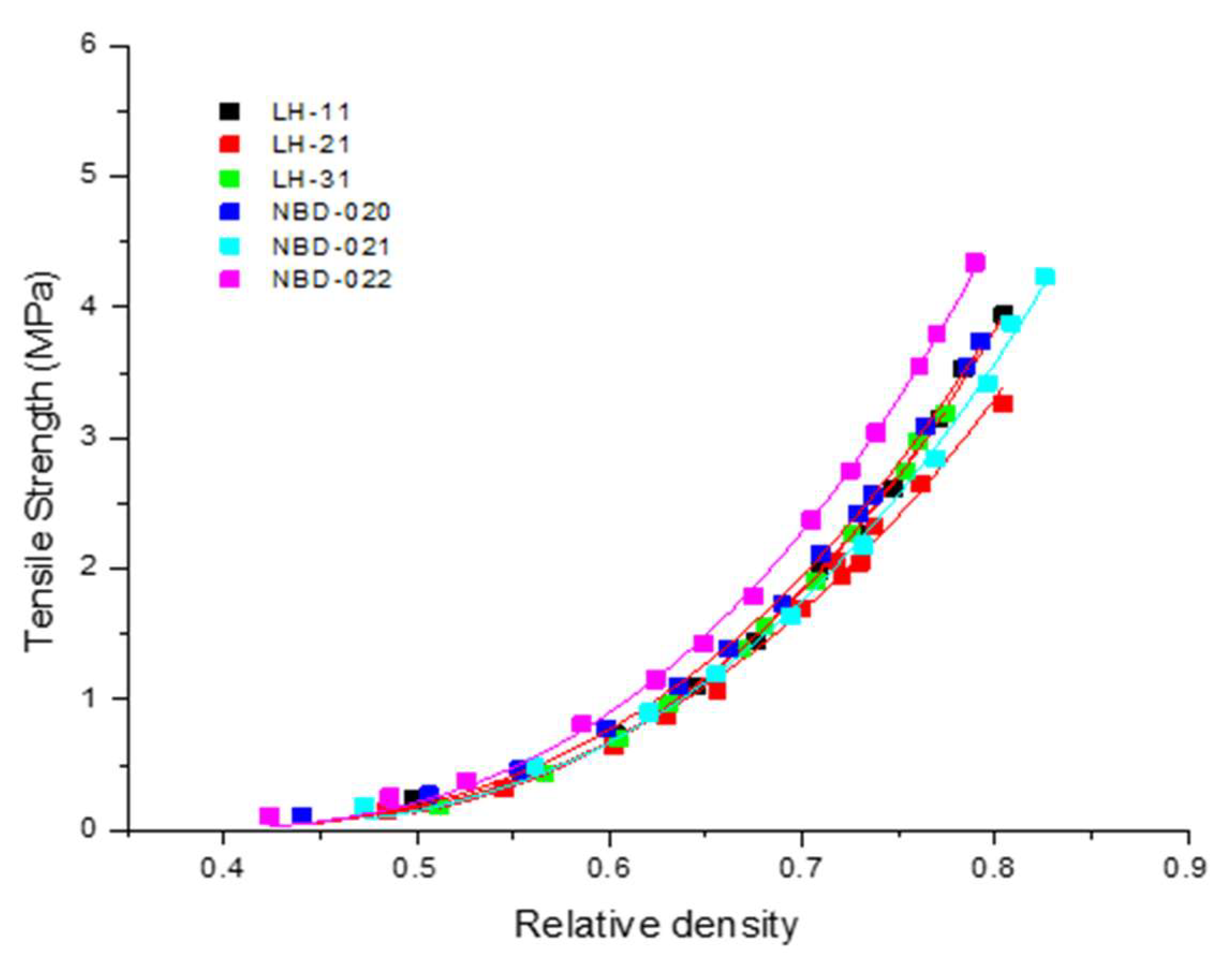
3.3. Compactibility of Different Grades of L-HPC
3.4. Disintegration Behavior of L-HPC Grades
3.4.1. Effect of Particle Size of L-HPC Grades and Compression Load on the Porosity and Disintegration Time of Tablets Using a 32 Full-Factorial Design
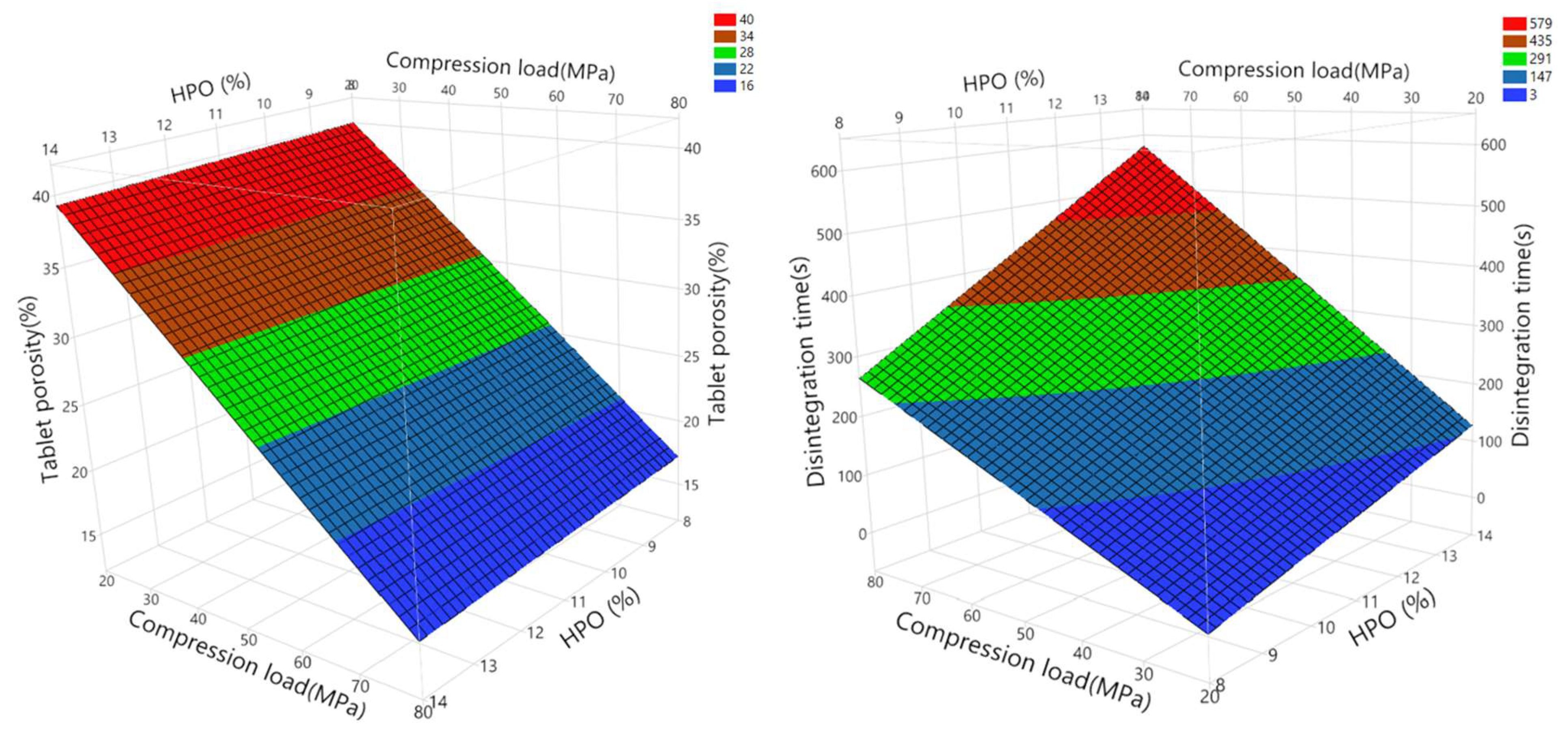
3.4.2. Effect of HPO (%) Content of L-HPC Grades and Compression Load on Porosity and Disintegration Time of Tablets Using 32 Full-Factorial Design
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dave, V.S.; Saoji, S.D.; Raut, N.A.; Haware, R.V. Excipient Variability and Its Impact on Dosage Form Functionality. J. Pharm. Sci. 2015, 104, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; FUKUOKA, E.; NAKAJIMA, S.; HASEGAWA, J. Crystallinity and Physical Characteristics of Microcrystalline Cellulose. Chem. Pharm. Bull. 1977, 25, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline Cellulose, a Direct Compression Binder in a Quality by Design Environment—A Review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Thoorens, G.; Krier, F.; Rozet, E.; Carlin, B.; Evrard, B. Understanding the Impact of Microcrystalline Cellulose Physicochemical Properties on Tabletability. Int. J. Pharm. 2015, 490, 47–54. [Google Scholar] [CrossRef]
- Krueger, C.; Thommes, M.; Kleinebudde, P. “Mcc Sanaq® Burst”—A New Type of Cellulose and Its Suitability to Prepare Fast Disintegrating Pellets. J. Pharm. Innov. 2010, 5, 45–57. [Google Scholar] [CrossRef]
- Pinakin, P.; Pandey, N.K.; Singh, S.K.; Garg, V. Mcc Sanaq® Burst: A Unique Carrier for Formulation of Sublingual Tablets. Int. J. Pharm. Tech. Res. 2016, 9, 15–22. [Google Scholar]
- Pazesh, S.; Persson, A.S.; Alderborn, G. Atypical Compaction Behaviour of Disordered Lactose Explained by a Shift in Type of Compact Fracture Pattern. Int. J. Pharm. X 2019, 1, 100037. [Google Scholar]
- Omar, C.S.; Dhenge, R.M.; Palzer, S.; Hounslow, M.J.; Salman, A.D. Roller Compaction: Effect of Relative Humidity of Lactose Powder. Eur. J. Pharm. Biopharm. 2016, 106, 26–37. [Google Scholar] [CrossRef]
- Kleinebudde, P. Application of Low Substituted Hydroxypropylcellulose (L-Hpc) in the Production of Pellets Using Extrusion/Spheronization. Int. J. Pharm. 1993, 96, 119–128. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; et al. Safety of Low-Substituted Hydroxypropyl Cellulose (L-Hpc) to Be Used as a Food Additive in Food Supplements in Tablet Form. EFSA J. 2018, 16, e05062. [Google Scholar]
- ElShaer, A.; Al-Khattawi, A.; Mohammed, A.R.; Warzecha, M.; Lamprou, D.A.; Hassanin, H. Understanding the Compaction Behaviour of Low-Substituted Hpc: Macro, Micro, and Nano-Metric Evaluations. Pharm. Dev. Technol. 2018, 23, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, L.; Lin, X.; Wang, Y.; Feng, Y. A Model to Simultaneously Evaluate the Compressibility and Compactibility of a Powder Based on the Compression Ratio. Int. J. Pharm. 2020, 577, 119023. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.M.; Rohera, B.D. Mechanics of Tablet Formation: A Comparative Evaluation of Percolation Theory with Classical Concepts. Pharm. Dev. Technol. 2019, 24, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Gomez-Amoza, J.L.; Martınez-Pacheco, R.; Souto, C.; Concheiro, A. Evaluation of Low-Substituted Hydroxypropylcelluloses (L-Hpcs) as Filler-Binders for Direct Compression. Int. J. Pharm. 2000, 197, 107–116. [Google Scholar] [CrossRef]
- Schaller, B.E.; Moroney, K.M.; Castro-Dominguez, B.; Cronin, P.; Belen-Girona, J.; Ruane, P.; Croker, D.M.; Walker, G.M. Systematic Development of a High Dosage Formulation to Enable Direct Compression of a Poorly Flowing Api: A Case Study. Int. J. Pharm. 2019, 566, 615–630. [Google Scholar] [CrossRef]
- Low Substituted Hydroxypropyl Cellulose Nf. 2016. Available online: https://www.metolose.jp/en/pharmaceutical/l-hpc.html (accessed on 9 March 2021).
- Sun, C.C. Quantifying Errors in Tableting Data Analysis Using the Ryshkewitch Equation Due to Inaccurate True Density. J. Pharm. Sci. 2005, 94, 2061–2068. [Google Scholar] [CrossRef]
- Sun, C.C. A Novel Method for Deriving True Density of Pharmaceutical Solids Including Hydrates and Water-Containing Powders. J. Pharm. Sci. 2004, 93, 646–653. [Google Scholar] [CrossRef]
- Mishra, S.M.; Rohera, B.D. An Integrated, Quality by Design (Qbd) Approach for Design, Development and Optimization of Orally Disintegrating Tablet Formulation of Carbamazepine. Pharm. Dev. Technol. 2017, 22, 889–903. [Google Scholar] [CrossRef]
- Mamidi, H.K.; Mishra, S.M.; Rohera, B.D. Determination of Maximum Flowable Liquid-Loading Potential of Neusilin® Us2 and Investigation of Compressibility and Compactibility of Its Liquisolid Blends with Peg (400). J. Drug Deliv. Sci. Technol. 2019, 54, 101285. [Google Scholar] [CrossRef]
- Tye, C.K.; Sun, C.C.; Amidon, G.E. Evaluation of the Effects of Tableting Speed on the Relationships between Compaction Pressure, Tablet Tensile Strength, and Tablet Solid Fraction. J. Pharm. Sci. 2005, 94, 465–472. [Google Scholar] [CrossRef]
- Fell, J.T.; Newton, J.M. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef]
- Zhao, N.; Augsburger, L.L. The Influence of Swelling Capacity of Superdisintegrants in Different Ph Media on the Dissolution of Hydrochlorothiazide from Directly Compressed Tablets. AAPS Pharmscitech 2005, 6, E120–E126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuenberger, H. The Compressibility and Compactibility of Powder Systems. Int. J. Pharm. 1982, 12, 41–55. [Google Scholar] [CrossRef]
- Leuenberger, H.; Rohera, B.D. Fundamentals of Powder Compression. I. The Compactibility and Compressibility of Pharmaceutical Powders. Pharm. Res. 1986, 3, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Heckel, R.W. Density-Pressure Relationships in Powder Compaction. Trans. Metall. Soc. AIME 1961, 221, 671–675. [Google Scholar]
- Paul, S.; Sun, C.C. The Suitability of Common Compressibility Equations for Characterizing Plasticity of Diverse Powders. Int. J. Pharm. 2017, 532, 124–130. [Google Scholar] [CrossRef]
- Kuentz, M.; Leuenberger, H. Pressure Susceptibility of Polymer Tablets as a Critical Property: A Modified Heckel Equation. J. Pharm. Sci. 1999, 88, 174–179. [Google Scholar] [CrossRef]
- Ryshkewitch, E. Compression Strength of Porous Sintered Alumina and Zirconia. J. Am. Ceram. Soc. 1953, 36, 65–68. [Google Scholar] [CrossRef]
- van Veen, B.; van der Voort Maarschalk, K.; Bolhuis, G.K.; Frijlink, H.W. Predicting Mechanical Properties of Compacts Containing Two Components. Powder Technol. 2004, 139, 156–164. [Google Scholar] [CrossRef]
- Mishra, S.M. Investigation of Compaction Behavior of Pharmaceutical Powders: An Elucidation Based on Percolation Theory. Ph.D. Thesis, Saint John’s University, New York, NY, USA, 2019. [Google Scholar]
- Leuenberger, H.; Leu, R. Formation of a Tablet: A Site and Bond Percolation Phenomenon. J. Pharm. Sci. 1992, 81, 976–982. [Google Scholar] [CrossRef]
- Guyon, E.; Roux, S.; Hansen, A.; Bideau, D.; Troadec, J.P.; Crapo, H. Non-Local and Non-Linear Problems in the Mechanics of Disordered Systems: Application to Granular Media and Rigidity Problems. Rep. Prog. Phys. 1990, 53, 373. [Google Scholar] [CrossRef]
- Kuentz, M.; Leuenberger, H. A New Theoretical Approach to Tablet Strength of a Binary Mixture Consisting of a Well and a Poorly Compactable Substance. Eur. J. Pharm. Biopharm. 2000, 49, 151–159. [Google Scholar] [CrossRef]
- Bates, D.M.; Watts, D.G. Nonlinear Regression Analysis and Its Applications; Wiley: New York, NY, USA, 1988; Volume 2. [Google Scholar]
- Pabari, R.M.; Ramtoola, Z. Application of Face Centred Central Composite Design to Optimise Compression Force and Tablet Diameter for the Formulation of Mechanically Strong and Fast Disintegrating Orodispersible Tablets. Int. J. Pharm. 2012, 430, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Solaiman, A.; Suliman, A.S.; Shinde, S.; Naz, S.; Elkordy, A.A. Application of General Multilevel Factorial Design with Formulation of Fast Disintegrating Tablets Containing Croscaremellose Sodium and Disintequick Mcc-25. Int. J. Pharm. 2016, 501, 87–95. [Google Scholar] [CrossRef]
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An Overview of the Different Excipients Useful for the Direct Compression of Tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Mattsson, S. Pharmaceutical Binders and Their Function in Directly Compressed Tablets: Mechanistic Studies on the Effect of Dry Binders on Mechanical Strength, Pore Structure and Disintegration of Tablets. Ph.D. Thesis, Acta Universitatis Upsaliensis, Uppsala, Sweden, 2000. [Google Scholar]
- Osei-Yeboah, F.; Chang, S.Y.; Sun, C.C. A Critical Examination of the Phenomenon of Bonding Area-Bonding Strength Interplay in Powder Tableting. Pharm. Res. 2016, 33, 1126–1132. [Google Scholar] [CrossRef]
- Olsson, H.; Mattsson, S.; Nyström, C. Evaluation of the Effect of Addition of Polyethylene Glycols of Differing Molecular Weights on the Mechanical Strength of Sodium Chloride and Sodium Bicarbonate Tablets. Int. J. Pharm. 1998, 171, 31–44. [Google Scholar] [CrossRef]
- Reus-Medina, M.; Lanz, M.; Kumar, V.; Leuenberger, H. Comparative Evaluation of the Powder Properties and Compression Behaviour of a New Cellulose-Based Direct Compression Excipient and Avicel Ph-102. J. Pharm. Pharmacol. 2004, 56, 951–956. [Google Scholar] [CrossRef]
- Ghori, M.U.; Conway, B.R. Powder Compaction: Compression Properties of Cellulose Ethers. Br. J. Pharm. 2016, 1, 19–29. [Google Scholar] [CrossRef]
- Sano, S.; Iwao, Y.; Noguchi, S.; Kimura, S.; Itai, S. Design and Evaluation of Microwave-Treated Orally Disintegrating Tablets Containing Polymeric Disintegrant and Mannitol. Int. J. Pharm. 2013, 448, 132–141. [Google Scholar] [CrossRef]
- Roberts, R.J.; Rowe, R.C. The Effect of the Relationship between Punch Velocity and Particle Size on the Compaction Behaviour of Materials with Varying Deformation Mechanisms. J. Pharm. Pharmacol. 1986, 38, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.S. Modified Celluloses as Multifunctional Excipients in Rapidly Dissolving Immediate Release Tablets. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2008. [Google Scholar]
- Ghori, M.U.; Grover, L.M.; Asare-Addo, K.; Smith, A.M.; Conway, B.R. Evaluating the Swelling, Erosion, and Compaction Properties of Cellulose Ethers. Pharm. Dev. Technol. 2018, 23, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Riepma, K.A.; Lerk, C.F.; De Boer, A.H.; Bolhuis, G.K.; Kussendrager, K.D. Consolidation and Compaction of Powder Mixtures. I. Binary Mixtures of Same Particle Size Fractions of Different Types of Crystalline Lactose. Int. J. Pharm. 1990, 66, 47–52. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Best, S.M.; Bentham, A.C.; Hancock, B.C.; Bonfield, W. Predicting the tensile strength of compacted multi-component mixtures of pharmaceutical powders. Pharm. Res. 2006, 23, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Leane, M.; Pitt, K.; Reynolds, G.; Manufacturing Classification System (MCS) Working Group. A Proposal for a Drug Product Manufacturing Classification System (Mcs) for Oral Solid Dosage Forms. Pharm. Dev. Technol. 2015, 20, 12–21. [Google Scholar] [CrossRef]
- Wu, C.Y.; Best, S.M.; Bentham, A.C.; Hancock, B.C.; Bonfield, W. A Simple Predictive Model for the Tensile Strength of Binary Tablets. Eur. J. Pharm. Sci. 2005, 25, 331–336. [Google Scholar] [CrossRef]
- Sun, C.C. Decoding Powder Tabletability: Roles of Particle Adhesion and Plasticity. J. Adhes. Sci. Technol. 2011, 25, 483–499. [Google Scholar] [CrossRef]
- Queiroz, A.L.; Faisal, W.; Devine, K.; Garvie-Cook, H.; Vucen, S.; Crean, A.M. The Application of Percolation Threshold Theory to Predict Compaction Behaviour of Pharmaceutical Powder Blends. Powder Technol. 2019, 354, 188–198. [Google Scholar] [CrossRef]
- Desai, P.M.; Liew, C.V.; Heng, P.W. Review of Disintegrants and the Disintegration Phenomena. J. Pharm. Sci. 2016, 105, 2545–2555. [Google Scholar] [CrossRef] [Green Version]
- Berardi, A.; Bisharat, L.; Quodbach, J.; Rahim, S.A.; Perinelli, D.R.; Cespi, M. Advancing the Understanding of the Tablet Disintegration Phenomenon-an Update on Recent Studies. Int. J. Pharm. 2021, 598, 120390. [Google Scholar] [CrossRef]
- Zhao, N.; Augsburger, L.L. Functionality Comparison of 3 Classes of Superdisintegrants in Promoting Aspirin Tablet Disintegration and Dissolution. AAPS Pharmscitech 2005, 6, E634–E640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bele, M.H.; Derle, D.V. Mechanism of Disintegrant Action of Polacrilin Potassium: Swelling or Wicking? Acta Pharm. Sin. B 2012, 2, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Smallenbroek, A.J.; Bolhuis, G.K.; Lerk, C.F. The Effect of Particle Size of Disintegrants on the Disintegration of Tablets. Pharm. Weekbl. 1981, 3, 1048–1051. [Google Scholar] [CrossRef]
- Markl, D.; Zeitler, J.A. A Review of Disintegration Mechanisms and Measurement Techniques. Pharm. Res. 2017, 34, 890–917. [Google Scholar] [CrossRef] [Green Version]
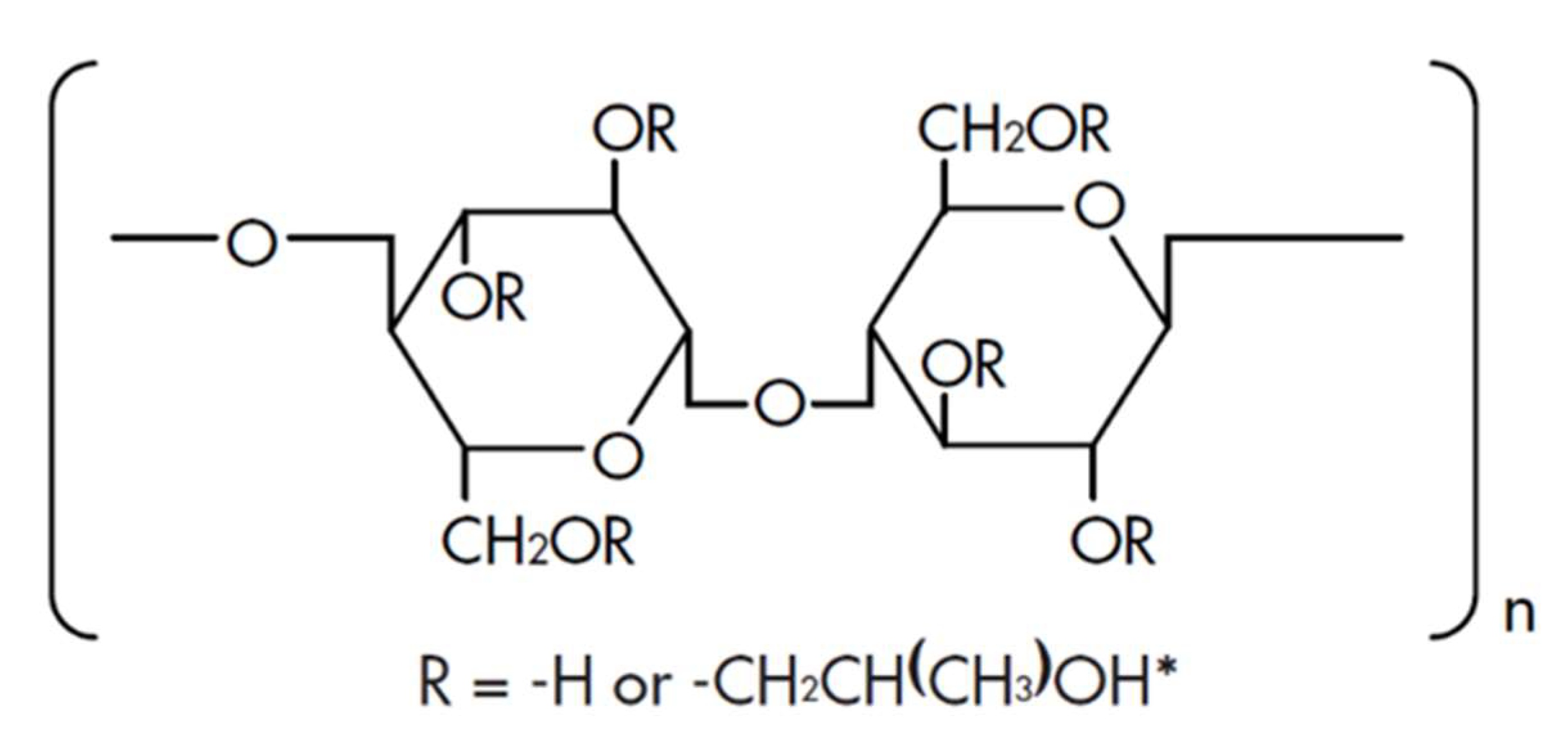

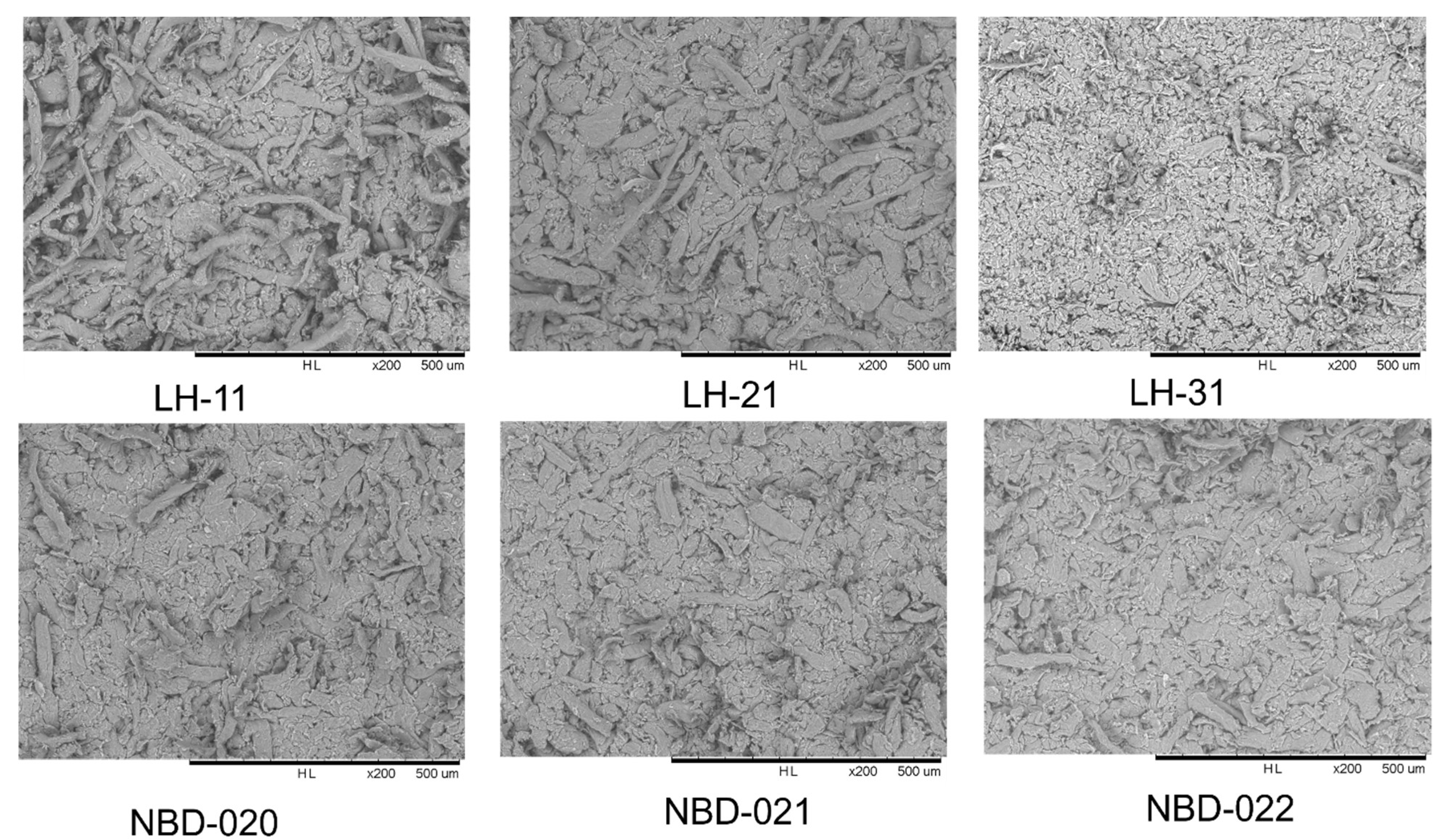


| Grade | Particle Size (D50) (µm) | HPO Content (%) |
|---|---|---|
| LH-11 | 52 | 10.8 |
| LH-21 | 49.8 | 11 |
| LH-31 | 19.4 | 11.3 |
| NBD-020 | 42.3 | 13.8 |
| NBD-021 | 39.6 | 10.9 |
| NBD-022 | 38.7 | 8.2 |
| Design I: Effect of Particle Size and Compression Load on Tablet Porosity and Disintegration Time | |||
| Independent Variables | Level | ||
| Low (−1) | Medium (0) | High (+1) | |
| Particle size (µm) | 19.4 (LH-31) | 49.8 (LH-21) | 52 (LH-11) |
| Compression load (MPa) | 25 | 50 | 75 |
| Design II: Effect of % HPO Content and Compression Load on Tablet Porosity and Disintegration Time | |||
| Independent Variables | Level | ||
| Low (−1) | Medium (0) | High (+1) | |
| Hydroxypropyl content (%) | 8.2 (NBD-022) | 10.9 (NBD-021) | 13.8 (NBD-020) |
| Compression load (MPa) | 25 | 50 | 75 |
| Responses | |||
| Tablet Porosity (%) | |||
| Disintegration time (s) | |||
| Grades | Bulk Density (g/cm3) | Tapped Density (g/cm3) | True Density (g/cm3) |
|---|---|---|---|
| LH-11 | 0.314 ± 0.020 | 0.489 ± 0.030 | 1.3955 ± 0.0007 |
| LH-21 | 0.375 ± 0.022 | 0.618 ± 0.034 | 1.4446 ± 0.0004 |
| LH-31 | 0.295 ± 0.018 | 0.519 ± 0.029 | 1.4252 ± 0.0003 |
| NDB-020 | 0.317 ± 0.014 | 0.491 ± 0.021 | 1.4466 ± 0.0004 |
| NBD-021 | 0.310± 0.019 | 0.480± 0.021 | 1.3948 ± 0.0007 |
| NBD-022 | 0.313 ± 0.015 | 0.491 ± 0.024 | 1.4768 ± 0.0012 |
| L-HPC Grade | Compressibility (1/C) (MPa) | Percolation Threshold (ρc) (-) | R2 | Adjusted R2 | Root Mean Square Error (RMSE) |
|---|---|---|---|---|---|
| LH-11 | 152.21 ± 6.55 | 0.246 ± 0.013 | 0.9975 | 0.9973 | 1.35 |
| LH-21 | 172.12 ± 32.88 | 0.237 ± 0.057 | 0.9486 | 0.9439 | 6.08 |
| LH-31 | 204.92 ± 14.03 | 0.292 ± 0.018 | 0.9931 | 0.9925 | 2.22 |
| NBD-020 | 188.68 ± 13.76 | 0.229 ± 0.021 | 0.9928 | 0.9922 | 2.27 |
| NBD-021 | 98.52 ± 4.45 | 0.170 ± 0.017 | 0.9976 | 0.9973 | 1.32 |
| NBD-022 | 176.99 ± 9.29 | 0.196 ± 0.016 | 0.9963 | 0.9960 | 1.63 |
| L-HPC Grade | Compactibility σ0, (MPa) | Percolation Threshold (ρr) (-) | R2 | Adjusted R2 | Root Mean Square Error (RMSE) |
|---|---|---|---|---|---|
| LH-11 | 10.88 ± 0.26 | 0.377 ± 0.008 | 0.9976 | 0.9973 | 0.07 |
| LH-21 | 8.91 ± 0.34 | 0.350 ± 0.013 | 0.9931 | 0.9924 | 0.08 |
| LH-31 | 10.82 ± 0.15 | 0.378 ± 0.004 | 0.9992 | 0.9991 | 0.03 |
| NBD-020 | 10.55 ± 0.14 | 0.355 ± 0.004 | 0.9992 | 0.9991 | 0.04 |
| NBD-021 | 9.95 ± 0.25 | 0.366 ± 0.010 | 0.9973 | 0.9970 | 0.08 |
| NBD-022 | 12.51 ± 0.21 | 0.358 ± 0.005 | 0.9989 | 0.9988 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, S.M.; Sauer, A. Effect of Physical Properties and Chemical Substitution of Excipient on Compaction and Disintegration Behavior of Tablet: A Case Study of Low-Substituted Hydroxypropyl Cellulose (L-HPC). Macromol 2022, 2, 113-130. https://doi.org/10.3390/macromol2010007
Mishra SM, Sauer A. Effect of Physical Properties and Chemical Substitution of Excipient on Compaction and Disintegration Behavior of Tablet: A Case Study of Low-Substituted Hydroxypropyl Cellulose (L-HPC). Macromol. 2022; 2(1):113-130. https://doi.org/10.3390/macromol2010007
Chicago/Turabian StyleMishra, Saurabh M, and Andreas Sauer. 2022. "Effect of Physical Properties and Chemical Substitution of Excipient on Compaction and Disintegration Behavior of Tablet: A Case Study of Low-Substituted Hydroxypropyl Cellulose (L-HPC)" Macromol 2, no. 1: 113-130. https://doi.org/10.3390/macromol2010007
APA StyleMishra, S. M., & Sauer, A. (2022). Effect of Physical Properties and Chemical Substitution of Excipient on Compaction and Disintegration Behavior of Tablet: A Case Study of Low-Substituted Hydroxypropyl Cellulose (L-HPC). Macromol, 2(1), 113-130. https://doi.org/10.3390/macromol2010007







