Neat Linear Polysiloxane-Based Ionic Polymers: Insights into Structure-Based Property Modifications and Applications
Abstract
:1. Introduction
1.1. A Brief History of Polysiloxanes, Including an Overview of Their Properties
1.2. Ionic Polymers and Polysiloxanes
2. Types of Polysiloxane-Based Ionic Polymers
2.1. Polyelectrolytes Where All Monomers Except the Terminal Group Contain an Ion
2.2. Polysiloxane-Based Polyelectrolytes with Some Monomers not Containing Ion Pairs
3. Melt Rheology of Polysiloxane-Based Ionic Polymers
3.1. Melt Rheology
3.2. Melt Rheology of Polysiloxane-Based Ionic Iolymers
4. Applications of Polysiloxane-Based Ionic Polymers
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Thomas, N.R. Frederic Kipping—Pioneer in Silicon Chemistry: His Life & Legacy. Silicon 2010, 2, 187–193. [Google Scholar]
- Rochow, E.G. Methyl Aryl Silicones and Insulated Conductors and Other Products Utilizing the Same. U.S. Patent 2,258,222, 7 October 1941. [Google Scholar]
- Rochow, E.G. Methyl Silicones and Related Products. U.S. Patent 2,258,218, 7 October 1941. [Google Scholar]
- Hyde, J.F. Organo-Silicon Polymers and Method of Making Them. U.S. Patent 2,371,050, 6 March 1945. [Google Scholar]
- Currie, C.C. Leather Water Repellent. U.S. Patent 2,672,455, 16 March 1954. [Google Scholar]
- Currie, C.C.; Keil, J.W. Organopolysiloxane Adhesive and Pressure-Sensitive Adhesive Tape Containing Same. U.S. Patent 2,814,601, 26 November 1957. [Google Scholar]
- Rauner, L.A. Method for Reducing or Preventing Foam in Liquid Mediums. U.S. Patent 3,455,839, 15 July 1969. [Google Scholar]
- Hartlein, R. Epoxy Silane Coupling Agent. U.S. Patent 3,702,783, 14 November 1972. [Google Scholar] [CrossRef]
- Cobb, V.S.; Rauscher, W.W.; Stanga, M.A.; Stevens, R.E.; Whitmarsh, R.H.; Wiese, K.D. Silicone Polyether Surfactants. U.S. Patent 5,830,970, 3 November 1998. [Google Scholar]
- Ahn, D.; Schulz, W.; Thompson, J. Silicone Compositions Comprising a Swollen Silicone Gel. U.S. Patent 9,243,113B2, 26 January 2016. [Google Scholar]
- Magalhäes, S.; Alves, L.; Medronho, B.; Fonseca, A.C.; Romano, A.; Coelho, J.F.J.; Norgren, M. Brief Overview on Bio-based Adhesives and Sealants. Polymers 2019, 11, 1685–1705. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.M. Alex. Poly(Dimethylsiloxane). In Polymer Data Handbook; Mark, J., Ed.; Oxford University Press: New York, NY, USA, 1999; pp. 411–435. [Google Scholar]
- Noll, W. CHAPTER 5—Preparation of Polyorganosiloxanes. In Chemistry and Technology of Silicones; Academic Press INC.: New York, NY, USA, 1968; pp. 190–245. [Google Scholar] [CrossRef]
- Noll, W. The Polymeric Organosiloxanes. In Chemistry and Technology of Silicones; Academic Press INC.: New York, NY, USA, 1968; pp. 246–331. [Google Scholar] [CrossRef]
- Smith, J.S.; Borodin, O.; Smith, G.D. A Quantum Chemistry Based Force Field for Poly(Dimethylsiloxane). J. Phys. Chem. B 2004, 108, 20340–20350. [Google Scholar] [CrossRef]
- Weinhold, F.; West, R. The Nature of the Silicon-Oxygen Bond. Organometallics 2011, 30, 5815–5824. [Google Scholar] [CrossRef]
- Karstedt, B.D. Platinum Complexes of Unsaturated Siloxanes and Platinum Containing Organopolysiloxanes. U.S. Patent 3,775,452, 27 November 1973. [Google Scholar]
- Stochmal, E.; Strzezik, J.; Krowiak, A. Physicochemical and Catalytic Properties of Polysiloxane Network–Pt Systems. RSC Adv. 2017, 7, 26342–26360. [Google Scholar] [CrossRef] [Green Version]
- Shanmuga sundar, D.; Sivanantha Raja, A.; Sanjeeviraja, C.; Jeyakumar, D. Highly Transparent Flexible Polydimethylsiloxane Films—A Promising Candidate for Optoelectronic Devices. Polym. Int. 2016, 65, 535–543. [Google Scholar] [CrossRef]
- Neplokh, V.; Kochetkov, F.M.; Deriabin, K.V.; Fedorov, V.V.; Bolshakov, A.D.; Eliseev, I.E.; Mikhailovskii, V.Y.; Ilatovskii, D.A.; Krasnikov, D.V.; Tchernycheva, M.; et al. Modified Silicone Rubber for Fabrication and Contacting of Flexible Suspended Membranes of n-/p-GaP Nanowires with a Single-Walled Carbon Nanotube Transparent Contact. Available online: https://arxiv.org/abs/1910.13182 (accessed on 19 July 2020).
- CosIng—European Commission Database for Information on Cosmetics Substances and Ingredients. Available online: https://ec.europa.eu/growth/tools-databases/cosing/ (accessed on 19 July 2020).
- He, Y.; Zhao, H.; Yao, M.; Weiss, R.G. Complex New Materials from Simple Chemistry: Combining an Amino-Substituted Polysiloxane and Carboxylic Acids. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3851–3861. [Google Scholar] [CrossRef]
- Disapio, A.; Fridd, P. Silicones: Use of Substantive Properties on Skin and Hair. Int. J. Cosmet. Sci. 1988, 10, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.W.; Caibao, Q.; Grigoras, S.; Halloran, D.J.; Zimmerman, B.L. Fundamental Aspects of Aminoalkyl Siloxane Softeners by Molecular Modeling and Experimental Methods. Text. Res. J. 1999, 69, 935–943. [Google Scholar] [CrossRef]
- Allen, M.H.; Wang, S.; Hemp, S.T.; Chen, Y.; Madsen, L.A.; Winey, K.I.; Long, T.E. Hydroxyalkyl-Containing Imidazolium Homopolymers: Correlation of Structure with Conductivity. Macromolecules 2013, 46, 3037–3045. [Google Scholar] [CrossRef]
- Sanchez, J.Y.; Iojolu, C.; Alloin, F.; Guindet, J.; Lepretre, J.C. Fuel Cells—Proton Exchange Membrane Fuel Cells. Membranes: Non-Fluorinated. In Encyclopedia of Electrochemical Power Sources; Elsevier B.V.: Amsterdam, The Netherlands, 2009; Available online: https://www.sciencedirect.com/science/article/pii/B978044452745500887X (accessed on 12 December 2020).
- Zhang, L.; Brostowitz, N.R.; Cavicchi, K.A.; Weiss, R.A. Perspective: Ionomer Research and Applications. Macromol. React. Eng. 2014, 8, 81–99. [Google Scholar] [CrossRef]
- Choi, U.H.; Lee, M.; Wang, S.; Liu, W.; Winey, K.I.; Gibson, H.W.; Colby, R.H. Ionic Conduction and Dielectric Response of Poly(Imidazolium Acrylate) Ionomers. Macromolecules 2012, 45, 3974–3985. [Google Scholar] [CrossRef]
- Eisenberg, A.; Rinaudo, M. Polyelectrolytes and Ionomers. Polym. Bull. 1990, 24, 671. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Q.; Colby, R.H. Dynamics of Associative Polymers. Soft Matter 2018, 14, 2961–2977. [Google Scholar] [CrossRef]
- Jourdain, A.; Serghei, A.; Drockenmuller, E. Enhanced Ionic Conductivity of a 1,2,3-Triazolium-Based Poly(Siloxane Ionic Liquid) Homopolymer. ACS Macro Lett. 2016, 5, 1283–1286. [Google Scholar] [CrossRef]
- Bocharova, V.; Wojnarowska, Z.; Cao, P.F.; Fu, Y.; Kumar, R.; Li, B.; Novikov, V.N.; Zhao, S.; Kisliuk, A.; Saito, T.; et al. Influence of Chain Rigidity and Dielectric Constant on the Glass Transition Temperature in Polymerized Ionic Liquids. J. Phys. Chem. B 2017, 121, 11511–11519. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(Ionic Liquid)s: An Update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, F.; Agapov, A.L.; Yu, X.; Hong, K.; Mays, J.; Sokolov, A.P. Design of Superionic Polymers—New Insights from Walden Plot Analysis. Solid State Ion. 2014, 262, 782–784. [Google Scholar] [CrossRef]
- Wang, Y.; Sokolov, A.P. Design of Superionic Polymer Electrolytes. Curr. Opin. Chem. Eng. 2015, 7, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, Y.; Iyi, N.; Kurashima, K.; Matsumoto, T.; Fujita, T.; Kitamura, K. Hexagonal-Structured Polysiloxane Material Prepared by Sol-Gel Reaction of Aminoalkyltrialkoxysilane without Using Surfactants. Chem. Mater. 2004, 16, 3417–3423. [Google Scholar] [CrossRef]
- Kaneko, Y.; Iyi, N.; Matsumoto, T.; Kitamura, K. Preparation of Higher-Ordered Inorganic-Organic Nanocomposite Composed of Rodlike Cationic Polysiloxane and Polyacrylate. J. Mater. Chem. 2005, 15, 1572–1575. [Google Scholar] [CrossRef]
- Kaneko, Y.; Iyi, N.; Matsumoto, T.; Kitamura, K. Synthesis of Rodlike Polysiloxane with Hexagonal Phase by Sol-Gel Reaction of Organotrialkoxysilane Monomer Containing Two Amino Groups. Polymer 2005, 46, 1828–1833. [Google Scholar] [CrossRef]
- Kubo, T.; Koge, S.; Ohshita, J.; Kaneko, Y. Preparation of Imidazolium Salt Type IonicLiquids Containing CyclicSiloxane Frameworks. Chem. Lett. 2015, 44, 1362–1364. [Google Scholar] [CrossRef]
- Zuo, Y.; Gou, Z.; Li, Z.; Qi, J.; Feng, S. Unexpected Self-Assembly, Photoluminescence Behavior, and Film-Forming Properties of Polysiloxane-Based Imidazolium Ionic Liquids Prepared by One-Pot Thiol–Ene Reaction. New J. Chem. 2017, 41, 14545–14550. [Google Scholar] [CrossRef]
- Kaneko, Y.; Iyi, N.; Matsumoto, T.; Fujii, K.; Kurashima, K.; Fujita, T. Synthesis of Ion-Exchangeable Layered Polysiloxane by Sol-Gel Reaction of Aminoalkyltrialkoxysilane: A New Preparation Method for Layered Polysiloxane Materials. J. Mater. Chem. 2003, 13, 2058–2060. [Google Scholar] [CrossRef]
- Antonietti, M.; Burger, C.; Effing, J. Mesomorphous Polyelectrolyte-Surfactant Complexes. Adv. Mater. 1995, 7, 751–753. [Google Scholar] [CrossRef]
- Antonietti, M.; Conrad, J.; Thünemann, A. Polyelectrolyte-Surfactant Complexes: A New Type of Solid, Mesomorphous Material. Macromolecules 1994, 27, 6007–6011. [Google Scholar] [CrossRef]
- Sokolov, E.L.; Yeh, F.; Khokhlov, A.; Chu, B. Nanoscale Supramolecular Ordering in Gel-Surfactant Complexes: Sodium Alkyl Sulfates in Poly(Diallyldimethylammonium Chloride). Langmuir 1996, 12, 6229–6234. [Google Scholar] [CrossRef]
- De Oliveira, V.A.; Tiera, M.J.; Neumann, M.G. Interaction of Cationic Surfactants with Acrylic Acid-Ethyl Methacrylate Copolymers. Langmuir 1996, 12, 607–612. [Google Scholar] [CrossRef]
- Gainaru, C.; Stacy, E.W.; Bocharova, V.; Gobet, M.; Holt, A.P.; Saito, T.; Greenbaum, S.; Sokolov, A.P. Mechanism of Conductivity Relaxation in Liquid and Polymeric Electrolytes: Direct Link between Conductivity and Diffusivity. J. Phys. Chem. B 2016, 120, 11074–11083. [Google Scholar] [CrossRef]
- Marangoci, N.; Ardeleanu, R.; Ursu, L.; Ibanescu, C.; Danu, M.; Pinteala, M.; Simionescu, B.C. Polysiloxane Ionic Liquids as Good Solvents for Beta-Cyclodextrin-Polydimethylsiloxane Polyrotaxane Structures. Beilstein J. Org. Chem. 2012, 8, 1610–1618. [Google Scholar] [CrossRef]
- Snyder, J.F.; Hutchison, J.C.; Ratner, M.A.; Shriver, D.F. Synthesis of Comb Polysiloxane Polyelectrolytes Containing Oligoether and Perfluoroether Side Chains. Chem. Mater. 2003, 15, 4223–4230. [Google Scholar] [CrossRef]
- Li, L.; Feng, W.; Welle, A.; Levkin, P.A. UV-Induced Disulfide Formation and Reduction for Dynamic Photopatterning. Angew. Chem. 2016, 128, 13969–13973. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Bai, L.; Tang, X.; Gao, Y.; Pan, D.; He, X.; Meng, F. Ionic Liquid-Crystalline Network Polymers Formed by Sulfonic Acid-Containing Polysiloxanes and Pyridinium Compounds. Eur. Polym. J. 2018, 100, 146–152. [Google Scholar] [CrossRef]
- Mitov, M. Cholesteric Liquid Crystals in Living Matter. Soft Matter 2017, 13, 4176–4209. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, S.; Shiau, H.S.; Colby, R.H. Linear Viscoelastic and Dielectric Properties of Phosphonium Siloxane Ionomers. ACS Macro Lett. 2013, 2, 970–974. [Google Scholar] [CrossRef]
- Liang, S.; Oreilly, M.V.; Choi, U.H.; Shiau, H.S.; Bartels, J.; Chen, Q.; Runt, J.; Winey, K.I.; Colby, R.H. High Ion Content Siloxane Phosphonium Ionomers with Very Low Tg. Macromolecules 2014, 47, 4428–4437. [Google Scholar] [CrossRef]
- Choi, U.H.; Liang, S.; Chen, Q.; Runt, J.; Colby, R.H. Segmental Dynamics and Dielectric Constant of Polysiloxane Polar Copolymers as Plasticizers for Polymer Electrolytes. ACS Appl. Mater. Interfaces 2016, 8, 3215–3225. [Google Scholar] [CrossRef]
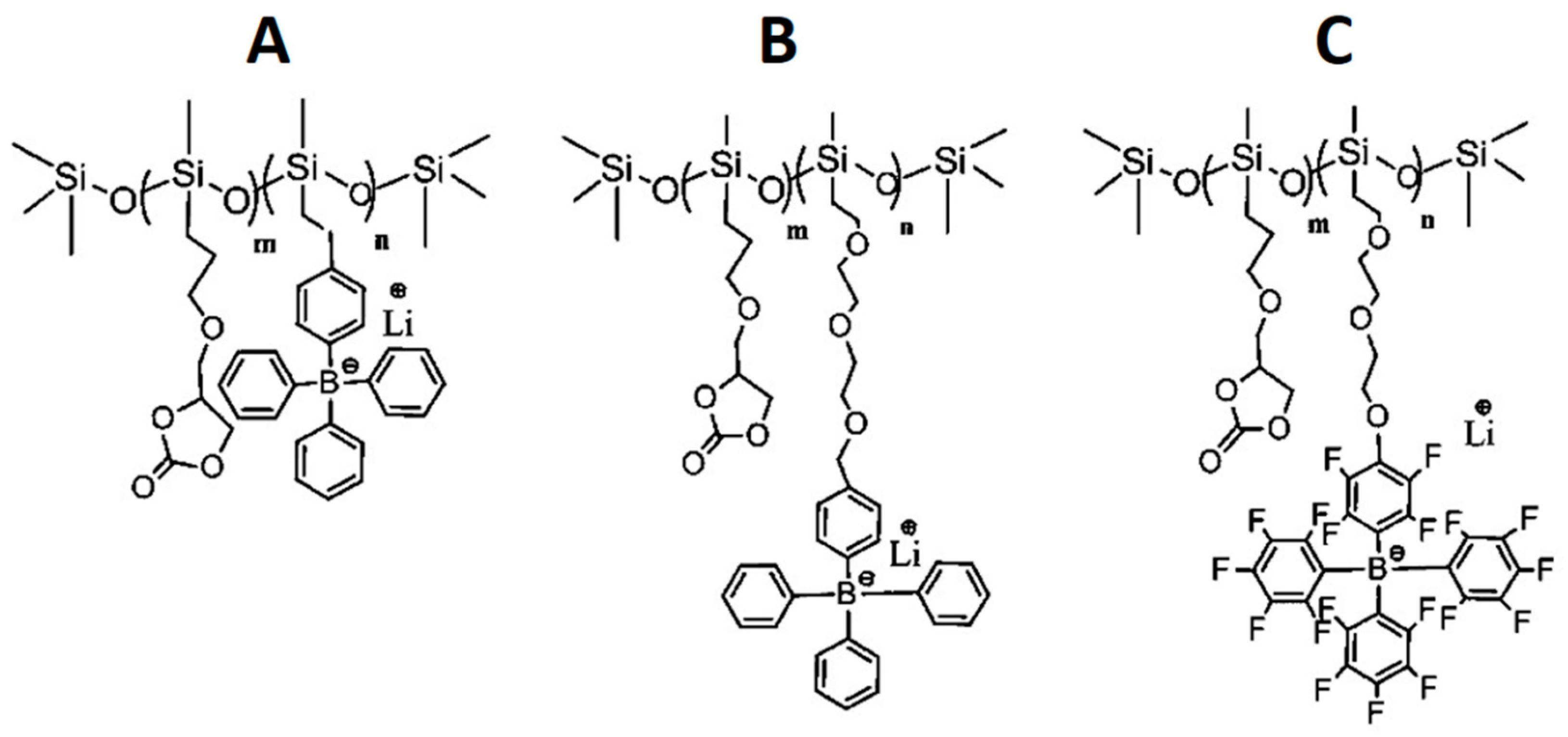
- Liang, S.; Choi, U.H.; Liu, W.; Runt, J.; Colby, R.H. Synthesis and Lithium Ion Conduction of Polysiloxane Single-Ion Conductors Containing Novel Weak-Binding Borates. Chem. Mater. 2012, 24, 2316–2323. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, Y.; Huang, Y. Ion Aggregation in the Polysiloxane Ionomers Bearing Pendant Quaternary Ammonium Groups. J. Appl. Polym. Sci. 2002, 83, 3099–3104. [Google Scholar] [CrossRef]
- Steinbach, J.C.; Schneider, M.; Hauler, O.; Lorenz, G.; Rebner, K.; Kandelbauer, A. A Process Analytical Concept for In-Line FTIR Monitoring of Polysiloxane Formation. Polymer 2020, 12, 2473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Xu, T.; Sima, H.; Hou, J. Effects of Polyhedral Oligomeric Silsesquioxane (POSS) on Thermal and Mechanical Properties of Polysiloxane Foam. Materials 2020, 13, 4570. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, Z.; Colby, R.H. Viscoelasticity of Entangled Random Polystyrene Ionomers. J. Rheol. 2016, 60, 1031–1040. [Google Scholar] [CrossRef]
- Chen, Q.; Tudryn, G.J.; Colby, R.H. Ionomer Dynamics and the Sticky Rouse Model. J. Rheol. 2013, 57, 1441–1462. [Google Scholar] [CrossRef]
- Leibler, L.; Rubinstein, M.; Colby, R.H. Dynamics of Reversible Networks. Macromolecules 1991, 24, 4701–4707. [Google Scholar] [CrossRef]
- Rouse, P.E. A Theory of the Linear Viscoelastic Properties of Dilute Solutions of Coiling Polymers. J. Chem. Phys. 1953, 21, 1272–1280. [Google Scholar] [CrossRef]
- Lindman, B.; Antunes, F.; Aidarova, S.; Miguel, M.; Nylander, T. Polyelectrolyte–Surfactant Association—from Fundamentals to Applications. Colloid J. 2014, 76, 635–644. [Google Scholar] [CrossRef]
- Zimm, B.H. Dynamics of Polymer Molecules in Dilute Solution: Viscoelasticity, Flow Birefringence and Dielectric Loss. J. Chem. Phys. 1956, 24, 269–278. [Google Scholar] [CrossRef]
- Colby, R.H. Structure and linear viscoelasticity of flexible polymer solutions: Comparison of polyelectrolyte and neutral polymer solutions. Rheol. Acta 2009, 49, 1441–1462. [Google Scholar] [CrossRef]
- Batra, A.; Cohen, C.; Duncan, T.M. Scaling Behavior of the Viscosity of Poly(Dimethylsiloxane) Ionomer Solutions. Macromolecules 2006, 39, 2398–2404. [Google Scholar] [CrossRef]
- Yu, T.; Wakuda, K.; Blair, D.L.; Weiss, R.G. Reversibly Cross-Linking Amino-Polysiloxanes by Simple Triatomic Molecules. Facile Methods for Tuning Thermal, Rheological, and Adhesive Properties. J. Phys. Chem. C 2009, 113, 11546–11553. [Google Scholar] [CrossRef]
- Gerhardt, L.J.; Manke, C.W.; Gulari, E. Rheology of Polydimethylsiloxane Swollen with Supercritical Carbon Dioxide. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 523–534. [Google Scholar] [CrossRef]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-Forming Liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Kasapis, S.; Mitchell, J.R. Definition of the Rheological Glass Transition Temperature in Association with the Concept of Iso-Free-Volume. Int. J. Biol. Macromol. 2001, 29, 315–321. [Google Scholar] [CrossRef]
- Johari, C.P.; Goidstein, M. Viscous Liquids and the Glass Transition. II. Secondary Relaxations in Glasses of Rigid Molecules. J. Chem. Phys. 1970, 53, 2372–2388. [Google Scholar] [CrossRef]
- Ngai, K.L. Relation between Some Secondary Relaxations and the α Relaxations in Glass-Forming Materials According to the Coupling Model. J. Chem. Phys. 1998, 109, 6982–6994. [Google Scholar] [CrossRef]
- Alegría, A.; Guerrica-Echevarría, E.; Goitiandía, L.; Telleria, I.; Colmenero, J. α-Relaxation in the Glass Transition Range of Amorphous Polymers. 1. Temperature Behavior across the Glass Transition. Macromolecules 1995, 28, 1516–1527. [Google Scholar] [CrossRef]
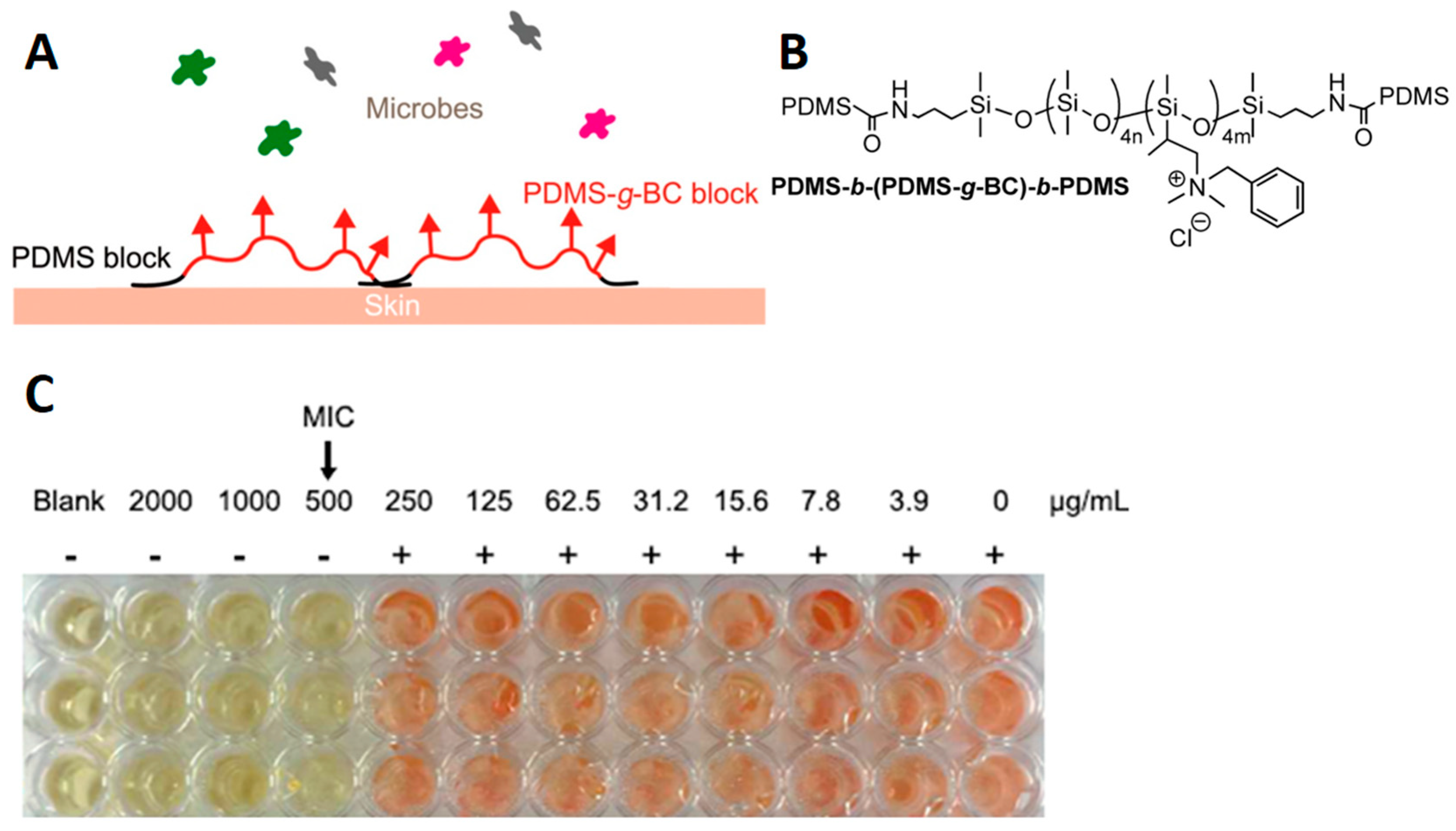
- Hazziza-Laskar, J.; Helary, G.; Sauvet, G. Biocidal Polymers Active by Contact. IV. Polyurethanes Based on Polysiloxanes with Pendant Primary Alcohols and Quaternary Ammonium Groups. J. Appl. Polym. Sci. 1995, 58, 77–84. [Google Scholar] [CrossRef]
- Novi, C.; Mourran, A.; Keul, H.; Möller, M. Ammonium-Functionalized Polydimethylsiloxanes: Synthesis and Properties. Macromol. Chem. Phys. 2006, 207, 273–286. [Google Scholar] [CrossRef]
- Sauvet, G.; Fortuniak, W.; Kazmierski, K.; Chojnowski, J. Amphiphilic Block and Statistical Siloxane Copolymers with Antimicrobial Activity. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2939–2948. [Google Scholar] [CrossRef]
- Domagk, G. Eine Neue Klasse von Desinfektionsmitteln. Dtsch. Med. Wochenschr. 1935, 61, 829–832. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary Ammonium-Based Biomedical Materials: State-of-the-Art, Toxicological Aspects and Antimicrobial Resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Cancouët, P.; Daudet, E.; Hélary, G.; Moreau, M.; Sauvet, G. Functional Polysiloxanes. I. Microstructure of Poly(Hydrogenmethylsiloxane-Co-Dimethylsiloxane)s Obtained by Cationic Copolymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 826–836. [Google Scholar] [CrossRef]
- Lei, Y.; Zhou, S.; Dong, C.; Zhang, A.; Lin, Y. PDMS Tri-Block Copolymers Bearing Quaternary Ammonium Salts for Epidermal Antimicrobial Agents: Synthesis, Surface Adsorption and Non-Skin-Penetration. React. Funct. Polym. 2018, 124, 20–28. [Google Scholar] [CrossRef]
- Fakirov, S. Flexibility of Polymer Chains and Its Origin. In Fundamentals of Polymer Science for Engineers; John Wiley & Sons, Inc.: Weinheim, Germany, 2017; pp. 43–58. [Google Scholar] [CrossRef]
- Choi, U.H.; Ye, Y.; Salas De La Cruz, D.; Liu, W.; Winey, K.I.; Elabd, Y.A.; Runt, J.; Colby, R.H. Dielectric and Viscoelastic Responses of Imidazolium-Based Ionomers with Different Counterions and Side Chain Lengths. Macromolecules 2014, 47, 777–790. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poon, L.; Hum, J.R.; Weiss, R.G. Neat Linear Polysiloxane-Based Ionic Polymers: Insights into Structure-Based Property Modifications and Applications. Macromol 2021, 1, 2-17. https://doi.org/10.3390/macromol1010002
Poon L, Hum JR, Weiss RG. Neat Linear Polysiloxane-Based Ionic Polymers: Insights into Structure-Based Property Modifications and Applications. Macromol. 2021; 1(1):2-17. https://doi.org/10.3390/macromol1010002
Chicago/Turabian StylePoon, Louis, Jacob R. Hum, and Richard G. Weiss. 2021. "Neat Linear Polysiloxane-Based Ionic Polymers: Insights into Structure-Based Property Modifications and Applications" Macromol 1, no. 1: 2-17. https://doi.org/10.3390/macromol1010002
APA StylePoon, L., Hum, J. R., & Weiss, R. G. (2021). Neat Linear Polysiloxane-Based Ionic Polymers: Insights into Structure-Based Property Modifications and Applications. Macromol, 1(1), 2-17. https://doi.org/10.3390/macromol1010002




