Drug-Induced Acute Generalized Exanthematous Pustulosis: Mechanisms, Diagnosis, and Clinical Differentiation from Other Pustular Eruptions
Abstract
1. Introduction
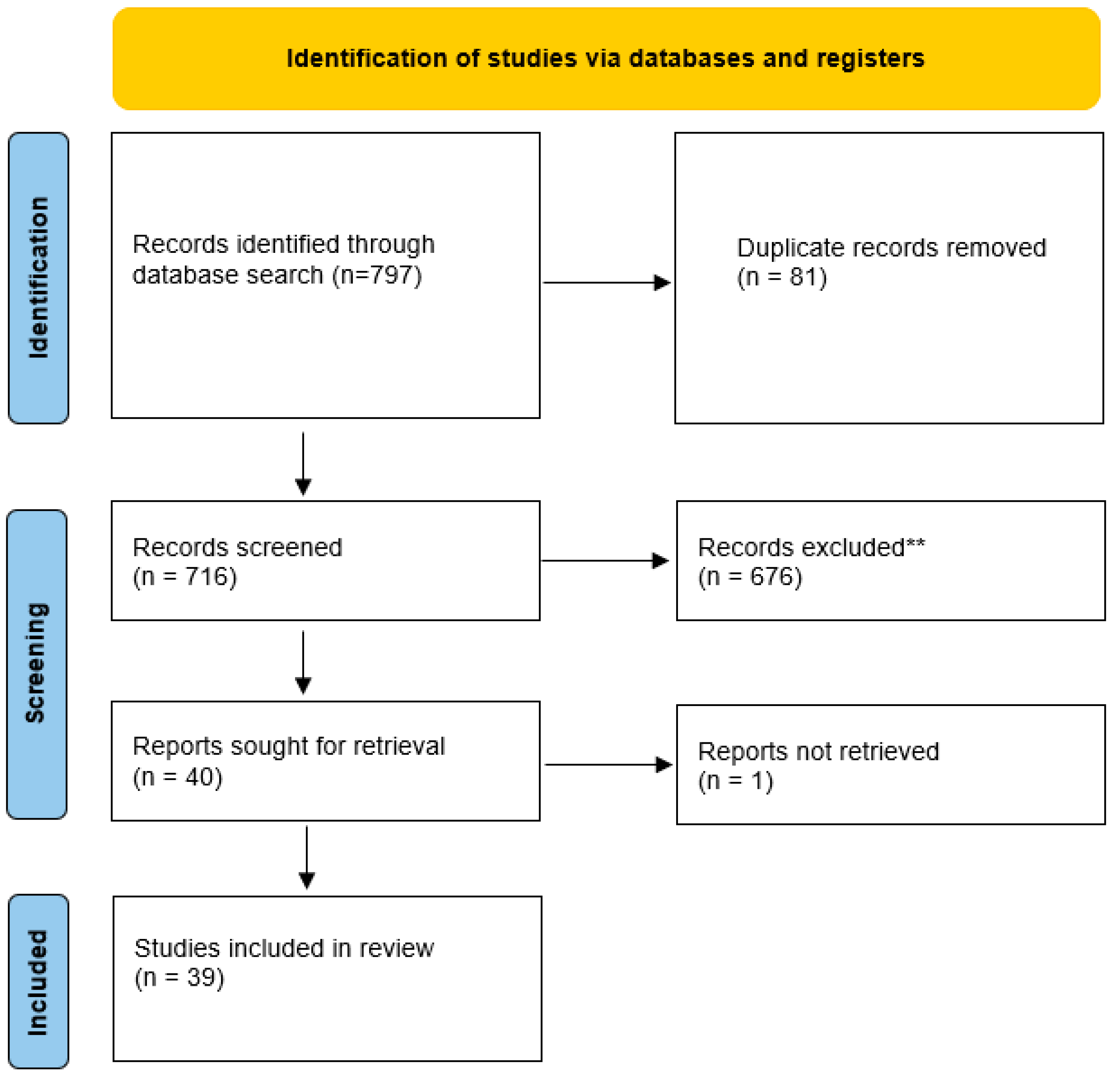
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Drug Selection
2.4. Data Extraction and Synthesis
2.5. Use of Generative Artificial Intelligence
3. Clinical and Pathophysiological Overview
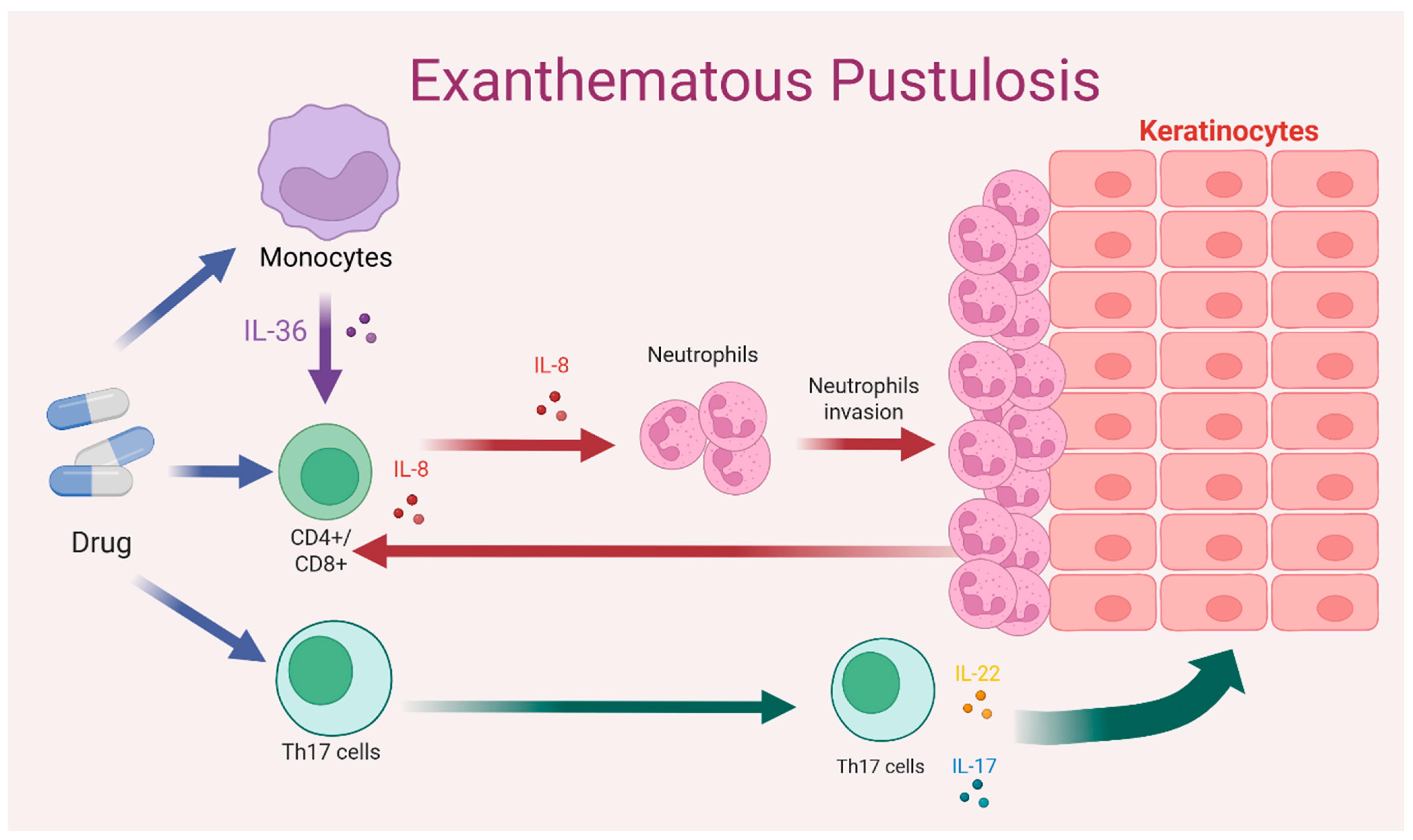
3.1. Immunopathogenesis
3.2. Clinical Features
3.3. Differential Diagnosis
3.3.1. AGEP vs. GPP
3.3.2. AGEP vs. DRESS/DIHS
3.3.3. AGEP vs. SJS/TEN
3.4. Histopathology and Biomarkers
| Biomarker/Finding | Description | Diagnostic Utility | Limitations |
|---|---|---|---|
| IL-36 (α, β, γ) [1,2] | Elevated in epidermis and dermis | Differentiates AGEP from other pustuloses; supports drug-dependent hyperinflammation [1,2] | Not routinely available; limited studies |
| IL36RN mutations [1,2,4,5] | Loss of IL-36 receptor antagonist function [1,2,4,5] | Suggests higher risk and greater inflammatory intensity | Requires genetic sequencing |
| Subcorneal/intraepidermal pustules [1,2,3,4,5] | Neutrophil-rich pustules in the superficial epidermis | Classic histologic feature of AGEP [1,2,3,4,5] | Overlaps with pustular psoriasis |
| Papillary dermal edema [1,2,4,5] | Prominent papillary dermal edema [1,2,4,5] | Compatible with AGEP | Also present in other drug reactions |
| Eosinophils in dermis [1,2,4,5] | Mixed inflammatory infiltrate with eosinophils [1,2,4,5] | Supports drug-related hypersensitivity | Not specific to AGEP |
| IL-8, CXCL1, CXCL2 [1,2,3,4,5,13,14] | Elevated neutrophil-attracting cytokines [1,2,3,4,5] | Associated with pustular extension | Not routinely available in clinical practice |
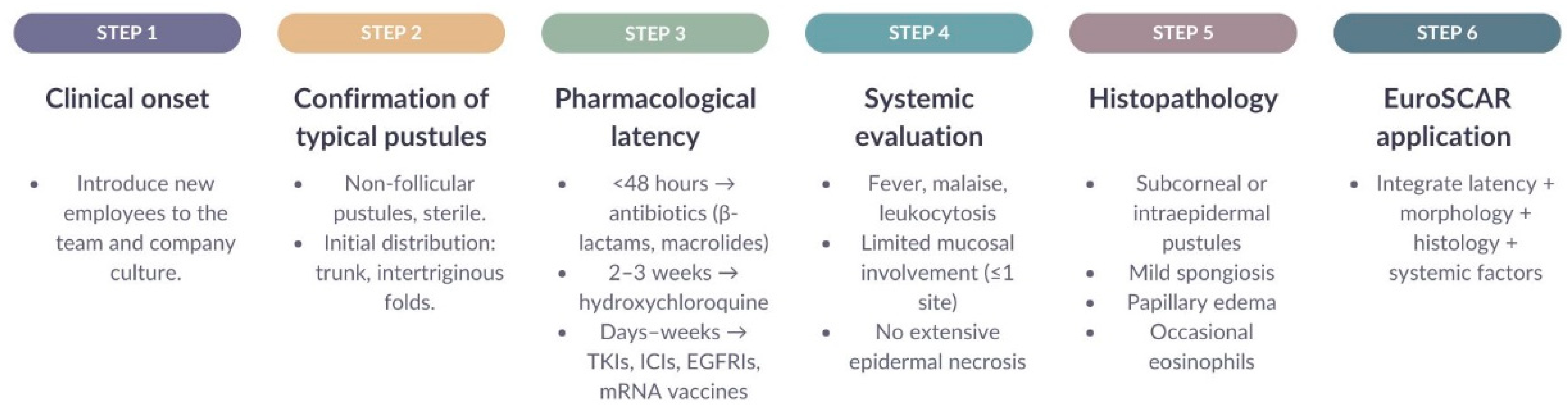
3.5. Diagnostic Algorithm and EuroSCAR Scoring System
3.6. Main Drugs Involved
3.6.1. Antibiotics
3.6.2. Antimalarial Agents
3.6.3. Targeted Therapies and Immunotherapies
3.6.4. Psychotropic Medications
3.6.5. Vaccines
3.7. Pharmacovigilance Findings and Emerging Drug-Association Signals
3.8. Limitations of the Evidence, Clinical Implications and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEP | Acute generalized exanthematous pustulosis |
| DRESS/DIHS | Drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome |
| EGFR | Epidermal Growth Factor Receptor |
| FAERS | FDA Adverse Event Reporting System |
| GPP | Generalized pustular psoriasis |
| HCQ | Hydroxychloroquine |
| ICI | Immune checkpoint inhibitors |
| IL | Interleukin |
| SJS/TEN | Stevens–Johnson syndrome/toxic epidermal necrolysis |
| TKI | Tyrosine kinase inhibitor |
Appendix A
| Database | Search Strategy (Exact Syntax) | Filters Applied in the Database | Date of Last Search |
|---|---|---|---|
| PubMed/MEDLINE | (“Acute Generalized Exanthematous Pustulosis”[MeSH] OR AGEP[tiab] OR “drug-induced pustulosis”[tiab]) AND (“Drug Hypersensitivity”[MeSH] OR “drug eruption”[tiab]) AND (“Interleukin-36”[MeSH] OR IL-36RN[tiab] OR “IL36RN mutation”[tiab]) | Humans; Adults ≥ 18 years; English/Spanish; Publication date 2000–2025 | 18 November 2025 |
| Scopus | TITLE-ABS-KEY(“acute generalized exanthematous pustulosis” OR AGEP OR “drug-induced pustulosis”) AND TITLE-ABS-KEY(“drug hypersensitivity” OR “adverse drug reaction”) AND TITLE-ABS-KEY(“IL-36” OR “IL-36RN”) | Article or Review; Human studies; 2000–2025 | 18 November 2025 |
| ScienceDirect | “acute generalized exanthematous pustulosis” AND “drug hypersensitivity” AND (“IL-36” OR “IL-36RN”) | Research articles; Clinical medicine; 2000–2025 | 18 November 2025 |
| SpringerLink | (“acute generalized exanthematous pustulosis” OR AGEP) AND (“drug-induced” OR “drug hypersensitivity”) AND (“IL-36” OR “IL36RN mutation”) | Medicine; Dermatology; 2000–2025 | 18 November 2025 |
References
- Parisi, R.; Shah, H.; Navarini, A.A.; Muehleisen, B.; Ziv, M.; Shear, N.H.; Dodiuk-Gad, R.P. Acute Generalized Exanthematous Pustulosis: Clinical Features, Differential Diagnosis, and Management. Am. J. Clin. Dermatol. 2023, 24, 557–575. [Google Scholar] [CrossRef] [PubMed]
- Sussman, M.; Napodano, A.; Huang, S.; Are, A.; Hsu, S.; Motaparthi, K. Pustular Psoriasis and Acute Generalized Exanthematous Pustulosis. Medicina 2021, 57, 1004. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.; Deshpande, P.; Campbell, C.N.; Krantz, M.S.; Mukherjee, E.; Mockenhaupt, M.; Pirmohamed, M.; Palubinsky, A.M.; Phillips, E.J. Updates on the Immunopathology and Genomics of Severe Cutaneous Adverse Drug Reactions. J. Allergy Clin. Immunol. 2023, 151, 289–300.e4. [Google Scholar] [CrossRef]
- Tetart, F.; Walsh, S.; Milpied, B.; Gaspar, K.; Vorobyev, A.; Tiplica, G.S.; Didona, B.; Welfringer-Morin, A.; Kucinskiene, V.; Bensaid, B.; et al. Acute Generalized Exanthematous Pustulosis: European Expert Consensus for Diagnosis and Management. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 2073–2081. [Google Scholar] [CrossRef]
- Stadler, P.-C.; Oschmann, A.; Kerl-French, K.; Maul, J.-T.; Oppel, E.M.; Meier-Schiesser, B.; French, L.E. Acute Generalized Exanthematous Pustulosis: Clinical Characteristics, Pathogenesis, and Management. Dermatology 2023, 239, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.J.; Akhtar, S.; Ahmad, M.; Hassan, I.; Wani, R. Etiopathological and Clinical Study of Acute Generalized Exanthematous Pustulosis: Experience from a Tertiary Care Hospital in North India. Indian Dermatol. Online J. 2020, 11, 391–397. [Google Scholar] [CrossRef]
- Marovt, M.; Marko, P.B. Denosumab-Induced Acute Generalized Exanthematous Pustulosis. Acta Derm. Venereol. 2024, 104, adv40430. [Google Scholar] [CrossRef]
- Rivera-Díaz, R.; Daudén, E.; Carrascosa, J.M.; Cueva, P.D.L.; Puig, L. Generalized Pustular Psoriasis: A Review on Clinical Characteristics, Diagnosis, and Treatment. Dermatol. Ther. 2023, 13, 673–688. [Google Scholar] [CrossRef]
- Casagranda, A.; Suppa, M.; Dehavay, F.; del Marmol, V. Overlapping DRESS and Stevens-Johnson Syndrome: Case Report and Review of the Literature. Case Rep. Dermatol. 2017, 9, 1–7. [Google Scholar] [CrossRef]
- Alfalah, M.; Alotaibi, Y.; Alotaibi, A.; Alharthi, R. Acute Generalized Exanthematous Pustulosis Induced by Iodinated Contrast Media: A Case Report. Dermatol. Rep. 2025, 17, 10217. [Google Scholar] [CrossRef]
- Moore, M.J.; Sathe, N.C.; Ganipisetti, V.M. Acute Generalized Exanthematous Pustulosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Benezeder, T.; Bordag, N.; Woltsche, J.; Falkensteiner, K.; Graier, T.; Schadelbauer, E.; Cerroni, L.; Meyersburg, D.; Mateeva, V.; Reich, A.; et al. IL-36-Driven Pustulosis: Transcriptomic Signatures Match between Generalized Pustular Psoriasis (GPP) and Acute Generalized Exanthematous Pustulosis (AGEP). J. Allergy Clin. Immunol. 2025, 155, 1913–1927. [Google Scholar] [CrossRef]
- Pospischil, I.; Hoetzenecker, W. Drug Eruptions with Novel Targeted Therapies—Immune Checkpoint and EGFR Inhibitors. JDDG J. Dtsch. Dermatol. Ges. 2021, 19, 1621–1643. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Yagi, M.; Wakasugi, Y.; Hattori, H.; Uno, K. A Study of Elevated Interleukin-8 (CXCL8) Detection of Leukocyte Migration Inhibitory Activity in Patients Allergic to Beta-Lactam Antibiotics. Allergol. Int. 2011, 60, 497–504. [Google Scholar] [CrossRef]
- Hsieh, C.; Yao, C.; Lo, Y. Acute Generalized Exanthematous Pustulosis Induced by Hydroxychloroquine. Kaohsiung J. Med. Sci. 2021, 37, 1122–1123. [Google Scholar] [CrossRef] [PubMed]
- Torres-Navarro, I.; Abril-Pérez, C.; Roca-Ginés, J.; Sánchez-Arráez, J.; Botella-Estrada, R. A Case of Cefditoren-Induced Acute Generalized Exanthematous Pustulosis during COVID-19 Pandemics. Severe Cutaneous Adverse Reactions Are an Issue. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e537–e539. [Google Scholar] [CrossRef]
- Shah, M.M.; Dhanani, S.; Patel, K.; Nair, P. Acute Generalized Exanthematous Pustulosis Following Ceftriaxone. Indian J. Pharmacol. 2024, 56, 297–298. [Google Scholar] [CrossRef]
- Klimas, N.; Quintanilla-Dieck, J.; Vandergriff, T. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis. In Cutaneous Drug Eruptions; Hall, J.C., Hall, B.J., Eds.; Springer: London, UK, 2015; pp. 259–269. ISBN 978-1-4471-6728-0. [Google Scholar]
- Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: MedlinePlus Genetics. Available online: https://medlineplus.gov/genetics/condition/stevens-johnson-syndrome-toxic-epidermal-necrolysis/ (accessed on 19 November 2025).
- Marwa, K.; Goldin, J.; Kondamudi, N.P. Type IV Hypersensitivity Reaction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Litaiem, N.; Hajlaoui, K.; Karray, M.; Slouma, M.; Zeglaoui, F. Acute Generalized Exanthematous Pustulosis after COVID-19 Treatment with Hydroxychloroquine. Dermatol. Ther. 2020, 33, e13565. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, S.; Mohammadpour, A.H.; Tavanaee, A.; Elyasi, S. Antibacterial Antibiotic-Induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Literature Review. Eur. J. Clin. Pharmacol. 2021, 77, 275–289. [Google Scholar] [CrossRef]
- Coleman, E.L.; Olamiju, B.; Leventhal, J.S. Potentially Life-threatening Severe Cutaneous Adverse Reactions Associated with Tyrosine Kinase Inhibitors. Oncol. Rep. 2021, 45, 891–898. [Google Scholar] [CrossRef]
- Frantz, R.; Huang, S.; Are, A.; Motaparthi, K. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Review of Diagnosis and Management. Medicina 2021, 57, 895. [Google Scholar] [CrossRef]
- Canhão, G.; Pinheiro, S.; Cabral, L. Toxic Epidermal Necrolysis: A Clinical and Therapeutic Review. Eur. Burn J. 2022, 3, 407–424. [Google Scholar] [CrossRef]
- Hama, N.; Abe, R.; Gibson, A.; Phillips, E.J. Drug-Induced Hypersensitivity Syndrome (DIHS)/Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Clinical Features and Pathogenesis. J. Allergy Clin. Immunol. Pract. 2022, 10, 1155–1167.e5. [Google Scholar] [CrossRef]
- Aiempanakit, K.; Apinantriyo, B. Clindamycin-Induced Acute Generalized Exanthematous Pustulosis: A Case Report. Medicine 2020, 99, e20389. [Google Scholar] [CrossRef] [PubMed]
- Mima, Y.; Ohtsuka, T. Acute Generalized Exanthematous Pustulosis Following the Administration of Cephalexin: A Case Report and Review of the Literature. Cureus 2025, 17, e79205. [Google Scholar] [CrossRef] [PubMed]
- Odorici, G.; Schenetti, C.; Pacetti, L.; Schettini, N.; Gaban, A.; Mantovani, L. Acute Generalized Exanthematous Pustulosis Due to Hydroxichloroquine. Dermatol. Ther. 2022, 35, e15520. [Google Scholar] [CrossRef] [PubMed]
- Komiya, N.; Takahashi, K.; Kato, G.; Kubota, M.; Tashiro, H.; Nakashima, C.; Nakamura, T.; Iwanaga, K.; Kimura, S.; Sueoka-Aragane, N. Acute Generalized Exanthematous Pustulosis Caused by Erlotinib in a Patient with Lung Cancer. Case Rep. Oncol. 2021, 14, 599–603. [Google Scholar] [CrossRef]
- Liquete, E.; Ali, S.; Kammo, R.; Ali, M.; Alali, F.; Challa, H.; Fata, F. Acute Generalized Exanthematous Pustulosis Induced by Erlotinib (Tarceva) with Superimposed Staphylococcus Aureus Skin Infection in a Pancreatic Cancer Patient: A Case Report. Case Rep. Oncol. 2012, 5, 253–259. [Google Scholar] [CrossRef]
- Roy, C.; Jaiswal, S.; Jayanath, B.P.; Paliwal, S.; Dey, P.; Mehta, V. Haloperidol-Induced Acute Generalized Exanthematous Pustulosis: A Rare Side Effect. Prim. Care Companion CNS Disord. 2022, 24, 21cr03051. [Google Scholar] [CrossRef]
- Jakhar, J.; Badyal, R.; Kumar, S.; Prasad, S. Olanzapine-Induced Acute Generalized Exanthematous Pustulosis: A Case Report. Indian J. Psychiatry 2021, 63, 411–413. [Google Scholar] [CrossRef]
- Wu, R.; Lin, T. Oxford-AstraZeneca COVID-19 Vaccine-induced Acute Localized Exanthematous Pustulosis. J. Dermatol. 2021, 48, e562. [Google Scholar] [CrossRef]
- Tay, W.C.; Lee, J.S.S.; Chong, W.-S. Tozinameran (Pfizer-BioNTech COVID-19 Vaccine)-Induced AGEP-DRESS Syndrome. Ann. Acad. Med. Singap. 2022, 51, 796–797. [Google Scholar] [CrossRef]
- Agaronov, A.; Makdesi, C.; Hall, C.S. Acute Generalized Exanthematous Pustulosis Induced by Moderna COVID-19 Messenger RNA Vaccine. JAAD Case Rep. 2021, 16, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; La Placa, M.; Poluzzi, E.; De Ponti, F. The Value of Case Reports and Spontaneous Reporting Systems for Pharmacovigilance and Clinical Practice. Br. J. Dermatol. 2021, 184, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Que, H.; Zheng, X.; Li, X.; Wang, W. Risk Factors for Drug-Induced Acute Generalized Exanthematous Pustulosis (AGEP) from 2004 to 2024: A Real-World Study Based on the FAERS. Clin. Cosmet. Investig. Dermatol. 2025, 18, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Martinez-De la Torre, A.; van Weenen, E.; Kraus, M.; Weiler, S.; Feuerriegel, S.; Burden, A.M. A Network Analysis of Drug Combinations Associated with Acute Generalized Exanthematous Pustulosis (AGEP). J. Clin. Med. 2021, 10, 4486. [Google Scholar] [CrossRef]
- Popiołek, I.; Piotrowicz-Wójcik, K.; Porebski, G. Hypersensitivity Reactions in Serious Adverse Events Reported for Paracetamol in the EudraVigilance Database, 2007–2018. Pharmacy 2019, 7, 12. [Google Scholar] [CrossRef]
| Characteristic | AGEP | GPP | DRESS | SJS/TEN |
|---|---|---|---|---|
| Onset/Latency | Rapid (24–48 h; delayed in HCQ) [1,15,16,17] | Subacute; recurrent [2] | Delayed (2–8 weeks) [1,3,5] | 1–3 weeks [18,19] |
| Type of pustules | Sterile, non-follicular [1,2,3] | Sterile, coalescent [1,2,3] | Rare pustules; polymorphic exanthema [1] | No pustules; epidermal necrosis [1,2,3,4,5,18,19,20] |
| Distribution | Generalized, over erythema [1,2,17,21] | Generalized, often with plaques [1,2] | Extensive eruption [1] | Extensive eruption with positive Nikolsky sign [1] |
| Systemic symptoms | Mild to moderate [1,2,3,4,17,21] | Marked fever [1,2] | High fever, lymphadenopathy, multiorgan involvement [1,22] | Very severe; high risk of mortality [1,23,24] |
| Mucosal involvement | Infrequent [1,2,4] | Absent or mild [1,2,5] | Occasional [1,22] | Frequent and severe [1,18,24] |
| Histology | Subcorneal or intraepidermal pustules; edema; eosinophils [1,2,3,4] | Munro microabscesses; acanthosis; pustules in spinous layer [2] | Eosinophilic infiltrate; interface dermatitis [1,20] | Massive epidermal necrosis [1,18,24] |
| Laboratory findings | Neutrophilia; variable eosinophilia [1,2,4,5] | Leukocytosis [2] | Marked eosinophilia [1,20] | Variable cytopenias [25] |
| Key diagnostic clue | Positive EuroSCAR score [1,2,3,4,5] | History of psoriasis [1,2] | HHV-6 reactivation possible [1,22,26] | Epidermal detachment [1,5,18,24,25] |
| Evolution | Resolution in 1–2 weeks [1,2,4,17,27] | Recurrent course [2] | Weeks to months [22,26] | Severe disease; high morbidity and mortality [18,24,25] |
| Category | Criteria | Score |
|---|---|---|
| Cutaneous morphology | Sterile non-follicular pustules over erythema | 0–2 |
| Distribution | Generalized; abrupt onset | 0–2 |
| Postpustular Desquamation | Present | 0–1 |
| Fever (>38 °C) | Present | 0–1 |
| Eosinophilia | >0.7 × 109/L | 0–1 |
| Neutrophilia | >7 × 109/L | 0–1 |
| Compatible Histopathology | Subcorneal or intraepidermal pustules; edema; neutrophilic and/or eosinophilic infiltrate | 0–2 |
| Compatible Latency | 24–48 h (some drugs, like HCQ, may require several weeks) | 0–1 |
| Rapid resolution | <15 days | 0–1 |
| Atypical evolution | Absence of key features | 1–2 |
| Pharmacological Class | Latency | Mechanism | Clinical Examples |
|---|---|---|---|
| Antibiotics | Rapid (24–48 h) [1,4,5] | Type IV T-cell-mediated hypersensitivity reaction; hapten–lymphocyte complex formation; neutrophilic and IL-36 activation [1,2,20]. | Penicillins (amoxicillin, cloxacillin), cephalosporins (ceftriaxone, cephalexin), macrolides (azithromycin, clarithromycin), quinolones [1,2,3,4,5,13] |
| Antimalarials | Prolonged [1,4,5] | Potentiation of the IL-36 pathway; exacerbation in carriers of IL36RN mutations; delayed neutrophilic activation [1,2] | HCQ, chloroquine [1,4,5,29] |
| Targeted therapies | Variable (days to weeks) [1,2,3,4,5,13] | Intense immunologic activation; dysregulation of the IL-36/IL-8 axis; mixed mechanisms between AGEP and drug-induced pustular psoriasis [1,2,13] | TKIs (imatinib, icotinib), EGFR inhibitors (erlotinib), BRAF/MEK inhibitors [13,23,30,31] |
| Immunotherapies | Variable; delayed | CTLA-4 or PD-1/PD-L1 blockade → T-cell hyperactivation; increased IL-36; atypical pustular reactions [3,13] | Ipilimumab, nivolumab, pembrolizumab [13] |
| Psychotropic drugs | Short (48–72 h) [1,4,5] | Uncertain mechanism; possible lymphocyte-mediated reaction with marked neutrophilia [1,32] | Haloperidol, olanzapine [32,33] |
| Vaccines (incl. mRNA COVID-19) | Irregular (1–14 días) [1] | Global immune activation rather than hapten-dependent mechanism; minimal systemic findings; good response to corticosteroids [34,35] | Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273), other viral vaccines [34,35,36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zavaleta-Monestel, E.; Escudero-Correa, A.; Mora-Jiménez, J.; Hernández-Vásquez, A.J.; Monge-Bogantes, L.C.; Hernández-López, J.; Arguedas-Chacón, S. Drug-Induced Acute Generalized Exanthematous Pustulosis: Mechanisms, Diagnosis, and Clinical Differentiation from Other Pustular Eruptions. Dermato 2026, 6, 3. https://doi.org/10.3390/dermato6010003
Zavaleta-Monestel E, Escudero-Correa A, Mora-Jiménez J, Hernández-Vásquez AJ, Monge-Bogantes LC, Hernández-López J, Arguedas-Chacón S. Drug-Induced Acute Generalized Exanthematous Pustulosis: Mechanisms, Diagnosis, and Clinical Differentiation from Other Pustular Eruptions. Dermato. 2026; 6(1):3. https://doi.org/10.3390/dermato6010003
Chicago/Turabian StyleZavaleta-Monestel, Esteban, Audry Escudero-Correa, Jeaustin Mora-Jiménez, Andy Jesús Hernández-Vásquez, Luis Carlos Monge-Bogantes, Josephine Hernández-López, and Sebastián Arguedas-Chacón. 2026. "Drug-Induced Acute Generalized Exanthematous Pustulosis: Mechanisms, Diagnosis, and Clinical Differentiation from Other Pustular Eruptions" Dermato 6, no. 1: 3. https://doi.org/10.3390/dermato6010003
APA StyleZavaleta-Monestel, E., Escudero-Correa, A., Mora-Jiménez, J., Hernández-Vásquez, A. J., Monge-Bogantes, L. C., Hernández-López, J., & Arguedas-Chacón, S. (2026). Drug-Induced Acute Generalized Exanthematous Pustulosis: Mechanisms, Diagnosis, and Clinical Differentiation from Other Pustular Eruptions. Dermato, 6(1), 3. https://doi.org/10.3390/dermato6010003








