Abstract
Adenosquamous carcinoma of the skin (ASCS) or primary cutaneous adenosquamous carcinoma is a rare malignant neoplasm. It is characterized by the presence of both glandular and squamous cell components and a propensity for aggressive clinical behavior. Due to its rarity, it continues to pose diagnostic challenges. To date, only a few cases of this tumor have been reported, and even fewer have been thoroughly investigated via immunohistochemistry.
Adenosquamous carcinoma of the skin a(ASCS), or primary cutaneous adenosquamous carcinoma, is a rare malignant neoplasm. It is best considered as a locally aggressive high-risk subtype of cutaneous squamous cell carcinoma. Compared to conventional SCCs, it is generally more aggressive.
This type of non-melanoma skin cancer is influenced by environmental stimuli, phenotypic characteristics (such as a less protective, lighter skin tone), and genetic factors. Exposure to ultraviolet radiation (UVR) through sunlight remains the most significant risk factor for its occurrence.
Histopathologically, adenosquamous carcinoma of the skin is characterized by the presence of both glandular and squamous cell components and a propensity for aggressive clinical behavior. Due to its rarity, it continues to pose diagnostic challenges. To date, only a few cases of this tumor have been reported, and even fewer have been thoroughly investigated through immunohistochemistry [1,2,3,4,5,6,7,8,9].
A 51-year-old man presented to the clinic with a scalp tumor that had progressed over the past 3 months. Moreover, our patient had a history of non-melanoma skin cancer on his shoulders 10 years ago, the nature of which was unfortunately unknown. The patient was not immunosuppressed and HIV-seronegative. He was not taking any medications. Neither heavy sunlight exposure nor family history of skin tumors were mentioned.
On physical examination, the patient had a 2.4 cm × 2 cm hyperkeratotic tumor with a dome-shaped growth (Figure 1). Initially, our differential diagnosis included a cutaneous squamous cell carcinoma (cSCC) and a malignant adnexal carcinoma.
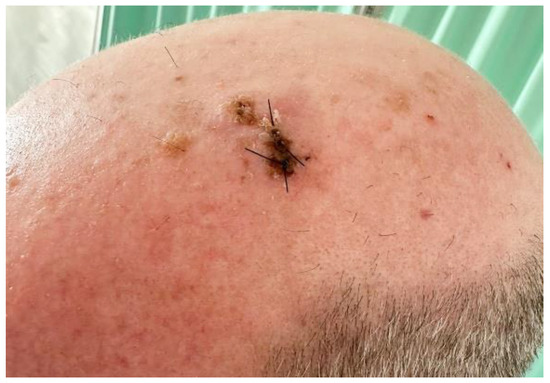
Figure 1.
Showing a crusty tumorous lesion in the left parietal region of the scalp.
The biopsy taken from the cutaneous mass revealed a dense proliferation of atypical epithelial cells with connection to the epidermis and surface ulceration. In the superficial aspect, tumor cells showed squamous differentiation; in deeper areas of the tumor, glandular/ductal differentiation was seen (Figure 2). On immunohistochemistry, the tumor was diffusely positive for CK7, p40, and GATA3 (Figure 2). Ductal differentiation was confirmed with CEA (Figure 3). However, it was negative for CK20, TTF1, and Melan A.
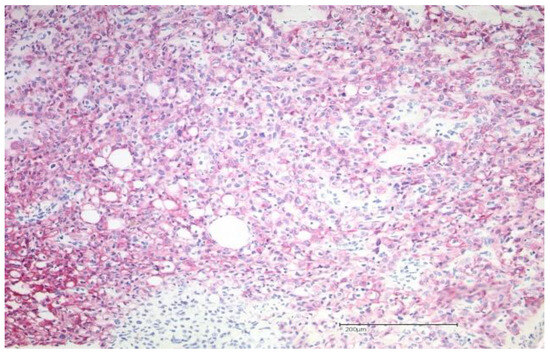
Figure 2.
Showing the hematoxylin–eosin histopathology (magnification: 200×) of an adenosquamous carcinoma of the skin with adenoid as well as squamous differentiation. Immunohistochemical staining demonstrates diffuse tumor positivity for CK7.
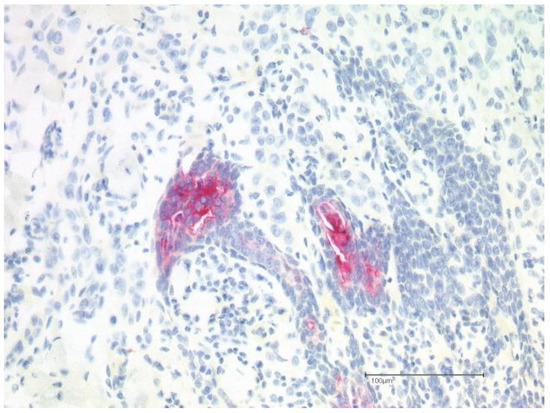
Figure 3.
Positive immunohistochemical staining for CEA confirming the presence of ductal differentiation.
Lymph node ultrasound, magnetic resonance imaging of the brain, and computed tomography of the thorax and abdomen did not reveal metastatic disease. Because of GATA3 positivity, a urothelial origin could be excluded via radiology workup [7]. The initial treatment consisted of complete surgical excision with adequate safety margins (>5 mm). The tumor thickness was 8 mm with perineural invasion and involvement of the galea. Nevertheless, there was no evidence of lymphovascular invasion. The patient was referred for local adjuvant radiation therapy.
ASCS consists of a rare malignant neoplasm of the skin, and it was first described in 1985 [1]. It is characterized by mixed squamous and glandular differentiation and a propensity for aggressive clinical behavior, such as extensive local invasion, recurrence, and rare metastasis [1,2,3]. The majority of risk factors are directly linked to individuals’ sunlight exposure behaviors or their sensitivity to solar radiation. DNA is damaged by UVR, especially UVB (290–320 nm), through the formation of cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts (6-4PPs).
If these products are not repaired through nucleotide excision, they interfere with proper base-pairing and obstruct essential cellular processes such as transcription and replication causing progressive gene alterations (including tumor suppressor genes and proto-oncogenes) and eventual tumor formation [9].
Only a few cases of ASCS have been reported so far in the literature [6]. Elderly patients, with a history of UV-related actinic damage through, typically present with an indurated, erythematous keratotic papule or plaque, located on the head or neck. In the present case, the tumor had a very similar appearance to incipient squamous cell carcinoma. It tends to affect elderly or immunocompromised individuals [4]. Lesion thickness, microscopic perineural invasion, and immunosuppression are important predictors of extensive local disease and recurrence [4]. Taking into account the previously investigated cases in the literature, our case is similar with respect to male sex, white race, and history of actinic damage [1,2]. However, the patient was neither old nor immunosuppressed. This case demonstrates that this entity might have diverse clinical presentations.
Patients need to be closely monitored since locoregional recurrence is frequent. However, distant metastasis remains rare [5]. Mohs surgery or other micrographic controlled approaches are considered the best initial treatments of ASCS. For the treatment of locally advanced carcinoma and particularly those cases with perineural involvement, adjuvant radiotherapy may also be useful [4]. Identifying and reporting high-risk features is important in order to reduce the morbidity and mortality associated with this disease.
Given the rarity of ASCS, data are sparse regarding its epidemiology and rates of recurrence and metastasis. This case highlights the fact that this entity often presents as non-specific lesion indistinguishable from cutaneous squamous cell carcinoma [8]. Prompt diagnosis and a complete work-up are important in order to manage this aggressive tumor adequately.
Author Contributions
Conceptualization, T.G. and R.J.; data interpretation, T.G., F.H. and A.T.; investigation, R.J., T.G., F.H., A.T. and S.B.; writing—original draft preparation, R.J. and T.G.; visualization, T.G. and F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This non-interventional case study was approved by Institutional Review Board at the Ruhr-University Bochum (IRB Study ID #16-5985). All procedures performed in studies involving human participants or their data were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Statement
Informed consent was obtained from the patient presented.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank the patient for consenting to the publication of images and data for this report.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weidner, N.; Foucar, E. Adenosquamous carcinoma of the skin: An aggressive mucin- and gland-forming squamous carcinoma. Arch. Dermatol. 1985, 121, 775–779. [Google Scholar] [CrossRef]
- Banks, E.R.; Cooper, P.H. Adenosquamous carcinoma of the skin: A report of 10 cases. J. Cutan. Pathol. 1991, 18, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, D.S.; Derienzo, D.P.; Barr, R.J. Cutaneous squamous cell carcinoma: A comprehensive clinicopathologic classification—Part two. J. Cutan. Pathol. 2006, 33, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.M.; McCalmont, T.; Siegrid, Y.S. Adenosquamouscarcinoma of the skin: A case series. Arch. Dermatol. 2009, 145, 1152–1158. [Google Scholar] [CrossRef]
- Patel, V.; Squires, S.M.; Liu, D.Y.; Fraga, G.R. Cutaneous adenosquamouscarcinoma: A rare neoplasm with biphasic differentiation. Cutis 2014, 94, 231–233. [Google Scholar] [PubMed]
- Alomran, H.; Cruel, T.; Harrou, O.; Kanitakis, J.; Balme, B. Cutaneous Adenosquamous Carcinoma of the Scalp With Intestinal Phenotype. Am. J. Dermatopathol. 2020, 42, e128–e130. [Google Scholar] [CrossRef] [PubMed]
- Lupinacci, R.M.; Santana, A.; Dias, A.R. Metastatic gallbladder adenosquamous carcinoma to the skin. J. Surg. Case Rep. 2014, 2014, rju130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Genois, A.; Maari, C.; Bouffard, D. Atypical presentation of adenosquamous carcinoma: A case report. SAGE Open Med. Case Rep. 2018, 6, 2050313X18801217. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, E.; Aloizou, A.M.; Zafiriou, E.; Bargiota, A.; Skaperda, Z.; Kouretas, D.; Roussaki-Schulze, A.V. Non-Melanoma Skin Cancer and Vitamin D: The “Lost Sunlight” Paradox and the Oxidative Stress Explanation. Antioxidants 2023, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).