Abstract
Acne tarda is defined as acne that develops (late-onset acne) or continues (persistent acne) after 25 years of age. The disease is more common in women. The etiology of acne tarda is still controversial, and a variety of factors such as endocrinological disorders including hyperandrogenism and hyperandrogenemia, stress, modern western diet, ultraviolet irradiation, drugs and cosmetics have been implicated. In particular, women with acne tarda and other symptoms of hyperandrogenism such as hirsutism and androgenetic alopecia have a high probability of endocrine abnormalities such as polycystic ovarian syndrome, primary ovarian insufficiency, Cushing’s syndrome and late-onset adrenogenital syndrome. Virilization is a relatively uncommon feature of hyperandrogenemia and its presence often suggests an androgen-producing tumor. Treatment is similar to that of acne in adolescence; however, long-term treatment over years or decades may be required. A thorough history, a focused clinical examination and an interdisciplinary approach together with gynecologists and endocrinologists are extremely helpful in diagnostic evaluation and therapy of patients with acne tarda.
1. Introduction
Acne, a chronic inflammatory disease of the pilosebaceous unit, is one of the most common diagnosed diseases in Europe and in the United States [1]. According to a systematic analysis for the Global Burden of Disease Study that provides a tool to quantify health loss from diseases, injuries, and risk factors worldwide, acne vulgaris was the 8th most prevalent disease globally in 2010 with a global prevalence of 650 million cases, following only two other skin disease categories on the list: fungal skin diseases (4th in global prevalence), and other skin and subcutaneous diseases (5th) [2,3].
The incidence of acne peaks between the ages of 15 and 18 years [1]. While spontaneous regression occurs in the majority of patients after puberty, the disease persists beyond the age of 25 years in approximately 10% of all cases [4]. The last type of acne is characterized as acne tarda that can develop (late-onset acne) or continue (persistent acne) after 25 years of age [5]. Women are more affected by acne tarda in comparison to men [4]. As acne tarda in its severe form can lead to scarring and post-inflammatory hyperpigmentation, the disease has often a subsequent impact on quality of life.
Although progress has been made in understanding the pathophysiology of acne and the mechanisms of actions of available drugs to treat the disease, the treatment of acne tarda still remains challenging. This is due to the fact, that acne tarda is in many cases resistant to therapy, and the dermatologists often have to deal with women at a reproductive age, who wish to have children, which minimizes therapy options. This is the reason why understanding the pathogenesis of this acne form is very important, as in several cases therapy should focus on the aetiological factors and not only on treating symptoms.
2. Pathogenesis
In recent years, many advances have been achieved in acne research, and several mechanisms have been suggested to be responsible for the initiation of the disease. Amongst others, key players that may orchestrate the development of acne tarda have been proposed, including disturbed sebaceous gland activity associated with hyperseborrhea [6] and alterations in sebum fatty acid composition [7], induction of inflammation cascades, dysregulation of the hormone microenvironment, interaction with neuropeptides [8,9], follicular hyperkeratinization and dysfunction of the innate and adaptive immunity reviewed in [6,10].
Consequently, the impaired natural cycling process in the sebaceous gland follicle leads to the transition of microcomedones to comedones, and this results in inflammatory lesions [11]. Propionibacterium acnes and associated antigens (lipopolysaccharides) can potentiate the inflammatory process [12]. Genetic studies of heterozygous and homozygous twins and family studies have produced a growing body of evidence for the role of hereditary factors [13,14]. Ultraviolet radiation and other environmental factors may play a role in some cases [15,16]. Dietary factors [17], smoking, stress [8,16,18] and modern lifestyle [19] have been also associated with higher acne risk.
Particularly, women with acne tarda have a high probability of endocrine abnormalities including hyperandrogenism and hyperandrogenemia [20]. The term hyperandrogenism describes all possible androgen-associated skin changes that can be attributed to an increased local androgen effect on the skin. In 20% of all cases, there is a combination of Seborrhoea, Acne, Hirsutism and androgenetic Alopecia—the so called SAHA syndrome—and occasionally polycystic ovaries are detectable [21,22,23]. The peripheral metabolism of androgens occurs in different areas within the sebaceous follicle [24,25,26,27] and is characterized by impaired activity of androgen-associated enzymes such as 5α-reductase, aromatase, and expression of androgen receptors [23].
Hyperandrogenemia occurs in cases in which circulating androgen levels are significantly elevated. In women, additionally to the typical symptoms of SAHA syndrome, accompanying systemic symptoms occur, such as irregular menstrual cycle, clitoral hypertrophy, virilization and changes in secondary sexual characteristics (voice pitch, hair pattern), polycystic ovarian syndrome (PCOS), cystic mastitis, infertility and metabolic syndrome [6,28]. The HAIR-AN syndrome describes an entity of Hyperandrogenemia, Insulin Resistance and Acanthosis Nigricans [21,29,30] and is often understood as a subtype of the polycystic ovarian syndrome.
The causes of hyperandrogenemia are diverse and are divided into endogenous and exogenous ones. Endogenous factors include (a) androgen-producing benign and malignant neoplasms of the ovaries/adrenal glands (adenomas/carcinomas), the pituitary gland and hypothalamus, as well as the paraneoplastic syndrome such as the production of adrenocorticotropic hormone (ACTH) from a bronchial carcinoma or (b) androgen excess without neoplasms due to congenital anomalies (adrenogenital syndrome—AGS, XY disorders), PCOS, Cushing’s disease, and endocrine diseases (e.g., hyperthyroidism) [6]. Lifestyle disorders usually observed in young lean women, e.g., high levels of stress, anorexia nervosa and physical stress, can often lead to typical SAHA syndrome symptoms—the so-called persistence of adrenarche syndrome [16]. Exogenous factors can also be involved, in particular hormonal contraceptives such as progestins with androgenic effects, androgens/anabolic steroids, antiepileptic drugs, corticosteroids or ACTH, metyrapone and certain cosmetics [21].
In a retrospective study, da Cunha et al. [31] performed a complete screening of circulating androgens in 835 women with late-onset acne over 15 years of age (mean age 26.64 years). The following androgens were measured: dehydroepiandrosterone sulfate (DHEAS), dehydroepiandrosterone (DHEA), 5α-dihydrotestosterone (DHT), andostenedione and total testosterone. Women between the ages of 15 and 18 years suffering from severe acne papulopustulosa or conglobata or a milder form with clinical signs of hyperandrogenemia (hypertrichosis, hirsutism, seborrheic dermatitis, obesity, alopecia) were also included in the study. Interestingly, hyperandrogenemia was found in 56.5% of the women. Significantly high DHEA levels were found in 39.6% of the cases, followed by androstenedione in 21.3% and total testosterone in 10.7%. In addition, 184 women had two or more abnormal androgen values (DHEA and androstenedione in 69 women; DHEA and total testosterone in 23 women). These results indicate the pivotal role of androgens in the initiation of acne in post-adolescents.
Additionally, increased serum levels of insulin-like growth factor I (IGF-I) have been observed in adult women and men with acne and the number of total acne lesions, inflammatory lesions, serum levels of DHT and DHEAS correlated with serum IGF-I levels in women with acne [32,33]. Mean facial sebum excretion rate and serum IGF-I levels were also correlated in post-adolescent acne patients [34]. IGF-I plays a key role in the induction of lipid synthesis in human sebocytes [35]. In addition, an interaction between the IGF-I and estradiol signalling pathway has been described in human SZ95 sebocytes, implicating that estrogens may have an indirect effect on the pathogenesis of acne. Western diet characterized by high glycemic load and dairy protein consumption has been correlated with activation of IGF-I signalling, followed by the promotion of mammalian targets of rapamycin (mTOR) signalling [36].
3. Clinical Presentation and Diagnostic Algorithm
The clinical features of acne tarda are quite specific: inflammatory acne that include superficial lesions such as papules and pustules (≤5 mm in diameter) and deep pustules or nodules in the lower facial region or macrocomedones (microcysts) spread over the face (Figure 1 and Figure 2). Involvement of the trunk is much more common in men [5]. The disease can be classified as mild, moderate or severe and in accordance with the lesions that predominate in a given patient: comedonal, papulopustular, nodular, nodulocystic or conglobate acne (acne conglobata) (Table 1) [10].
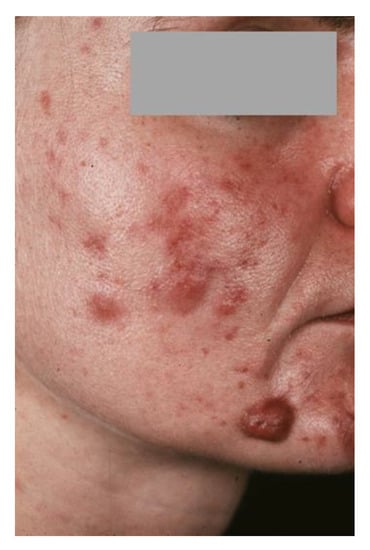
Figure 1.
31-year-old patient suffering from severe late-onset acne tarda.
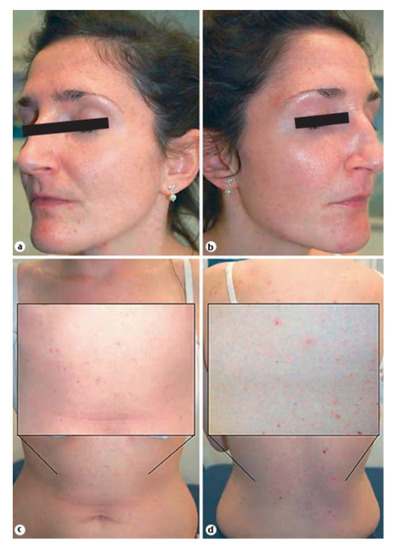
Figure 2.
30-year-old patient with acne tarda suffering from primary ovarian insufficiency (POI). Seborrhea and multiple skin coloured and erythematous follicular papules on the face (a,b) and the trunk (c,d) [37].

Table 1.
Clinical forms of acne [10].
For better clinical diagnosis of contributing factors to acne tarda, a diagnostic algorithm has been established (Figure 3). Elevated levels of DHEAS that do not decrease by a dexamethasone test indicate a tumor of the adrenal cortex, excessively elevated free testosterone indicates an ovarian tumor, and elevated 17α-hydroxyprogesterone indicates late-onset congenital adrenal hyperplasia (Figure 3). In particular, PCOS, primary ovarian insufficiency (POI) and other acne-associated syndromes such as HAIR-AN, SAHA, Cushing’s disease and late-onset androgenital syndrome should be considered [28]. In order to establish the correct diagnosis and early intervention in cooperation with other disciplines, other clinical symptoms such as amenorrhea, hypermenorrhea, oligomenorrhea, infertility and the metabolic syndrome must be taken into account [23] (Table 2).
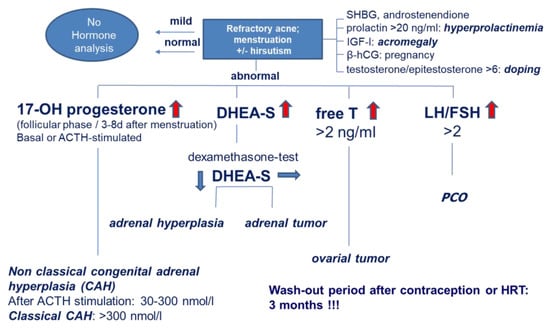
Figure 3.
Algorithm of serological hormone tests in patients with suspected hyperandrogenemia. 17-OH-P: 17-hydroxy-progesterone, ACTH: adrenocorticotropic hormone, DHEA-S: dehydroepiandrosterone sulfate, LH/FSH: luteinizing hormone/follicle stimulating hormone, PCO: polycystic ovary, SHBG: sex hormone-binding globulin, IGF-I: insulin -like growth hormone-I, β-hCG: β-human choriogonadotropin, HRT: hormone replacement therapy.

Table 2.
Diagnostic approach and endocrinological evaluation in suspected hyperandrogenemia.
4. PCOS
Polycystic ovaries are characterized by morphological changes in the ovaries in the sense of multiple cystic follicular structures, thickening of the cortex and the tunica albuginea or hyperplasia of the theca interna. In the case that other symptoms such as oligo/amenorrhoea, sterility/infertility due to chronic anovulation or androgenization symptoms (such as hirsutism, hyperandrogenemia, moderate/severe acne, insulin resistance and obesity) occur, this is referred to as PCOS [38,39,40]. Since PCOS is not a clinical entity, the diagnosis often proves to be very difficult. In addition, a number of differential diagnoses must be ruled out before considering PCOS, including adrenal hyperandrogenemia, the adult form of adrenogenital syndrome, androgen-forming tumors, corticosteroid excess (e.g., in Cushing’s syndrome) and hyperprolactinemia in pituitary processes (Figure 2).
The complex pathophysiology of PCOS includes the interaction of genetic and epigenetic changes, primary ovarian abnormalities, and endocrine and neuroendocrine imbalances [39]. The early onset of adrenarche may represent the first clinical feature of PCOS. Additional clinical symptoms such as hirsutism, moderate/severe treatment-resistant acne, menstrual disorders two years after menarche, and elevated androgen levels may contribute to the diagnosis. Increased ovarian volume in combination with menstrual disorders can indicate the development of PCOS [41]. Hyperinsulinemia, insulin resistance, and obesity may be present in adolescents with PCOS but are not diagnostic criteria. Adolescents with PCOS have an increased risk of developing type 2 diabetes [42].
Despite decades of research, the etiology of PCOS still remains unclear. Elevated levels of total and free testosterone indicate PCOS. Another biomarker that is more relevant in adults is the measurement of anti-Müllerian hormone (AMH)—a glycoprotein that is produced in the granulosa cells of smaller-growing follicles-. In addition, dysregulated secretion of gonadotropins (GnRH), which control ovarian steroidogenesis, is indicative of PCOS. Elevated levels of luteinizing hormone (LH), an elevated LH/follicle stimulating hormone (FSH) ratio and an increased frequency of LH pulses are also common [39]. Further necessary examinations are a dexamethasone suppression test to differentiate the androgens with regard to adrenal and ovarian origin and the determination of cortisol and 17-OH-progesterone in order to exclude Cushing’s or an adrenogenital syndrome.
The goal of therapy is to lower the elevated androgen levels by regulating ovarian androgenesis and reducing the signs of androgenization. First-line therapies also include lifestyle intervention based on a calorie-restricted diet and physical activity [39]. The aim is to detect PCOS as early as possible in order to initiate the appropriate treatment. In this way, long-term consequences of PCOS could be prevented and a better life quality could be ensured.
5. POI
POI, also known as premature menopause, premature ovarian failure or early menopause, is defined as the occurrence of amenorrhea before the age of 40 accompanied by increased follicle-stimulating hormone to menopausal levels (>40 mIU/mL) and decreased estradiol levels (<50 pg/mL). It occurs in about 1% of the female population under the age of 40 and is occasionally diagnosed after discontinuation of hormonal contraceptives [37,43]. Even though presenting with clinical findings similar to those of menopause, 50% of POI patients have varying and unpredictable ovarian function and only 5–10% are able to accomplish pregnancy [44].
Patients with POI often present with typical skin changes such as seborrhea, acne tarda and male-pattern alopecia, which are likely the results of the relative accompanying hyperandrogenism [37]. Estrogen deficiency directly affects skin homeostasis and may also result in dysregulation of peripheral androgen metabolism. Androgens have been documented to influence skin morphology and play a distinguished role in epidermal barrier homeostasis, wound healing, sebaceous gland growth, differentiation and hair growth [24]. Further common symptoms are amenorrhea, weight loss, mucosal xerosis and dyspareunia especially after discontinuation of hormonal contraception. Hormonal and immunologic serological and ultrasound examinations often reveal an autoimmune hypergonadotropic POI with ovarian fibrosis with a marked reduced follicle pool.
POI is first diagnosed when patients complain about irregularity of the menstrual cycle and after detection of high circulating gonadotropin levels as well as low estradiol and anti-Mueller hormone levels. Especially, AMH is considered an important marker of ovarian reserve [45]. Karyotypic abnormalities such as Turner syndrome (45,X or mosaic 45,X/46,XX), and trisomy or polysomy X may be detected in adolescents with POI, particularly those with primary amenorrhea [46].
Approximately 14.3–30% of POI cases have been associated with autoimmunity [47]. POI has been reported in Addison’s disease, Hashimoto’s thyroiditis, dry eye syndrome, myasthenia gravis, rheumatoid arthritis, diabetes mellitus or systemic lupus erythematosus [48,49]. The presence of so-called steroid cell autoantibodies against steroidogenic enzymes, such as 21β-hydroxylase, 17α-hydroxylase and side-chain cleavage enzyme, has been detected in 4% of women with POI, indicating an autoimmune oophoritis [49,50]. In some cases, POI is the first sign of general immune dysregulation such as an autosomal recessive disorder known as autoimmune polyglandular syndrome type I (including two of three major clinical symptoms: hypoparathyroidism, hypoadrenalism and mucocutaneous candidiasis). POI may be associated with hereditary galactosemia, an autosomal recessive disorder because of deficiency of galactose-1-phosphate uridyl transferase enzyme activity, which in most cases is diagnosed in infancy [51]. Radiation, chemotherapy, ovarian surgery and viral infections may also lead to POI [43].
Patients with POI are rarely evaluated by dermatologists, while gynecologists seem to rarely take into consideration concomitant skin changes [52]. However, early diagnosis of POI is of great importance for the patient, as there is a high probability of upcoming infertility due to oocyte depletion. In addition, healthy ovarian function is also essential for women’s general health, including the maintenance of bone mass, the cardiovascular system and cognition.
6. Cushing’s Syndrome
Cushing’s syndrome comprises a variety of clinical symptoms that are either triggered by endogenous factors such as chronic overproduction of glucocorticoids or by exogenic factors such as iatrogenic long-term treatment with steroids or ACTH [53]. In the case of an endogenous cause, an ACTH-dependent endogenous Cushing’s syndrome (i.e., increased ACTH production due to a pituitary tumor or paraneoplasia) can be distinguished from an ACTH-independent (i.e., due to an adrenal tumor), whereby in approx. 80% of the cases the glucocorticoid excess is the consequence of an autonomic ACTH-producing pituitary adenoma [53,54]. Other factors that can lead to hypercortisolism and should therefore be considered in the diagnosis include stress, excessive exercise, pregnancy, alcoholism and anorexia nervosa [55]. Women are 5:1 more likely to develop central Cushing’s disease due to increased ACTH production by pituitary adenomas. The disease occurs in all age groups but shows a peak between the ages of 30 and 50 [56].
The most common symptoms of Cushing’s syndrome include weight gain, centripetal obesity with full moon face and buffalo nape, typical striae rubrae distensae on the abdomen, thighs and armpit folds, fatigue and muscle weakness, depressive mood, sleep disorders and anxiety, menstrual disorders as well as acne tarda in women, hirsutism, osteoporosis with back pain and loss of libido in men. Pronounced virilization even indicates an adrenal cortex carcinoma [56]. In addition to the clinical examination, the diagnostic procedure includes endocrinological functional diagnostics and imaging procedures. A short, low-dose dexamethasone test is carried out to confirm the suspected clinical diagnosis. If the plasma cortisol levels are above 3 µg/dL, the suspicion can be confirmed by determining the free cortisol in the 24-h urine. If the diagnosis of Cushing’s syndrome is confirmed, the etiology can be clarified with functional tests that allow assessment of ACTH activity. When assessing plasma cortisol levels, it is important to consider the circadian rhythm of cortisol production, which is highest at 8:00 a.m. [57].
Excessive production of cortisone leads to inhibition of epidermal proliferation and collagen synthesis and can stimulate sebum production. Thus, the affected person can experience typical skin atrophy, an increased incidence of ecchymoses, acneiform dermatitis and connective tissue disorders. In the case of ectopic ACTH overproduction, multiple hyperpigmentation often occurs. Furthermore, an accompanying overproduction of androgens often leads to diffuse hair loss. Due to an increased morbidity rate, especially due to cardiovascular diseases, it is very important to diagnose Cushing’s syndrome as quickly as possible.
7. Adrenogenital Syndrome (AGS)
Congenital adrenal hyperplasia is attributed to autosomal recessive enzyme defects in the adrenal cortex. In more than 90% of cases, it is caused by a CYP21 (21-hydroxylase) enzyme defect, which can lead to reduced production of cortisol and occasionally also of aldosterone. The absence of cortisol negative feedback effect on the pituitary gland leads to increased ACTH secretion resulting in increased production of androgens in the adrenal cortex [58]. This leads to virilization in girls, and adrenal failure. If the disease remains undetected and accordingly untreated, the children will be conspicuous by the apparent development of puberty (pseudopubertas praecox) that begins very early. Typical signs of androgen excess in girls are the occurrence of hirsutism, acne, lack of breast development, disorders of the menstrual cycle and infertility. As bones mature prematurely, those affected suffer from short stature over the course of the disease [59]. AGS with salt wasting syndrome can lead to life-threatening metabolic imbalances in the first postnatal weeks.
The so-called late-onset AGS or non-classical AGS is characterized by mild symptoms without loss of salt. Premature pubic hair and acne occur, and women experience menstrual cycle and conception disorders. Short stature is not noticeable in those affected. If the symptoms lead to the suspicion of an adrenogenital syndrome, the diagnosis can first be confirmed by determining the hormone concentrations, in particular 17α-hydroxyprogesterone in serum (Figure 3), which, as an alternative metabolite, is significantly increased when cortisol synthesis is disturbed. In Germany, the determination of 17α-hydroxyprogesterone is part of the extended newborn screening and is routinely determined in all newborns. An ACTH test can be performed to identify heterozygous carriers who do not develop the disease themselves but can pass the mutation to their children. The adrenal glands are stimulated to produce more hormones by administering the adrenal cortex-stimulating hormone (Figure 3) [58].
8. Therapeutic Intervention
Treatment of acne tarda is rather challenging and should be a multidisciplinary task involving dermatologists, gynaecologists, endocrinologists and, if necessary, psychiatrists [60]. Adherence to evidence-based diagnostic and treatment strategies that address not only symptomatic improvement but also treatment of the underlying aetiology is essential to successfully treating affected women [10,61].
With regard to persistent, therapy-resistant acne, hormonal antiandrogen therapy is indicated, particularly in female patients who show signs of peripheral hyperandrogenism or hyperandrogenemia [6,10,16,28,62,63,64]. The primary effect of therapy is inhibition of the sebaceous gland production. In addition, hormonal antiandrogens reduce circulating testosterone by 40–50%. Regarding the fact that antiandrogens are teratogenic, contraception during treatment is essential [23,64].
Excess release of ovarian androgens is treated with ovarian suppression and antiandrogens [64]. Ovarian suppression includes the use of contraceptives containing an estrogen, ethinyl estradiol, and a progestogen. Chlormadinone acetate, cyproterone acetate and dienogest are potent, orally active progestogens, which have antiandrogenic instead of partial androgenic activity [65]. They exert their action through blocking of androgen receptors in skin, and reducing the activity of 5α-reductase, the enzyme responsible for converting testosterone to the more potent androgen, DHT, in sebaceous glands and hair follicles. Chlormadinone acetate and cyproterone acetate also suppress gonadotropin secretion, thereby reducing ovarian and adrenal androgen production [66]. Combined oral contraceptives containing 2 mg of chlormadinone acetate or cyproterone acetate plus 30 or 35 μg of ethinyl estradiol produced improvement or resolution of seborrhoea in 80% of users, acne in 59–70%, hirsutism in 36% and androgen-related alopecia in up to 86%. These combinations are well tolerated; however, some adverse effects have been reported including headache, breast tenderness and nausea [65]. Desogestrel and norgestimate are newer third-generation gonanes or synthetic progestins that have less activity at the androgen receptor and therefore more benefit in acne and hirsutism [67]. Drospirenone, a synthetic 17α-spironolactone-like molecule with antiandrogenic and antimineralocorticoid activity, is also available (3 mg) in combination with ethinyl estradiol (20 μg or 30 μg) and has also a significant impact on acne treatment [23,64,68]. Older synthetic first-generation progestins, such as the gonane norethindrone, and second-generation estranges, such as levonorgestrel and norgestrel, are derived from progesterone and may activate the androgen receptor, theoretically lessening the beneficial effects of these agents for acne [69]. Contraindications for oral contraception include hypertension and age >35 years, cerebrovascular disease, cigarette smoking and age >35 years, congestive heart failure, obesity and age >35 years, coronary artery disease, systemic lupus erythematous with vascular disease and/or nephritis history of thromboembolic disease and migraine headaches [70].
When polycystic ovary syndrome is associated with insulin resistance, metformin must be considered as a further treatment [71]. Adrenal-induced acne tarda is treated with adrenal suppression with glucocorticoids (0.125–0.375 mg oral dexamethasone) and antiandrogens. Hyperprolactinemic induced acne tarda should be treated with dopamine agonists such as the ergoline-derived agents, bromocriptine and cabergoline [72]. Acne tarda due to hypercortisolemia caused by Cushing’s disease should be promptly diagnosed. Gold standard treatment is surgical resection of adrenocorticotropic hormone-secreting pituitary adenoma, which is curative [73].
In addition, the initiation of systemic isotretinoin therapy under contraception should be considered [10,61]. Isotretinoin as the most effective systemic acne therapeutic drug influences all main pathogenetic factors. At the same time, it is also the most effective drug for inhibiting sebum production through a retinoid receptor-independent mechanism and leading to apoptosis [74]. The drug is only indicated for severe forms of acne that have proven to be resistant to adequate standard therapies with systemic antibiotics and topical therapy (second-line-therapeutic agent). Low-dose long-term treatments and moderate-dose intermittent treatments help in mild acne that is otherwise resistant to treatment or acne that recurs quickly after stopping systemic antibiotics and do not induce any adverse side effects such as mucocutaneous symptoms [75,76].
9. Conclusions
Refractory acne in post-adolescents may reflect the first signs of endocrinological disorders and can contribute to the diagnosis of internal diseases such as PCOS, POI, Cushing’s disease and AGS. A thorough history, a focused clinical examination and an interdisciplinary approach together with gynecologists and endocrinologists are of great importance in order to diagnose these diseases promptly and avoid late life-threatening complications such as diabetes mellitus, obesity and psychiatric diseases.
Author Contributions
E.M. and C.C.Z.: conceptualization, manuscript writing, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghodsi, S.Z.; Orawa, H.; Zouboulis, C.C. Prevalence, severity, and severity risk factors of acne in high school pupils: A community-based study. J. Investig. Dermatol. 2009, 129, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.D.; Umari, T.; Dunnick, C.A.; Dellavalle, R.P. The epidemiology of acne vulgaris in late adolescence. Adolesc. Health Med. Ther. 2016, 7, 13–25. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Goulden, V.; Stables, G.I.; Cunliffe, W.J. Prevalence of facial acne in adults. J. Am. Acad. Dermatol. 1999, 41, 577–580. [Google Scholar]
- Zouboulis, C.C. Adulte Akne (Acne tarda) der Frau: Eine Herausforderung fur den Dermatologen. J. Dtsch. Dermatol. Ges. 2018, 16, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C. Endocrinology and immunology of acne: Two sides of the same coin. Exp. Dermatol. 2020, 29, 840–859. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Jourdan, E.; Picardo, M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 527–532. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Bohm, M. Neuroendocrine regulation of sebocytes -- a pathogenetic link between stress and acne. Exp. Dermatol. 2004, 13 (Suppl. S4), 31–35. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Graziene, V.; Fimmel, S.; Zouboulis, C.C. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br. J. Dermatol. 2009, 160, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Moradi Tuchayi, S.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Primers 2015, 1, 15029. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Yoshida, G.J.; Wu, Y.; Xia, L.; Schneider, M.R. Sebaceous gland: Milestones of 30-year modelling research dedicated to the “brain of the skin”. Exp. Dermatol. 2020, 29, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C. Acne as a chronic systemic disease. Clin. Dermatol. 2014, 32, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Goulden, V.; McGeown, C.H.; Cunliffe, W.J. The familial risk of adult acne: A comparison between first-degree relatives of affected and unaffected individuals. Br. J. Dermatol. 1999, 141, 297–300. [Google Scholar] [CrossRef]
- Herane, M.I.; Ando, I. Acne in infancy and acne genetics. Dermatology 2003, 206, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Ju, Q.; Fimmel, S.; Hinz, N.; Stahlmann, R.; Xia, L.; Zouboulis, C.C. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters sebaceous gland cell differentiation in vitro. Exp. Dermatol. 2011, 20, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Zouboulis, C.C.; Tan, J.; Andersen, M.L.; Katta, R.; Lyu, X.; Aguilar, L.; Kerob, D.; Morita, A.; Krutmann, J.; et al. Adult skin acute stress responses to short-term environmental and internal aggression from exposome factors. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1963–1975. [Google Scholar] [CrossRef]
- Melnik, B.C.; John, S.M.; Schmitz, G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: Lessons learnt from laron syndrome. Nutr. Metab. 2011, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Borrel, V.; Thomas, P.; Catovic, C.; Racine, P.J.; Konto-Ghiorghi, Y.; Lefeuvre, L.; Duclairoir-Poc, C.; Zouboulis, C.C.; Feuilloley, M.G.J. Acne and Stress: Impact of Catecholamines on Cutibacterium acnes. Front. Med. 2019, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Wolkenstein, P.; Misery, L.; Amici, J.M.; Maghia, R.; Branchoux, S.; Cazeau, C.; Voisard, J.J.; Taieb, C. Smoking and dietary factors associated with moderate-to-severe acne in French adolescents and young adults: Results of a survey using a representative sample. Dermatology 2015, 230, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Zouboulis, C.C. Hyperandrogenism, adrenal dysfunction, and hirsutism. Hautarzt 2020, 71, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Orfanos, C.E.; Adler, Y.D.; Zouboulis, C.C. The SAHA syndrome. Horm. Res. 2000, 54, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Deplewski, D.; Rosenfield, R.L. Role of hormones in pilosebaceous unit development. Endocr. Rev. 2000, 21, 363–392. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Rabe, T. Hormonal antiandrogens in acne treatment. J. Dtsch. Dermatol. Ges. 2010, 8 (Suppl. S1), S60–S74. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. Androgens and ageing of the skin. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Zouboulis, C.C. Testosterone metabolism to 5alpha-dihydrotestosterone and synthesis of sebaceous lipids is regulated by the peroxisome proliferator-activated receptor ligand linoleic acid in human sebocytes. Br. J. Dermatol. 2007, 156, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tsai, S.J.; Sheu, H.M.; Tsai, J.C.; Zouboulis, C.C. Testosterone synthesized in cultured human SZ95 sebocytes derives mainly from dehydroepiandrosterone. Exp. Dermatol. 2010, 19, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C. The human skin as a hormone target and an endocrine gland. Hormones 2004, 3, 9–26. [Google Scholar] [CrossRef]
- Baroud, S.; Wu, J.; Zouboulis, C.C. Acne Syndromes and Mosaicism. Biomedicines 2021, 9, 1735. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Degitz, K. Androgen action on human skin -- from basic research to clinical significance. Exp. Dermatol. 2004, 13 (Suppl. S4), 5–10. [Google Scholar] [CrossRef] [PubMed]
- Vigouroux, C. What have we learned form monogenic forms of severe insulin resistance associated with PCOS/HAIRAN? Ann. Endocrinol. 2010, 71, 222–224. [Google Scholar] [CrossRef][Green Version]
- Da Cunha, M.G.; Fonseca, F.L.; Machado, C.D. Androgenic hormone profile of adult women with acne. Dermatology 2013, 226, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, J.M.; Jeong, M.K.; Zouboulis, C.C.; Lee, S.H. 11β-hydroxysteroid dehydrogenase type 1 is expressed in human sebaceous glands and regulates glucocorticoid-induced lipid synthesis and toll-like receptor 2 expression in SZ95 sebocytes. Br. J. Dermatol. 2013, 168, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Niimura, M. Elevated serum insulin-like growth factor-1 (IGF-1) levels in women with postadolescent acne. J. Dermatol. 1995, 22, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Cappel, M.; Mauger, D.; Thiboutot, D. Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. Arch. Dermatol. 2005, 141, 333–338. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Vogel, K.; Fimmel, S.; Oeff, M.; Seltmann, H.; Zouboulis, C.C. Interplay of IGF-I and 17beta-estradiol at age-specific levels in human sebocytes and fibroblasts in vitro. Exp. Gerontol. 2008, 43, 939–946. [Google Scholar] [CrossRef]
- Rosignoli, C.; Nicolas, J.C.; Jomard, A.; Michel, S. Involvement of the SREBP pathway in the mode of action of androgens in sebaceous glands in vivo. Exp. Dermatol. 2003, 12, 480–489. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Achenbach, A.; Makrantonaki, E. Acne tarda and male-pattern baldness unmasking primary ovarian insufficiency: A case and review. Dermatology 2014, 229, 51–54. [Google Scholar] [CrossRef]
- Azziz, R.; Dumesic, D.A.; Goodarzi, M.O. Polycystic ovary syndrome: An ancient disorder? Fertil. Steril. 2011, 95, 1544–1548. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- Witchel, S.F.; Recabarren, S.E.; Gonzalez, F.; Diamanti-Kandarakis, E.; Cheang, K.I.; Duleba, A.J.; Legro, R.S.; Homburg, R.; Pasquali, R.; Lobo, R.A.; et al. Emerging concepts about prenatal genesis, aberrant metabolism and treatment paradigms in polycystic ovary syndrome. Endocrine 2012, 42, 526–534. [Google Scholar] [CrossRef]
- Bentzen, J.G.; Forman, J.L.; Johannsen, T.H.; Pinborg, A.; Larsen, E.C.; Andersen, A.N. Ovarian antral follicle subclasses and anti-mullerian hormone during normal reproductive aging. J. Clin. Endocrinol. Metab. 2013, 98, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Dunaif, A.; Graf, M.; Mandeli, J.; Laumas, V.; Dobrjansky, A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J. Clin. Endocrinol. Metab. 1987, 65, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yu, Y.; Huang, H. An update on primary ovarian insufficiency. Sci. China Life Sci. 2012, 55, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Falorni, A.; Brozzetti, A.; Aglietti, M.C.; Esposito, R.; Minarelli, V.; Morelli, S.; Sbroma Tomaro, E.; Marzotti, S. Progressive decline of residual follicle pool after clinical diagnosis of autoimmune ovarian insufficiency. Clin. Endocrinol. 2012, 77, 453–458. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Devroey, P.; Fauser, B.C. Primary ovarian insufficiency. Lancet 2010, 376, 911–921. [Google Scholar] [CrossRef]
- Bakalov, V.K.; Gutin, L.; Cheng, C.M.; Zhou, J.; Sheth, P.; Shah, K.; Arepalli, S.; Vanderhoof, V.; Nelson, L.M.; Bondy, C.A. Autoimmune disorders in women with turner syndrome and women with karyotypically normal primary ovarian insufficiency. J. Autoimmun. 2012, 38, 315–321. [Google Scholar] [CrossRef]
- Sammaritano, L.R. Menopause in patients with autoimmune diseases. Autoimmun. Rev. 2012, 11, A430–A436. [Google Scholar] [CrossRef]
- Smith, J.A.; Vitale, S.; Reed, G.F.; Grieshaber, S.A.; Goodman, L.A.; Vanderhoof, V.H.; Calis, K.A.; Nelson, L.M. Dry eye signs and symptoms in women with premature ovarian failure. Arch. Ophthalmol. 2004, 122, 151–156. [Google Scholar] [CrossRef][Green Version]
- Hoek, A.; Schoemaker, J.; Drexhage, H.A. Premature ovarian failure and ovarian autoimmunity. Endocr. Rev. 1997, 18, 107–134. [Google Scholar] [CrossRef]
- Bakalov, V.K.; Anasti, J.N.; Calis, K.A.; Vanderhoof, V.H.; Premkumar, A.; Chen, S.; Furmaniak, J.; Smith, B.R.; Merino, M.J.; Nelson, L.M. Autoimmune oophoritis as a mechanism of follicular dysfunction in women with 46,XX spontaneous premature ovarian failure. Fertil. Steril. 2005, 84, 958–965. [Google Scholar] [CrossRef]
- Cervera, R.; Balasch, J. Bidirectional effects on autoimmunity and reproduction. Hum. Reprod. Update 2008, 14, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.M. Clinical practice. Primary ovarian insufficiency. N. Engl. J. Med. 2009, 360, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Tatsi, C.; Flippo, C.; Stratakis, C.A. Cushing syndrome: Old and new genes. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101418. [Google Scholar] [CrossRef]
- Kairys, N.; Schwell, A. Cushing Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Newell-Price, J.; Bertagna, X.; Grossman, A.B.; Nieman, L.K. Cushing’s syndrome. Lancet 2006, 367, 1605–1617. [Google Scholar] [CrossRef]
- Valassi, E.; Santos, A.; Yaneva, M.; Toth, M.; Strasburger, C.J.; Chanson, P.; Wass, J.A.; Chabre, O.; Pfeifer, M.; Feelders, R.A.; et al. The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur. J. Endocrinol. 2011, 165, 383–392. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M. The diagnosis of Cushing’s syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef]
- Claahsen-van der Grinten, H.L.; Hoefsloot, L.H. From gene to disease: Adrenogenital syndrome and the CYP21A2 gene. Ned. Tijdschr. Geneeskd. 2007, 151, 1174–1177. [Google Scholar]
- Von Muhlendahl, K.E. Problems of delayed diagnosis of uncomplicated adrenogenital syndrome. Dtsch. Med. Wochenschr. 1998, 123, 1295. [Google Scholar]
- Chernyshov, P.V.; Zouboulis, C.C.; Tomas-Aragones, L.; Jemec, G.B.; Manolache, L.; Tzellos, T.; Sampogna, F.; Evers, A.W.M.; Dessinioti, C.; Marron, S.E.; et al. Quality of life measurement in acne. Position Paper of the European Academy of Dermatology and Venereology Task Forces on Quality of Life and Patient Oriented Outcomes and Acne, Rosacea and Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 194–208. [Google Scholar] [CrossRef]
- Descamps, V. Clinical Guidelines for Management of Acne Vulgaris. JAMA 2017, 317, 213. [Google Scholar] [CrossRef]
- Chen, W.; Obermayer-Pietsch, B.; Hong, J.B.; Melnik, B.C.; Yamasaki, O.; Dessinioti, C.; Ju, Q.; Liakou, A.I.; Al-Khuzaei, S.; Katsambas, A.; et al. Acne-associated syndromes: Models for better understanding of acne pathogenesis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C. Treatment of acne with antiandrogens--an evidence-based review. J. Dtsch. Dermatol. Ges. 2003, 1, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Bettoli, V. Management of severe acne. Br. J. Dermatol. 2015, 172 (Suppl. S1), 27–36. [Google Scholar] [CrossRef] [PubMed]
- Raudrant, D.; Rabe, T. Progestogens with antiandrogenic properties. Drugs 2003, 63, 463–492. [Google Scholar] [CrossRef]
- Worret, I.; Arp, W.; Zahradnik, H.P.; Andreas, J.O.; Binder, N. Acne resolution rates: Results of a single-blind, randomized, controlled, parallel phase III trial with EE/CMA (Belara) and EE/LNG (Microgynon). Dermatology 2001, 203, 38–44. [Google Scholar] [CrossRef]
- Salvaggio, H.L.; Zaenglein, A.L. Examining the use of oral contraceptives in the management of acne. Int. J. Womens Health 2010, 2, 69–76. [Google Scholar] [CrossRef][Green Version]
- Joish, V.N.; Boklage, S.; Lynen, R.; Schmidt, A.; Lin, J. Use of drospirenone/ethinyl estradiol (DRSP/EE) among women with acne reduces acne treatment-related resources. J. Med. Econ. 2011, 14, 681–689. [Google Scholar] [CrossRef]
- Harper, J.C. Use of Oral Contraceptives for Management of Acne Vulgaris: Practical Considerations in Real World Practice. Dermatol. Clin. 2016, 34, 159–165. [Google Scholar] [CrossRef]
- Tyler, K.H.; Zirwas, M.J. Contraception and the dermatologist. J. Am. Acad. Dermatol. 2013, 68, 1022–1029. [Google Scholar] [CrossRef]
- Dalamaga, M.; Papadavid, E.; Basios, G.; Vaggopoulos, V.; Rigopoulos, D.; Kassanos, D.; Trakakis, E. Ovarian SAHA syndrome is associated with a more insulin-resistant profile and represents an independent risk factor for glucose abnormalities in women with polycystic ovary syndrome: A prospective controlled study. J. Am. Acad. Dermatol. 2013, 69, 922–930. [Google Scholar] [CrossRef]
- Verhelst, J.; Abs, R. Hyperprolactinemia: Pathophysiology and management. Treat Endocrinol. 2003, 2, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bray, D.P.; Rindler, R.S.; Dawoud, R.A.; Boucher, A.B.; Oyesiku, N.M. Cushing Disease: Medical and Surgical Considerations. Otolaryngol. Clin. N. Am. 2022, 55, 315–329. [Google Scholar] [CrossRef]
- Nelson, A.M.; Gilliland, K.L.; Cong, Z.; Thiboutot, D.M. 13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J. Investig. Dermatol. 2006, 126, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Goulden, V.; Clark, S.M.; McGeown, C.; Cunliffe, W.J. Treatment of acne with intermittent isotretinoin. Br. J. Dermatol. 1997, 137, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Goulden, V.; Layton, A.M.; Cunliffe, W.J. Long-term safety of isotretinoin as a treatment for acne vulgaris. Br. J. Dermatol. 1994, 131, 360–363. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).