Effects of Bifidobacterium animalis subsp. lactis Bl-04 on Skin Wrinkles and Dryness: A Randomized, Triple-Blinded, Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Ethical Approval and Registry
2.3. Sample Size
2.4. Inclusion and Exclusion Criteria
- Korean female subjects aged 30 to 60 years (by Korean age).
- Dry skin on cheek (hydration value < 48 arbitrary units (AU) on a Corneometer®).
- Greater than grade 3 skin wrinkle per a DERMAPRO standard photograph.
- No chronic or acute disease, including skin disease.
- Signed informed consent for study participation.
- Cooperation and availability for follow-up during the study period.
- Consumption of probiotics as dietary supplements, food, or beverage during the previous 2 weeks.
- Pregnancy, planned pregnancy, or lactation.
- Irritation or symptomatic allergy to food, including ingredients of cosmetic, medical, and test products.
- Use of oral or topical antibiotics during the previous 3 months.
- Use of oral retinoid/steroid drug or topical steroid application during the previous 6 months.
- Use of functional cosmetics to improve skin wrinkles, hydration, or elasticity within the past 3 months.
- Having had skin treatment on the test site (e.g., decortication and Botox treatment).
- Participation in a previous study without an appropriate intervening period (3 months) between studies.
- Presence of disease that could affect the aim of the study (e.g., cardiovascular, kidney, liver, thyroid, gastrointestinal disease, gout).
- Presence of any skin disease (e.g., AD) on test site.
- Presence of any chronic disease (e.g., diabetes, asthma, high blood pressure) or psychiatric disorder (e.g., depression, schizophrenia).
- Use of medication for obesity (e.g., antidepressants, anorectics), contraceptives, hormones, or diuretics.
- Excessive alcohol use (over 30 g alcohol per day) or drug abuse.
- Sensitive or hypersensitive skin.
- Damaged skin in or around the test area (including sunburn, tattoos, scars, or other disfiguration of the test area).
- Abnormal clinical chemical analysis result at V1, per a medical specialist.
- Presence of a problem that could interfere with the aim of the study, based on the judgment of the principal investigator.
2.5. Interventions
2.6. Randomization and Blinding
2.7. Study Assessments
2.7.1. Primary Outcomes: Facial Wrinkles and Skin Hydration
2.7.2. Secondary Outcomes
2.7.3. Safety Outcomes and Other Evaluations
2.8. Statistical Methods
2.9. Post Hoc Analyses
3. Results
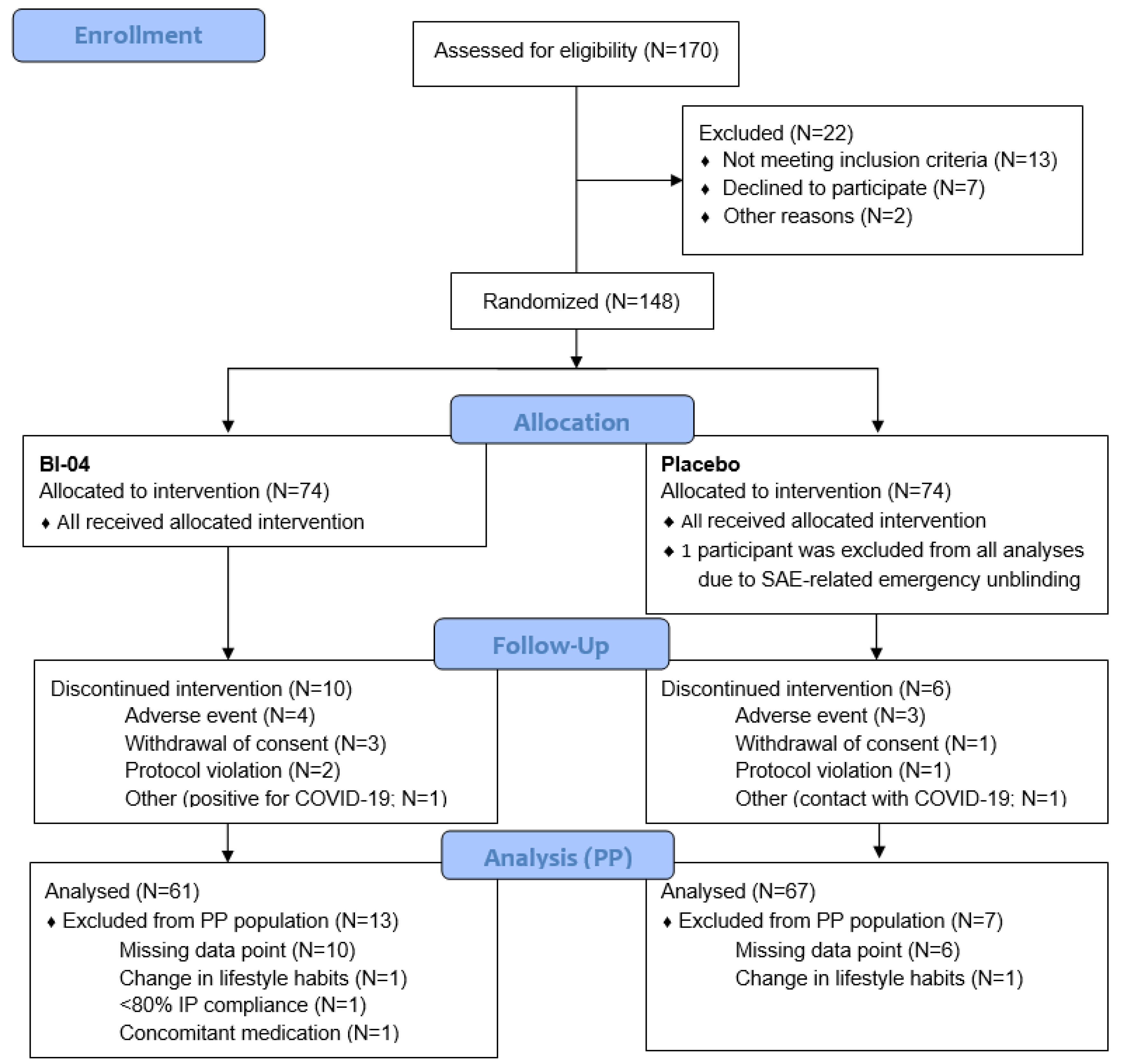
3.1. Recruitment and Study Populations
3.2. Baseline Data
3.3. Primary Outcomes
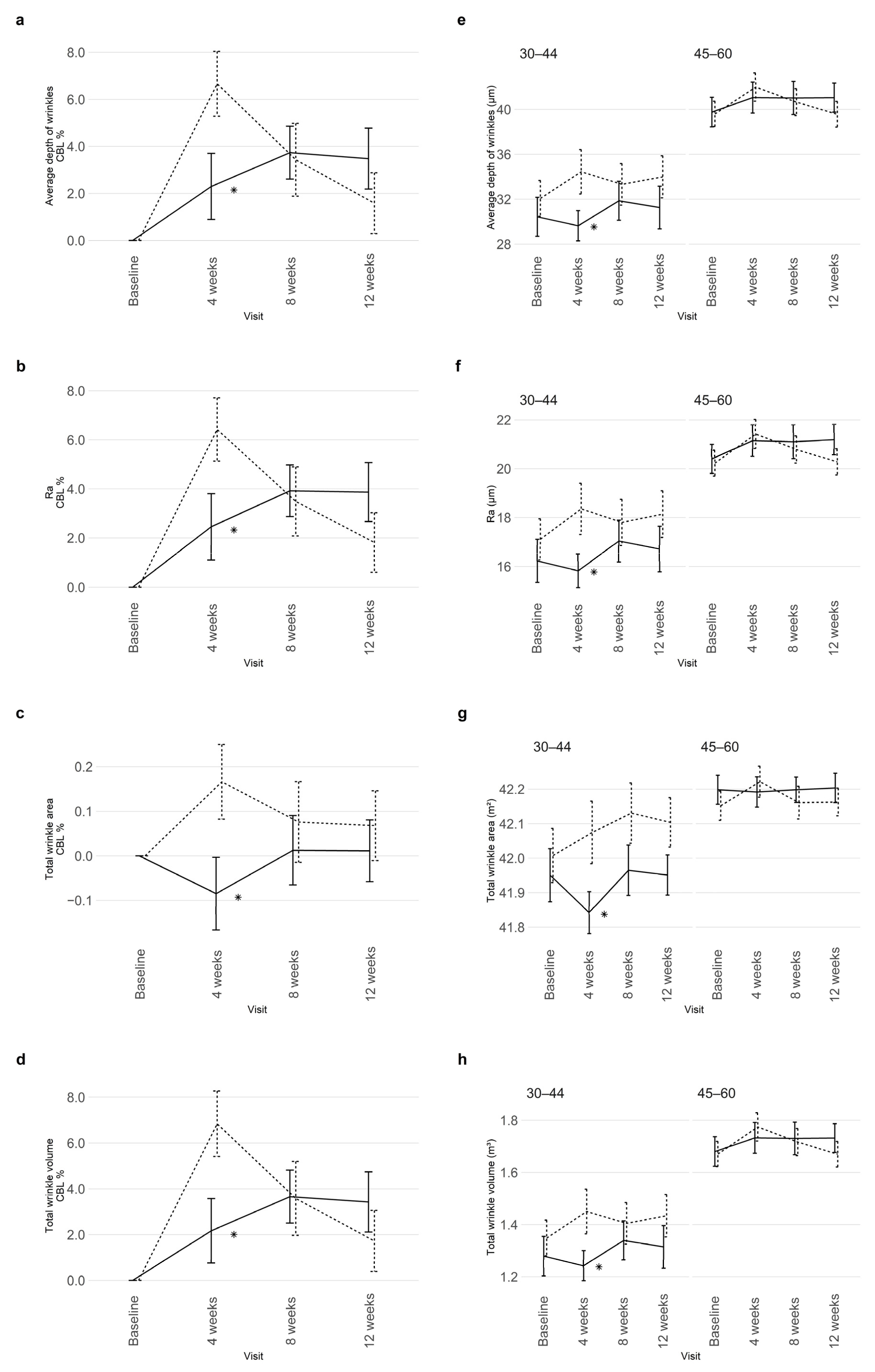
3.3.1. Skin Wrinkles
3.3.2. Skin Hydration
3.4. Secondary Outcomes
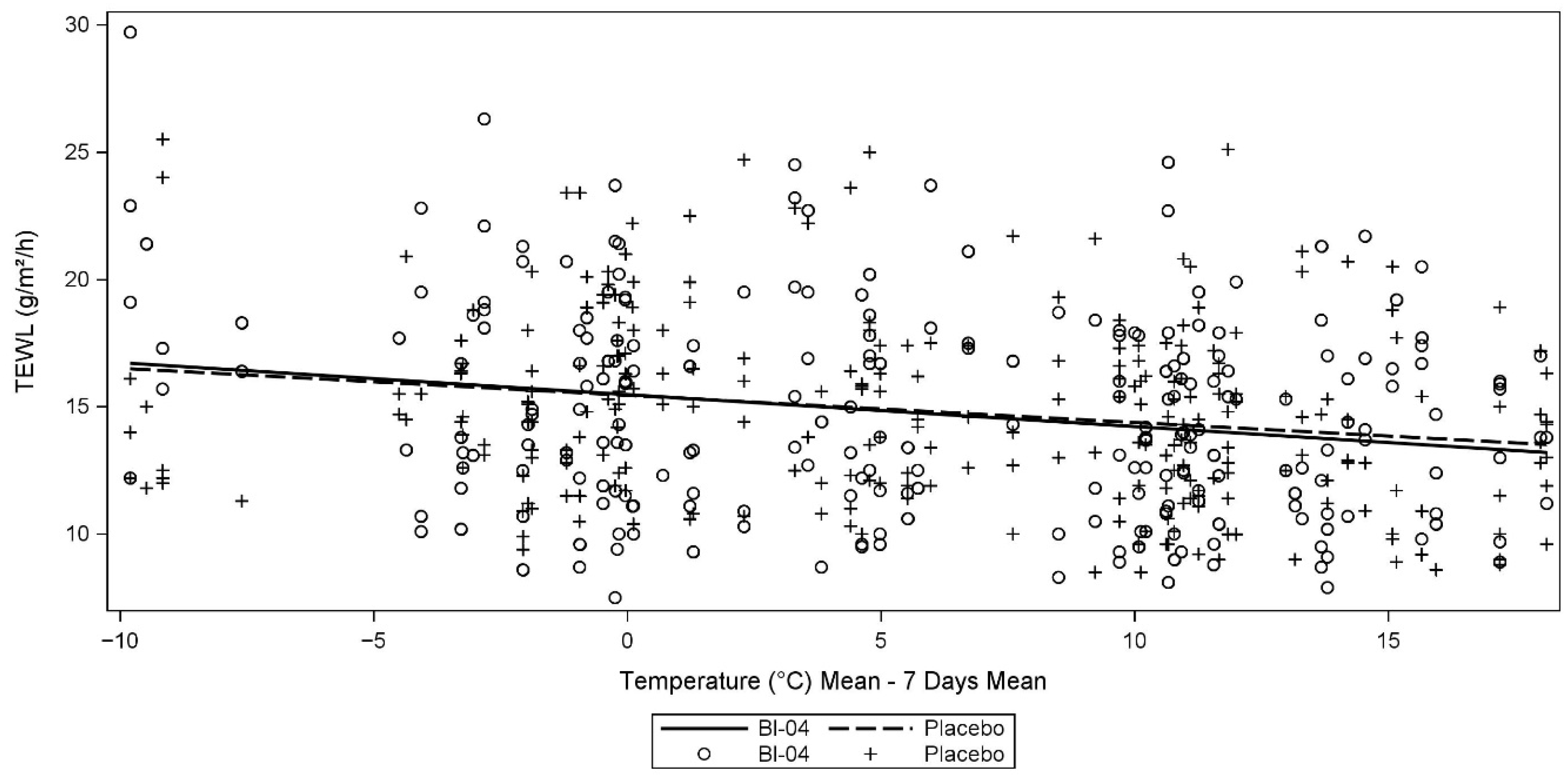
3.5. Ancillary Analyses
3.6. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy Dermatol. Alergol. 2016, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fore, J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manag. 2006, 52, 24–35. [Google Scholar] [PubMed]
- Liska, D.; Mah, E.; Brisbois, T.; Barrios, P.L.; Baker, L.B.; Spriet, L.L. Narrative Review of Hydration and Selected Health Outcomes in the General Population. Nutrients 2019, 11, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging-From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [Green Version]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Szanto, M.; Dozsa, A.; Antal, D.; Szabo, K.; Kemeny, L.; Bai, P. Targeting the gut-skin axis-Probiotics as new tools for skin disorder management? Exp. Dermatol. 2019, 28, 1210–1218. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, M.L.; Romanuk, T.N. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS ONE 2012, 7, e34938. [Google Scholar] [CrossRef] [Green Version]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Velez, E.; Perdigon, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Lee, D.E.; Huh, C.S.; Ra, J.; Choi, I.D.; Jeong, J.W.; Kim, S.H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.H.; et al. Clinical Evidence of Effects of Lactobacillus plantarum HY7714 on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tseng, Y.P.; Chan, L.P.; Liang, C.H. The potential of Streptococcus thermophiles (TCI633) in the anti-aging. J. Cosmet. Dermatol. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gueniche, A.; Philippe, D.; Bastien, P.; Reuteler, G.; Blum, S.; Castiel-Higounenc, I.; Breton, L.; Benyacoub, J. Randomised double-blind placebo-controlled study of the effect of Lactobacillus paracasei NCC 2461 on skin reactivity. Benef. Microbes 2014, 5, 137–145. [Google Scholar] [CrossRef]
- Peguet-Navarro, J.; Dezutter-Dambuyant, C.; Buetler, T.; Leclaire, J.; Smola, H.; Blum, S.; Bastien, P.; Breton, L.; Gueniche, A. Supplementation with oral probiotic bacteria protects human cutaneous immune homeostasis after UV exposure-double blind, randomized, placebo controlled clinical trial. Eur. J. Dermatol. 2008, 18, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; West, N.P.; Horn, P.L.; Lehtinen, M.J.; Koerbin, G.; Pyne, D.B.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Effects of probiotic supplementation over 5 months on routine haematology and clinical chemistry measures in healthy active adults. Eur. J. Clin. Nutr. 2014, 68, 1255–1257. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Acid, bile, and heat tolerance of free and microencapsulated probiotic bacteria. J. Food Sci. 2007, 72, M446–M450. [Google Scholar] [CrossRef] [PubMed]
- Forssten, S.D.; Ouwehand, A.C. Simulating colonic survival of probiotics in single-strain products compared to multi-strain products. Microb Ecol Health Dis 2017, 28, 1378061. [Google Scholar] [CrossRef] [Green Version]
- Forssten, S.; Ouwehand, A.C. Dose-Response Recovery of Probiotic Strains in Simulated Gastro-Intestinal Passage. Microorganisms 2020, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Ouwehand, A.C.; Nermes, M.; Collado, M.C.; Rautonen, N.; Salminen, S.; Isolauri, E. Specific probiotics alleviate allergic rhinitis during the birch pollen season. World J. Gastroenterol. 2009, 15, 3261–3268. [Google Scholar] [CrossRef]
- Taverniti, V.; Koirala, R.; Dalla Via, A.; Gargari, G.; Leonardis, E.; Arioli, S.; Guglielmetti, S. Effect of Cell Concentration on the Persistence in the Human Intestine of Four Probiotic Strains Administered through a Multispecies Formulation. Nutrients 2019, 11, 285. [Google Scholar] [CrossRef] [Green Version]
- Paineau, D.; Carcano, D.; Leyer, G.; Darquy, S.; Alyanakian, M.A.; Simoneau, G.; Bergmann, J.F.; Brassart, D.; Bornet, F.; Ouwehand, A.C. Effects of seven potential probiotic strains on specific immune responses in healthy adults: A double-blind, randomized, controlled trial. FEMS Immunol. Med Microbiol. 2008, 53, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.B.; Woodfolk, J.A.; Borish, L.; Steinke, J.W.; Patrie, J.T.; Muehling, L.M.; Lahtinen, S.; Lehtinen, M.J. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection—A randomised controlled trial. Benef. Microbes 2017, 8, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muehling, L.M.; Turner, R.B.; Brown, K.B.; Wright, P.W.; Patrie, J.T.; Lahtinen, S.J.; Lehtinen, M.J.; Kwok, W.W.; Woodfolk, J.A. Single-Cell Tracking Reveals a Role for Pre-Existing CCR5+ Memory Th1 Cells in the Control of Rhinovirus-A39 After Experimental Challenge in Humans. J. Infect. Dis. 2018, 217, 381–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehtinen, M.J.; Hibberd, A.A.; Männikkö, S.; Yeung, N.; Kauko, T.; Forssten, S.; Lehtoranta, L.; Lahtinen, S.J.; Stahl, B.; Lyra, A.; et al. Nasal microbiota clusters associate with inflammatory response, viral load, and symptom severity in experimental rhinovirus challenge. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M.; Saiki, A.; Matsui, Y.; Tsuchimoto, N.; Nakakita, Y.; Takata, Y.; Nakamura, T. Effects of oral intake of heat-killed Lactobacillus brevis SBC8803 (SBL88) on dry skin conditions: A randomized, double-blind, placebo-controlled study. Exp. Ther. Med. 2016, 12, 3863–3872. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.R.; Jeong, B.J.; Lee, S.S.; Kim, T.R.; Jeong, J.H.; Lee, M.; Lee, S.; Lee, J.S.; Chung, D.K. Effects of oral intake of kimchi-derived Lactobacillus plantarum K8 lysates on skin moisturizing. J. Microbiol. Biotechnol. 2015, 25, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Kimoto-Nira, H.; Aoki, R.; Sasaki, K.; Suzuki, C.; Mizumachi, K. Oral intake of heat-killed cells of Lactococcus lactis strain H61 promotes skin health in women. J. Nutr. Sci. 2012, 1, e18. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Mihara, T.; Maruyama, K.; Saito, J.; Ikeda, M.; Tomonaga, A.; Kumagai, T. Effects of intake of Lactobacillus casei subsp. casei 327 on skin conditions: A randomized, double-blind, placebo-controlled, parallel-group study in women. Biosci. Microbiota Food Health 2017, 36, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.H.; Chang, U.J.; Kim, Y.S.; Jung, E.Y.; Suh, H.J. Dietary galacto-oligosaccharides improve skin health: A randomized double blind clinical trial. Asia Pac. J. Clin. Nutr. 2017, 26, 613–618. [Google Scholar] [CrossRef]
- Kim, D.U.; Chung, H.C.; Choi, J.; Sakai, Y.; Lee, B.Y. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef] [Green Version]
- Foligne, B.; Nutten, S.; Grangette, C.; Dennin, V.; Goudercourt, D.; Poiret, S.; Dewulf, J.; Brassart, D.; Mercenier, A.; Pot, B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J. Gastroenterol. 2007, 13, 236–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leech, B.; Schloss, J.; Steel, A. Association between increased intestinal permeability and disease: A systematic review. Adv. Integr. Med. 2019, 6, 23–34. [Google Scholar] [CrossRef]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Therap. Adv. Gastroenterol. 2021, 14, 1756284821993586. [Google Scholar] [CrossRef] [PubMed]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Farre, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef] [Green Version]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Egawa, G.; Honda, T.; Kabashima, K. SCFAs Control Skin Immune Responses via Increasing Tregs. J. Invest. Dermatol. 2017, 137, 800–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The Short-Chain Fatty Acid Sodium Butyrate Functions as a Regulator of the Skin Immune System. J. Invest. Dermatol. 2017, 137, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riviere, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, P.R.; Bittencourt, L.F.F.; Larochelle, S.; Andrade, S.P.; Mackay, C.R.; Slevin, M.; Moulin, V.J.; Barcelos, L.S. GPR43 regulates sodium butyrate-induced angiogenesis and matrix remodeling. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1066–H1079. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.R.; Kim, N.R.; Park, S.J.; Lee, M.; Kim, O.K. Combination of Bifidobacterium longum and Galacto-Oligosaccharide Protects the Skin from Photoaging. J. Med. Food 2021, 24, 606–616. [Google Scholar] [CrossRef]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Invest. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Humbert, P.; Viennet, C.; Legagneux, K.; Grandmottet, F.; Robin, S.; Oddos, T.; Muret, P. In the shadow of the wrinkle: Theories. J. Cosmet. Dermatol. 2012, 11, 72–78. [Google Scholar] [CrossRef]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef]
- Hillebrand, G.G.; Liang, Z.; Yan, X.; Yoshii, T. New wrinkles on wrinkling: An 8-year longitudinal study on the progression of expression lines into persistent wrinkles. Br. J. Dermatol. 2010, 162, 1233–1241. [Google Scholar] [CrossRef]
- Huuskonen, L.; Anglenius, H.; Tiihonen, K.; Ouwehand, A.C. Probiotics and Their Various Forms Supporting Skin Health. In Probiotic Research in Therapeutics: Volume 3: Probiotics and Gut Skin Axis–Inside Out and Outside In; Beri, K., Deol, P.K., Sandhu, S.K., Eds.; Springer: Singapore, 2022; pp. 57–109. [Google Scholar]
- Fanian, F.; Mac-Mary, S.; Jeudy, A.; Lihoreau, T.; Messikh, R.; Ortonne, J.P.; Sainthillier, J.M.; Elkhyat, A.; Guichard, A.; Kenari, K.H.; et al. Efficacy of micronutrient supplementation on skin aging and seasonal variation: A randomized, placebo-controlled, double-blind study. Clin. Interv. Aging 2013, 8, 1527–1537. [Google Scholar] [CrossRef] [Green Version]
- Nam, G.W.; Baek, J.H.; Koh, J.S.; Hwang, J.K. The seasonal variation in skin hydration, sebum, scaliness, brightness and elasticity in Korean females. Skin Res. Technol. 2015, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Egawa, M.; Oguri, M.; Kuwahara, T.; Takahashi, M. Effect of exposure of human skin to a dry environment. Skin Res. Technol. 2002, 8, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Gefeller, O.; Schwanitz, H.J. An epidemiological study of the influence of season (cold and dry air) on the occurrence of irritant skin changes of the hands. Br. J. Dermatol. 1998, 138, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Goad, N.; Gawkrodger, D.J. Ambient humidity and the skin: The impact of air humidity in healthy and diseased states. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1285–1294. [Google Scholar] [CrossRef]
- Black, D.; Del Pozo, A.; Lagarde, J.M.; Gall, Y. Seasonal variability in the biophysical properties of stratum corneum from different anatomical sites. Skin Res. Technol. 2000, 6, 70–76. [Google Scholar] [CrossRef]
- Wan, M.J.; Su, X.Y.; Zheng, Y.; Gong, Z.J.; Yi, J.L.; Zhao, Y.; Guan, X.M.; Lai, W. Seasonal variability in the biophysical properties of forehead skin in women in Guangzhou City, China. Int. J. Dermatol. 2015, 54, 1319–1324. [Google Scholar] [CrossRef]
- Engebretsen, K.A.; Johansen, J.D.; Kezic, S.; Linneberg, A.; Thyssen, J.P. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 223–249. [Google Scholar] [CrossRef]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef]
- Dabrowska, A.K.; Adlhart, C.; Spano, F.; Rotaru, G.M.; Derler, S.; Zhai, L.; Spencer, N.D.; Rossi, R.M. In vivo confirmation of hydration-induced changes in human-skin thickness, roughness and interaction with the environment. Biointerphases 2016, 11, 031015. [Google Scholar] [CrossRef] [Green Version]
- Akdeniz, M.; Tomova-Simitchieva, T.; Dobos, G.; Blume-Peytavi, U.; Kottner, J. Does dietary fluid intake affect skin hydration in healthy humans? A systematic literature review. Skin Res. Technol. 2018, 24, 459–465. [Google Scholar] [CrossRef]
- Rogiers, V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E. EEMCO guidance for the assessment of stratum corneum hydration: Electrical methods. Skin Res. Technol. 1997, 3, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Loden, M.; Serup, J.; Masson, P.; Rodrigues, L.M. The revised EEMCO guidance for the in vivo measurement of water in the skin. Skin Res. Technol. 2018, 24, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Cho, C.; Cho, E.; Kim, N.; Shin, J.; Woo, S.; Lee, E.; Hwang, J.; Ha, J. Age-related biophysical changes of the epidermal and dermal skin in Korean women. Skin Res. Technol. 2019, 25, 504–511. [Google Scholar] [CrossRef]
- Galzote, C.; Estanislao, R.; Suero, M.O.; Khaiat, A.; Mangubat, M.I.; Moideen, R.; Tagami, H.; Wang, X. Characterization of facial skin of various Asian populations through visual and non-invasive instrumental evaluations: Influence of age and skincare habits. Skin Res. Technol. 2013, 19, 454–465. [Google Scholar] [CrossRef]
- Baek, J.H.; Lee, M.Y.; Koh, J.S. Relationship between clinical features of facial dry skin and biophysical parameters in Asians. Int. J. Cosmet. Sci. 2011, 33, 222–227. [Google Scholar] [CrossRef]
- Akdeniz, M.; Gabriel, S.; Lichterfeld-Kottner, A.; Blume-Peytavi, U.; Kottner, J. Transepidermal water loss in healthy adults: A systematic review and meta-analysis update. Br. J. Dermatol. 2018, 179, 1049–1055. [Google Scholar] [CrossRef]
- Galzote, C.; Estanislao, R.; Suero, M.O.; Khaiat, A.; Mangubat, M.I.; Moideen, R.; Tagami, H.; Wang, X. Characterization of facial skin of various Asian populations through visual and non-invasive instrumental evaluations: Influence of seasons. Skin Res. Technol. 2014, 20, 453–462. [Google Scholar] [CrossRef]
- Cravello, B.; Ferri, A. Relationships between skin properties and environmental parameters. Skin Res. Technol. 2008, 14, 180–186. [Google Scholar] [CrossRef]
- Singh, B.; Maibach, H. Climate and skin function: An overview. Skin Res. Technol. 2013, 19, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Mayuzumi, J.; Kikuchi, N.; Arai, S. Seasonal variations in skin temperature, skin pH, evaporative water loss and skin surface lipid values on human skin. Chem Pharm Bull 1980, 28, 387–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasic-Kostov, M.; Martinovic, M.; Ilic, D.; Cvetkovic, M. Cotton versus medical face mask influence on skin characteristics during COVID-19 pandemic: A short-term study. Skin Res. Technol. 2022, 28, 66–70. [Google Scholar] [CrossRef]
- Park, S.R.; Han, J.; Yeon, Y.M.; Kang, N.Y.; Kim, E. Effect of face mask on skin characteristics changes during the COVID-19 pandemic. Skin Res. Technol. 2021, 27, 554–559. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, S.; Kwon, O.S.; Jeong, E.T.; Lim, J.M.; Park, S.G. Influence of quarantine mask use on skin characteristics: One of the changes in our life caused by the COVID-19 pandemic. Skin Res. Technol. 2021, 27, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, H.; Kim, S.; Lee, J.; Kim, S.; Byun, J.W.; Hwang-Bo, J.; Park, K.H. Changes in skin wrinkles and pores due to long-term mask wear. Skin Res. Technol. 2021, 27, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Cuenca-Barrales, C.; Martinez-Lopez, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin adverse events related to personal protective equipment: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1994–2006. [Google Scholar] [CrossRef]
- Reid, G.; Gaudier, E.; Guarner, F.; Huffnagle, G.B.; Macklaim, J.M.; Munoz, A.M.; Martini, M.; Ringel-Kulka, T.; Sartor, B.; Unal, R.; et al. Responders and non-responders to probiotic interventions: How can we improve the odds? Gut Microbes 2010, 1, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Mazidi, M.; Rezaie, P.; Ferns, G.A.; Vatanparast, H. Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients 2017, 9, 20. [Google Scholar] [CrossRef]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Dore, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef]
- Van den Munckhof, I.C.L.; Kurilshikov, A.; Ter Horst, R.; Riksen, N.P.; Joosten, L.A.B.; Zhernakova, A.; Fu, J.; Keating, S.T.; Netea, M.G.; de Graaf, J.; et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: A systematic review of human studies. Obes. Rev. 2018, 19, 1719–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajamian, M.; Steer, D.; Rosella, G.; Gibson, P.R. Serum zonulin as a marker of intestinal mucosal barrier function: May not be what it seems. PLoS ONE 2019, 14, e0210728. [Google Scholar] [CrossRef] [PubMed]
- Grubb, D.S.; Wrigley, S.D.; Freedman, K.E.; Wei, Y.; Vazquez, A.R.; Trotter, R.E.; Wallace, T.C.; Johnson, S.A.; Weir, T.L. PHAGE-2 Study: Supplemental Bacteriophages Extend Bifidobacterium animalis subsp. lactis BL04 Benefits on Gut Health and Microbiota in Healthy Adults. Nutrients 2020, 12, 2474. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.; Kim, S.A.; Park, S.D.; Kim, H.J.; Kim, J.S.; Bae, C.H.; Kim, J.Y.; Nam, W.; Lee, J.L.; Sim, J.H. Regulatory effects of Lactobacillus plantarum HY7714 on skin health by improving intestinal condition. PLoS ONE 2020, 15, e0231268. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]

| Item | Classification | Bl-04 (N = 61) N (%) 1 | Placebo (N = 67) N (%) 1 | Total (N = 128) N (%) 1 |
|---|---|---|---|---|
| Facial skin type | Dry | 35 (57.4) | 41 (61.2) | 76 (59.4) |
| Normal | 19 (31.1) | 18 (26.9) | 37 (28.9) | |
| Oily | 1 (1.6) | 1 (1.5) | 2 (1.6) | |
| Dry and oily | 6 (9.8) | 7 (10.4) | 13 (10.2) | |
| Problematic (acne or other skin disease) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| Body skin type | Dry | 35 (57.4) | 39 (58.2) | 74 (57.8) |
| Normal | 23 (37.7) | 24 (35.8) | 47 (36.7) | |
| Oily | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| Dry and oily | 3 (4.9) | 4 (6.0) | 7 (5.5) | |
| Problematic (acne or other skin disease) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| Facial skin hydration 2 | Sufficient | 1 (1.6) | 0 (0.0) | 1 (0.8) |
| Normal | 27 (44.3) | 25 (37.3) | 52 (40.6) | |
| Deficient | 32 (52.5) | 42 (62.7) | 74 (57.8) | |
| Body skin hydration 3 | Sufficient | 0 (0.0) | 1 (1.5) | 1 (0.8) |
| Normal | 28 (45.9) | 30 (44.8) | 58 (45.3) | |
| Deficient | 32 (52.5) | 35 (52.2) | 67 (52.3) | |
| Facial skin sebum | Glossy | 3 (4.9) | 2 (3.0) | 5 (3.9) |
| Normal | 29 (47.5) | 37 (55.2) | 66 (51.6) | |
| Deficient | 29 (47.5) | 28 (41.8) | 57 (44.5) | |
| Body skin sebum | Glossy | 1 (1.6) | 1 (1.5) | 2 (1.6) |
| Normal | 27 (44.3) | 37 (55.2) | 64 (50.0) | |
| Deficient | 33 (54.1) | 29 (43.3) | 62 (48.4) | |
| Facial skin surface roughness | Smooth | 9 (14.8) | 20 (29.9) | 29 (22.7) |
| Normal | 47 (77.0) | 37 (55.2) | 84 (65.6) | |
| Rough | 5 (8.2) | 10 (14.9) | 15 (11.7) | |
| Body skin surface roughness | Smooth | 8 (13.1) | 13 (19.4) | 21 (16.4) |
| Normal | 43 (70.5) | 46 (68.7) | 89 (69.5) | |
| Rough | 10 (16.4) | 8 (11.9) | 18 (14.1) | |
| Facial skin thickness | Thin | 17 (27.9) | 25 (37.3) | 42 (32.8) |
| Normal | 40 (65.6) | 40 (59.7) | 80 (62.5) | |
| Thick | 4 (6.6) | 2 (3.0) | 6 (4.7) | |
| Average daily UV exposure time | Less than 1 h | 25 (41.0) | 32 (47.8) | 57 (44.5) |
| 1–3 h | 33 (54.1) | 33 (49.3) | 66 (51.6) | |
| More than 3 h | 3 (4.9) | 2 (3.0) | 5 (3.9) |
| Parameter | Time Point | Bl-04 (N = 61) | Placebo (N = 67) | p-Value 2 | ||
|---|---|---|---|---|---|---|
| Mean (SD) 4 | p-Value 1 | Mean (SD) 4 | p-Value 1 | |||
| Average depth of wrinkles, µm | Baseline | 37.01 (9.28) | - | 38.14 (8.37) | - | 0.470 |
| 4 weeks | 37.69 (9.71) | 0.237 | 40.52 (9.30) | <0.001 * | 0.026 * | |
| 8 weeks | 38.32 (9.92) | 0.003 * | 39.23 (8.88) | 0.056 | 0.755 | |
| 12 weeks | 38.16 (9.45) | 0.031 * | 38.48 (8.37) | 0.466 | 0.252 | |
| Mean depth largest wrinkle, µm | Baseline | 52.43 (20.00) | - | 56.20 (19.12) | - | 0.279 |
| 4 weeks | 53.79 (20.34) | 0.385 | 58.03 (20.84) | 0.149 | 0.810 | |
| 8 weeks | 53.81 (20.67) | 0.310 | 58.32 (18.97) | 0.097 | 0.685 | |
| 12 weeks | 53.92 (20.08) | 0.314 | 56.31 (19.94) | 0.912 | 0.447 | |
| Max. depth largest wrinkle, µm | Baseline | 152.84 (77.98) | - | 157.18 (66.18) | - | 0.734 |
| 4 weeks | 163.05 (88.98) | 0.747 3 | 163.23 (74.72) | 0.211 | 0.607 | |
| 8 weeks | 153.87 (81.68) | 2.497 3 | 165.34 (70.37) | 0.069 | 0.365 | |
| 12 weeks | 153.47 (71.88) | 2.572 3 | 163.59 (77.12) | 0.279 | 0.499 | |
| Total wrinkle area, mm2 | Baseline | 42.13 (0.31) | - | 42.12 (0.31) | - | 0.982 |
| 4 weeks | 42.09 (0.32) | 0.292 | 42.19 (0.33) | 0.054 | 0.034 * | |
| 8 weeks | 42.13 (0.28) | 0.897 | 42.16 (0.34) | 0.418 | 0.598 | |
| 12 weeks | 42.13 (0.29) | 0.894 | 42.15 (0.29) | 0.406 | 0.597 | |
| Total wrinkle volume, mm3 | Baseline | 1.56 (0.40) | - | 1.61 (0.36) | - | 0.490 |
| 4 weeks | 1.59 (0.42) | 0.280 | 1.71 (0.40) | <0.001 * | 0.019 * | |
| 8 weeks | 1.61 (0.42) | 0.004 * | 1.66 (0.38) | 0.057 | 0.863 | |
| 12 weeks | 1.61 (0.40) | 0.041 * | 1.62 (0.36) | 0.445 | 0.309 | |
| Total length of wrinkles, mm | Baseline | 109.23 (16.26) | - | 106.00 (11.80) | - | 0.205 |
| 4 weeks | 108.46 (15.05) | 0.367 | 105.42 (11.64) | 0.441 | 0.778 | |
| 8 weeks | 108.74 (15.22) | 0.511 | 104.99 (12.32) | 0.163 | 0.388 | |
| 12 weeks | 108.08 (15.45) | 0.203 | 105.70 (11.36) | 0.724 | 0.787 | |
| Ra, µm | Baseline | 19.17 (4.28) | - | 19.62 (3.92) | - | 0.533 |
| 4 weeks | 19.58 (4.59) | 0.138 | 20.83 (4.41) | <0.001 * | 0.031 * | |
| 8 weeks | 19.90 (4.65) | 0.001 * | 20.21 (4.14) | 0.030 * | 0.661 | |
| 12 weeks | 19.87 (4.49) | 0.005 * | 19.86 (3.93) | 0.294 | 0.170 | |
| Ry, µm | Baseline | 307.84 (109.65) | - | 311.52 (91.50) | - | 0.836 |
| 4 weeks | 324.39 (116.72) | 0.010 *3 | 328.49 (104.48) | 0.007 * | 0.963 | |
| 8 weeks | 316.17 (108.98) | 0.654 3 | 314.40 (93.84) | 0.636 | 0.530 | |
| 12 weeks | 310.13 (94.70) | 1.372 3 | 314.02 (95.41) | 0.719 | 0.983 | |
| Rz, µm | Baseline | 234.86 (57.64) | - | 240.68 (53.42) | - | 0.554 |
| 4 weeks | 241.84 (59.58) | 0.053 | 249.61 (55.36) | 0.007 * | 0.684 | |
| 8 weeks | 241.89 (61.75) | 0.019 * | 242.73 (51.97) | 0.497 | 0.238 | |
| 12 weeks | 240.32 (57.54) | 0.108 | 241.23 (50.45) | 0.869 | 0.298 | |
| Location | Time Point | Bl-04 (N = 61) | Placebo (N = 67) | p-Value 2 | ||
|---|---|---|---|---|---|---|
| Mean (SD) | p-Value 1 | Mean (SD) | p-Value 1 | |||
| Cheek, AU | Baseline | 44.87 (2.68) | - | 45.24 (2.01) | - | 0.697 4 |
| 4 weeks | 45.47 (6.47) | 2.940 3 | 46.27 (6.16) | 1.045 3 | 0.615 4 | |
| 8 weeks | 45.97 (7.96) | 2.488 3 | 46.87 (7.35) | 0.587 3 | 0.580 4 | |
| 12 weeks | 46.12 (6.94) | 0.797 3 | 46.67 (7.12) | 0.485 3 | 0.780 4 | |
| Forearm, AU | Baseline | 35.63 (7.89) | - | 37.40 (8.61) | - | 0.227 |
| 4 weeks | 34.51 (7.20) | 0.154 | 35.60 (6.65) | 0.071 | 0.588 | |
| 8 weeks | 34.37 (6.78) | 0.153 | 36.01 (7.53) | 0.156 | 0.917 | |
| 12 weeks | 33.98 (7.20) | 0.061 | 34.28 (6.83) | 0.003 * | 0.277 | |
| Hand, AU | Baseline | 40.36 (8.39) | - | 40.44 (7.41) | - | 0.957 |
| 4 weeks | 37.34 (7.24) | <0.001 * | 38.41 (7.35) | 0.005 * | 0.349 | |
| 8 weeks | 37.59 (7.23) | 0.002 * | 38.51 (7.95) | 0.019 * | 0.473 | |
| 12 weeks | 37.69 (6.90) | 0.001 * | 36.81 (7.69) | <0.001 * | 0.386 | |
| Location | Time Point | Bl-04 (N = 61) | Placebo (N = 67) | p-Value 2 | ||
|---|---|---|---|---|---|---|
| Mean (SD) | p-Value 1 | Mean (SD) | p-Value 1 | |||
| Cheek, AU | Baseline | 44.20 (3.41) | - | 44.33 (3.56) | - | 0.832 |
| 4 weeks | 44.83 (4.40) | 0.191 | 45.74 (5.03) | 0.087 3 | 0.288 | |
| 8 weeks | 43.67 (4.79) | 0.388 | 44.82 (4.74) | 1.060 3 | 0.213 | |
| 12 weeks | 45.00 (3.97) | 0.150 | 45.47 (4.41) | 0.049 *3 | 0.634 | |
| Forearm, AU | Baseline | 31.18 (2.98) | - | 31.35 (3.07) | - | 0.754 |
| 4 weeks | 30.95 (3.07) | 0.470 | 31.19 (3.25) | 0.594 | 0.872 | |
| 8 weeks | 30.52 (2.98) | 0.041 * | 30.61 (3.34) | 0.026 * | 0.864 | |
| 12 weeks | 30.61 (3.15) | 0.081 | 30.53 (3.38) | 0.016 * | 0.600 | |
| Hand, AU | Baseline | 38.65 (4.52) | - | 37.96 (4.20) | - | 0.371 |
| 4 weeks | 38.70 (4.53) | 0.903 | 38.60 (4.75) | 0.106 | 0.303 | |
| 8 weeks | 38.54 (4.20) | 0.754 | 37.89 (4.66) | 0.871 | 0.930 | |
| 12 weeks | 38.70 (4.25) | 0.917 | 37.74 (4.77) | 0.576 | 0.644 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huuskonen, L.; Lyra, A.; Lee, E.; Ryu, J.; Jeong, H.; Baek, J.; Seo, Y.; Shin, M.; Tiihonen, K.; Pesonen, T.; et al. Effects of Bifidobacterium animalis subsp. lactis Bl-04 on Skin Wrinkles and Dryness: A Randomized, Triple-Blinded, Placebo-Controlled Clinical Trial. Dermato 2022, 2, 30-52. https://doi.org/10.3390/dermato2020005
Huuskonen L, Lyra A, Lee E, Ryu J, Jeong H, Baek J, Seo Y, Shin M, Tiihonen K, Pesonen T, et al. Effects of Bifidobacterium animalis subsp. lactis Bl-04 on Skin Wrinkles and Dryness: A Randomized, Triple-Blinded, Placebo-Controlled Clinical Trial. Dermato. 2022; 2(2):30-52. https://doi.org/10.3390/dermato2020005
Chicago/Turabian StyleHuuskonen, Laura, Anna Lyra, Eunju Lee, Jahyun Ryu, Hyunjin Jeong, Jihwoon Baek, Youngkyoung Seo, Minkyung Shin, Kirsti Tiihonen, Tommi Pesonen, and et al. 2022. "Effects of Bifidobacterium animalis subsp. lactis Bl-04 on Skin Wrinkles and Dryness: A Randomized, Triple-Blinded, Placebo-Controlled Clinical Trial" Dermato 2, no. 2: 30-52. https://doi.org/10.3390/dermato2020005
APA StyleHuuskonen, L., Lyra, A., Lee, E., Ryu, J., Jeong, H., Baek, J., Seo, Y., Shin, M., Tiihonen, K., Pesonen, T., Lauerma, A., Reimari, J., Ibarra, A., & Anglenius, H. (2022). Effects of Bifidobacterium animalis subsp. lactis Bl-04 on Skin Wrinkles and Dryness: A Randomized, Triple-Blinded, Placebo-Controlled Clinical Trial. Dermato, 2(2), 30-52. https://doi.org/10.3390/dermato2020005






