Investigating the Early Events after Skin-Barrier Disruption Using Microdialysis—A Human Ex Vivo Skin Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Skin Specimens
2.2. Skin Barrier Disruption
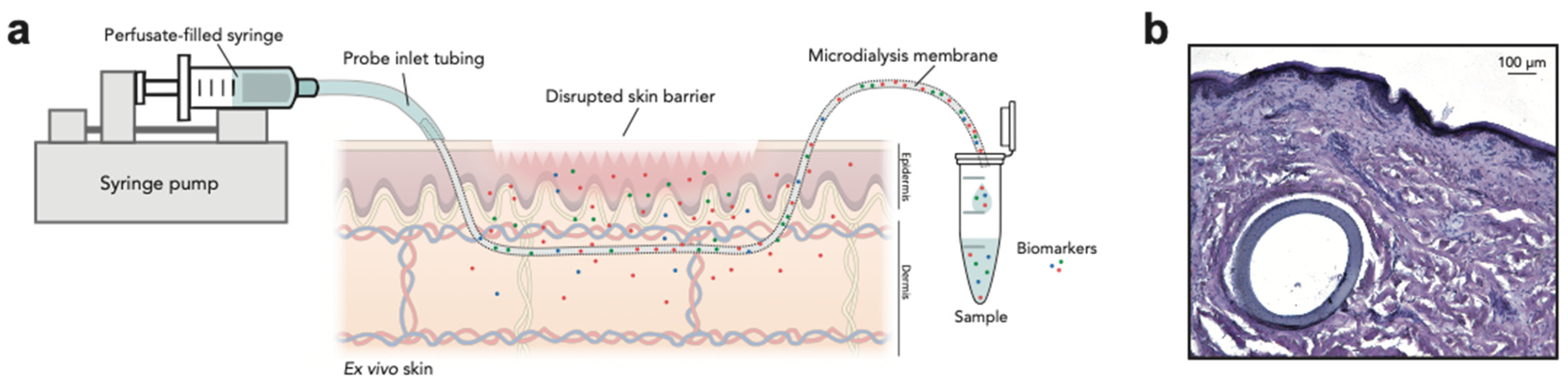
2.3. Microdialysis Setup
2.4. Protein Extraction from Punch Biopsies
2.5. Sample Analysis
2.6. Histology of Microdialysis Membranes within the Skin
2.7. Statistical Analysis
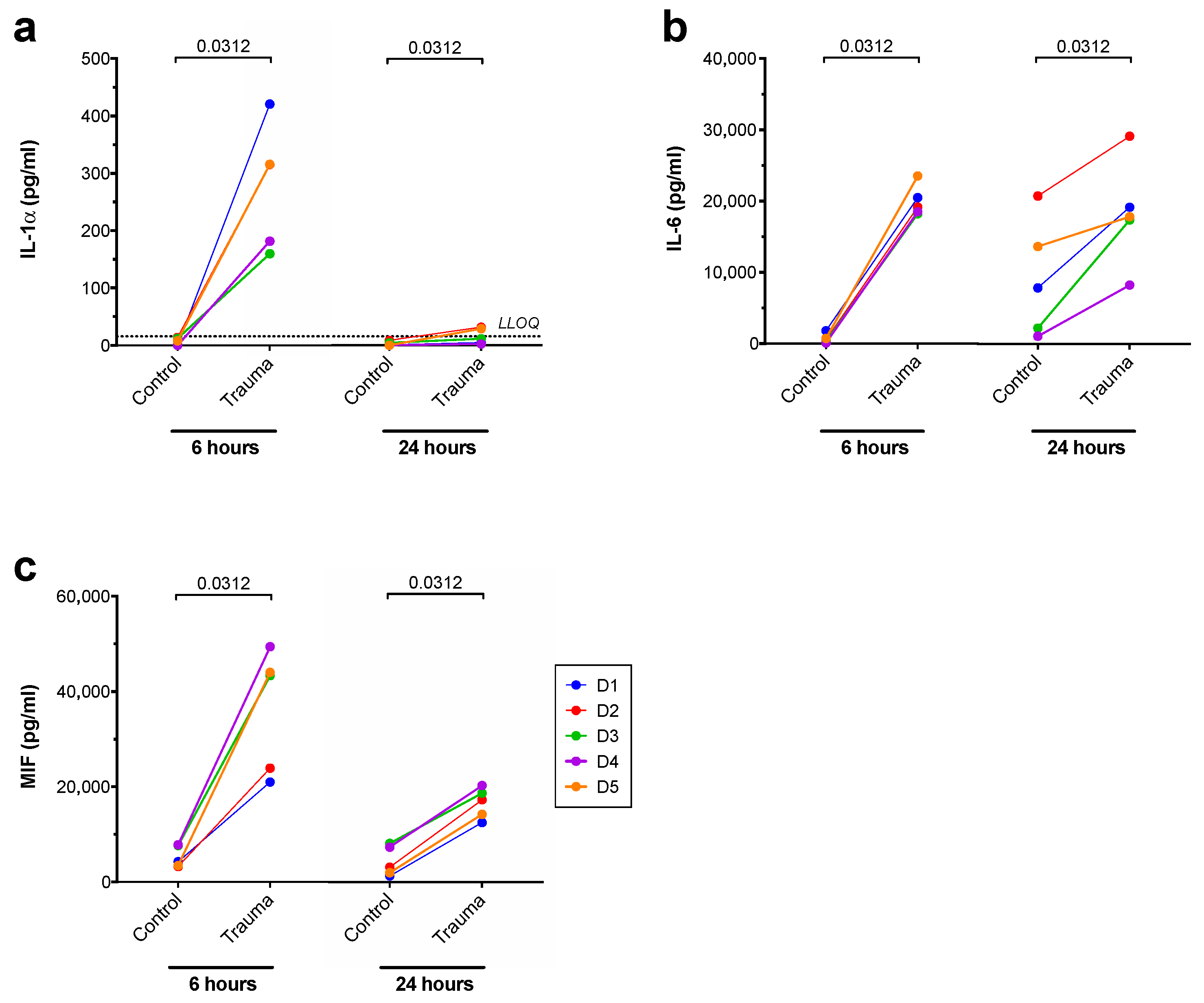
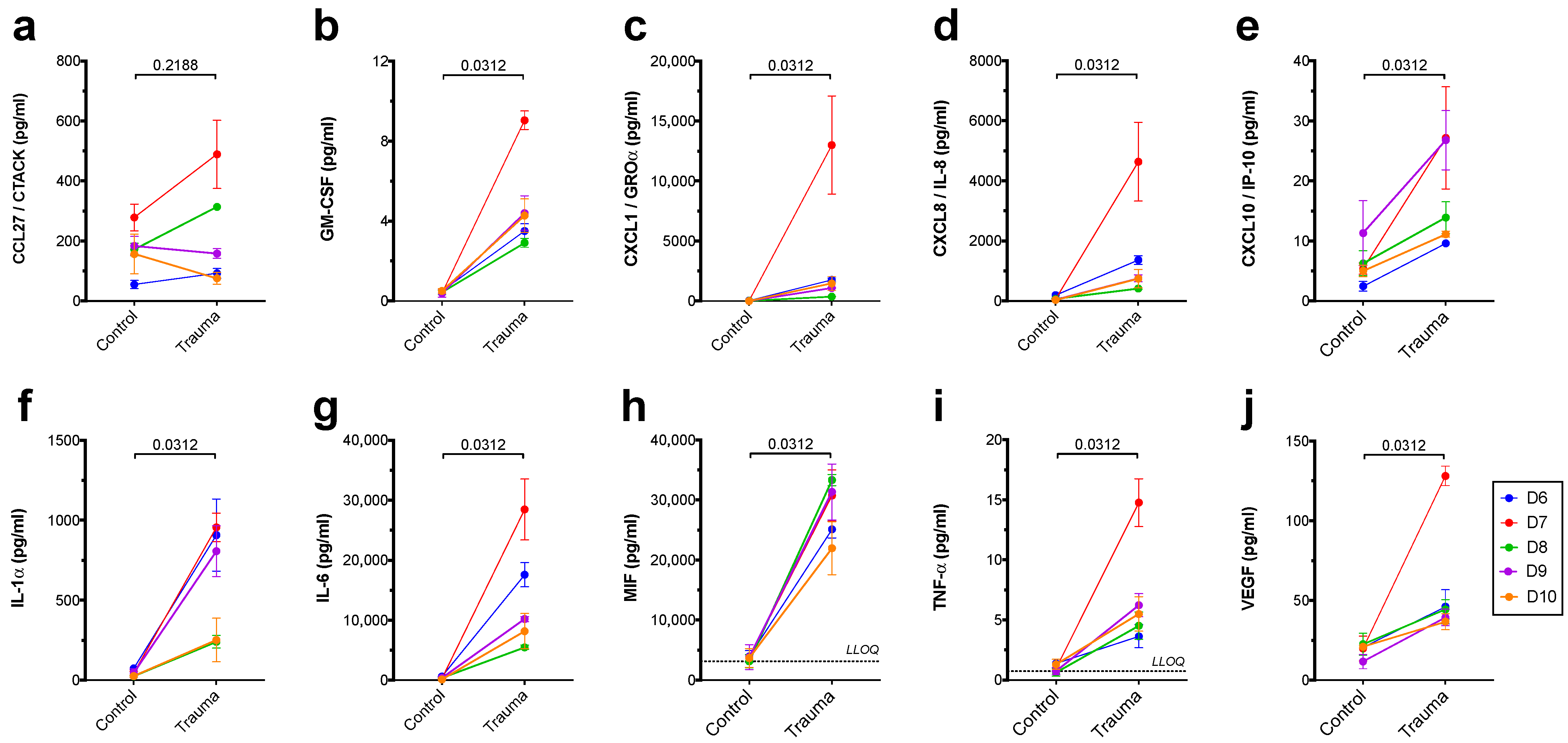
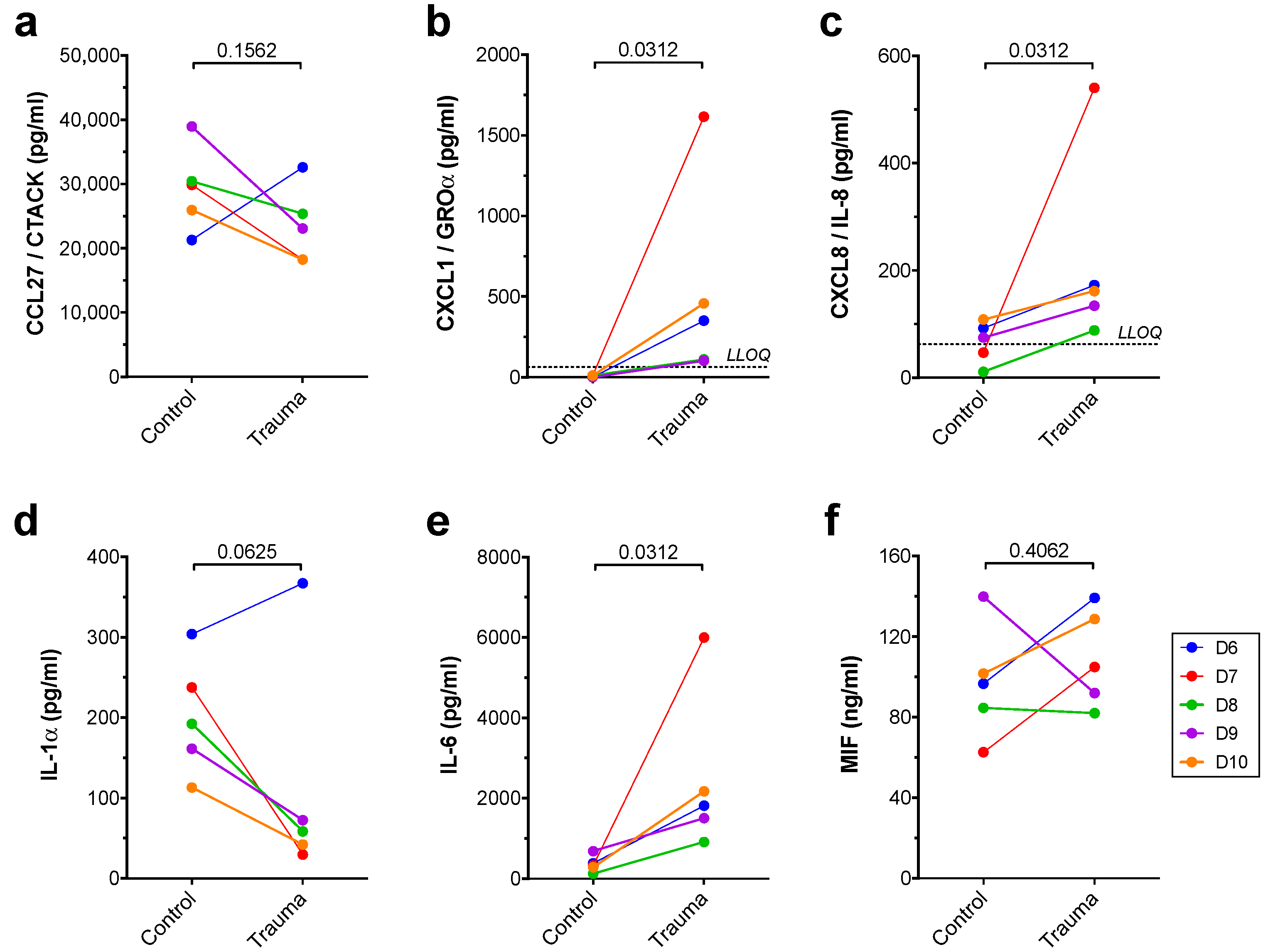
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Hänel, K.; Cornelissen, C.; Lüscher, B.; Baron, J. Cytokines and the Skin Barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, E.; Toksoy, A.; Goebeler, M.; Debus, S.; Bröcker, E.-B.; Gillitzer, R. Chemokines IL-8, GROα, MCP-1, IP-10, and Mig Are Sequentially and Differentially Expressed during Phase-Specific Infiltration of Leukocyte Subsets in Human Wound Healing. Am. J. Pathol. 1998, 153, 1849–1860. [Google Scholar] [CrossRef]
- Antonov, D.; Schliemann, S.; Elsner, P. Methods for the Assessment of Barrier Function. Skin Barrier Funct. 2016, 49, 61–70. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Chen, S.; Li, S.; Liu, X. Acute skin barrier disruption with repeated tape stripping: Anin vivomodel for damage skin barrier. Ski. Res. Technol. 2013, 19, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.Y.; Church, M.K.; Clough, G.F.; Quist, S.R.; Schmelz, M.; Skov, P.S.; Anderson, C.D.; Tannert, L.K.; Giménez-Arnau, A.M.; Frischbutter, S.; et al. Skin microdialysis: Methods, applications and future opportunities—An EAACI position paper. Clin. Transl. Allergy 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, H.; Kirby, B. A Review of Cutaneous Microdialysis of Inflammatory Dermatoses. Acta Derm. Venereol. 2019, 99, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Sjögren, F.; Anderson, C.D. Are Cutaneous Microdialysis Cytokine Findings Supported by End Point Biopsy Immunohistochemistry Findings? AAPS J. 2010, 12, 741–749. [Google Scholar] [CrossRef] [Green Version]
- Plock, N.; Kloft, C. Microdialysis—Theoretical background and recent implementation in applied life-sciences. Eur. J. Pharm. Sci. 2005, 25, 1–24. [Google Scholar] [CrossRef]
- Ud-Din, S.; Bayat, A. Non-animal models of wound healing in cutaneous repair: In silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regen. 2017, 25, 164–176. [Google Scholar] [CrossRef]
- Bartek, M.J.; Labudde, J.A.; Maibach, H.I. Skin Permeability In Vivo: Comparison in Rat, Rabbit, Pig and Man. J. Investig. Dermatol. 1972, 58, 114–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef]
- Danso, M.O.; Berkers, T.; Mieremet, A.; Hausil, F.; Bouwstra, J.A. Anex vivo humanskin model for studying skin barrier repair. Exp. Dermatol. 2015, 24, 48–54. [Google Scholar] [CrossRef]
- Berkers, T.; Boiten, W.; Absalah, S.; van Smeden, J.; Lavrijsen, A.; Bouwstra, J. Compromising human skin in vivo and ex vivo to study skin barrier repair. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2019, 1864, 1103–1108. [Google Scholar] [CrossRef]
- Berkers, T.; Visscher, D.; Gooris, G.; Bouwstra, J. Degree of Skin Barrier Disruption Affects Lipid Organization in Regenerated Stratum Corneum. Acta Derm. Venereol. 2018, 98, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Döge, N.; Hönzke, S.; Schumacher, F.; Balzus, B.; Colombo, M.; Hadam, S.; Rancan, F.; Blume-Peytavi, U.; Schäfer-Korting, M.; Schindler, A.; et al. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin—Assessment by intradermal microdialysis and extraction from the different skin layers. J. Control. Release 2016, 242, 25–34. [Google Scholar] [CrossRef]
- Pfannes, E.K.; Weiss, L.; Hadam, S.; Gonnet, J.; Combardiere, B.; Blume-Peytavi, U.; Vogt, A. Physiological and Molecular Effects of in vivo and ex vivo Mild Skin Barrier Disruption. Ski. Pharmacol. Physiol. 2018, 31, 115–124. [Google Scholar] [CrossRef]
- Kidwell, M.J.; Arpey, C.J.; Messingham, M.J. A Comparison of Histologic Effectiveness and Ultrastructural Properties of the Electrocautery Scratch Pad to Sandpaper for Manual Dermabrasion. Dermatol. Surg. 2008, 34, 1194–1199. [Google Scholar] [CrossRef]
- Pavlidis, L.; Spyropoulou, G.-A. A Simple Technique to Perform Manual Dermabrasion with Sandpaper. Dermatol. Surg. 2012, 38, 2016–2017. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-M.; Todo, H.; Sugibayashi, K. Effects of pretreatment of needle puncture and sandpaper abrasion on the in vitro skin permeation of fluorescein isothiocyanate (FITC)-dextran. Int. J. Pharm. 2006, 316, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.; Falkencrone, S.; Knudsen, N.P.; Woetmann, A.; Dabelsteen, S.; Skov, P.S. The Skin Reservoir Model: A Tool for Evaluating Microdialysis Sampling of Large Biomarkers from Human Skin. Acta Derm. Venereol. 2019, 100, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Holmgaard, R.; Nielsen, J.; Benfeldt, E. Microdialysis Sampling for Investigations of Bioavailability and Bioequivalence of Topically Administered Drugs: Current State and Future Perspectives. Ski. Pharmacol. Physiol. 2010, 23, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Stenken, J.A.; Poschenrieder, A. Bioanalytical chemistry of cytokines—A review. Anal. Chim. Acta 2015, 853, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Dabitao, D.; Margolick, J.B.; Lopez, J.; Bream, J.H. Multiplex measurement of proinflammatory cytokines in human serum: Comparison of the Meso Scale Discovery electrochemiluminescence assay and the Cytometric Bead Array. J. Immunol. Methods 2011, 372, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Wood, L.C.; Jackson, S.M.; Elias, P.M.; Grunfeld, C.; Feingold, K.R. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J. Clin. Investig. 1992, 90, 482–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirmacher, P.; Mann, A.; Breuhahn, K.; Blessing, M. Keratinocyte-Derived Granulocyte-Macrophage Colony Stimulating Factor Accelerates Wound Healing: Stimulation of Keratinocyte Proliferation, Granulation Tissue Formation, and Vascularization. J. Investig. Dermatol. 2001, 117, 1382–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickoloff, B.J.; Naidu, Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J. Am. Acad. Dermatol. 1994, 30, 535–546. [Google Scholar] [CrossRef]
- Döge, N.; Avetisyan, A.; Hadam, S.; Pfannes, E.K.B.; Rancan, F.; Blume-Peytavi, U.; Vogt, A. Assessment of skin barrier function and biochemical changes of ex vivo human skin in response to physical and chemical barrier disruption. Eur. J. Pharm. Biopharm. 2017, 116, 138–148. [Google Scholar] [CrossRef]
- Dickel, H.; Gambichler, T.; Kamphowe, J.; Altmeyer, P.; Skrygan, M. Standardized tape stripping prior to patch testing induces upregulation of Hsp90, Hsp70, IL-33, TNF-α and IL-8/CXCL8 mRNA: New insights into the involvement of ‘alarmins’. Contact Dermat. 2010, 63, 215–222. [Google Scholar] [CrossRef]
- Kroeze, K.L.; Boink, M.A.; Sampat-Sardjoepersad, S.C.; Waaijman, T.; Scheper, R.J.; Gibbs, S. Autocrine Regulation of Re-Epithelialization After Wounding by Chemokine Receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J. Investig. Dermatol. 2012, 132, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-P.; Schunck, M.; Kallen, K.-J.; Neumann, C.; Trautwein, C.; Rose-John, S.; Proksch, E. The Interleukin-6 Cytokine System Regulates Epidermal Permeability Barrier Homeostasis. J. Investig. Dermatol. 2004, 123, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Grellner, W. Time-dependent immunohistochemical detection of proinflammatory cytokines (IL-1β, IL-6, TNF-α) in human skin wounds. Forensic Sci. Int. 2002, 130, 90–96. [Google Scholar] [CrossRef]
- Brown, L.F.; Yeo, K.T.; Berse, B.; Yeo, T.K.; Senger, D.R.; Dvorak, H.F.; Van De Water, L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J. Exp. Med. 1992, 176, 1375–1379. [Google Scholar] [CrossRef] [Green Version]
- Nissen, N.N.; Polverini, P.J.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445–1452. [Google Scholar]
- Elias, P.M.; Arbiser, J.; Brown, B.E.; Rossiter, H.; Man, M.-Q.; Cerimele, F.; Crumrine, D.; Gunathilake, R.; Choi, E.H.; Uchida, Y.; et al. Epidermal Vascular Endothelial Growth Factor Production Is Required for Permeability Barrier Homeostasis, Dermal Angiogenesis, and the Development of Epidermal Hyperplasia: Implications for the Pathogenesis of Psoriasis. Am. J. Pathol. 2008, 173, 689–699. [Google Scholar] [CrossRef] [Green Version]
- Wood, L.C.; Elias, P.M.; Calhoun, C.; Tsai, J.C.; Grunfeld, C.; Feingold, K.R. Barrier Disruption Stimulates Interleukin-1α Expression and Release from a Pre-Formed Pool in Murine Epidermis. J. Investig. Dermatol. 1996, 106, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.T.; Briggs, W.H.; Cheng, G.C.; Rossiter, H.B.; Libby, P.; Kupper, T. Mechanical deformation promotes secretion of IL-1 alpha and IL-1 receptor antagonist. J. Immunol. 1997, 159, 5084–5088. [Google Scholar]
- Gilliver, S.C.; Emmerson, E.; Bernhagen, J.; Hardman, M.J. MIF: A key player in cutaneous biology and wound healing. Exp. Dermatol. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- Grieb, G.; Simons, D.; Eckert, L.; Hemmrich, M.; Steffens, G.; Bernhagen, J.; Pallua, N. Levels of macrophage migration inhibitory factor and glucocorticoids in chronic wound patients and their potential interactions with impaired wound endothelial progenitor cell migration. Wound Repair Regen. 2012, 20, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Shimizu, T.; Ohkawara, A.; Nishihira, J. Enhancement of macrophage migration inhibitory factor (MIF) expression in injured epidermis and cultured fibroblasts. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 1999, 1500, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bünemann, E.; Hoff, N.-P.; Buhren, B.A.; Wiesner, U.; Meller, S.; Bölke, E.; Müller-Homey, A.; Kubitza, R.; Ruzicka, T.; Zlotnik, A.; et al. Chemokine ligand–receptor interactions critically regulate cutaneous wound healing. Eur. J. Med Res. 2018, 23, 4. [Google Scholar] [CrossRef]
- Inokuma, D.; Abe, R.; Fujita, Y.; Sasaki, M.; Shibaki, A.; Nakamura, H.; McMillan, J.R.; Shimizu, T.; Shimizu, H. CTACK/CCL27 Accelerates Skin Regeneration via Accumulation of Bone Marrow-Derived Keratinocytes. Stem Cells 2006, 24, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, F.; Anderson, C. Sterile Trauma to Normal Human Dermis Invariably Induces IL1beta, IL6 and IL8 in an Innate Response to “Danger”. Acta Derm. Venereol. 2009, 89, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Stenken, J.A.; Church, M.K.; Gill, C.A.; Clough, G.F. How Minimally Invasive is Microdialysis Sampling? A Cautionary Note for Cytokine Collection in Human Skin and other Clinical Studies. AAPS J. 2010, 12, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, M.A.; Osterhues, M.A.; Farage, M.A.; Robinson, M.K. A noninvasive method to assess skin irritation and compromised skin conditions using simple tape adsorption of molecular markers of inflammation. Ski. Res. Technol. 2001, 7, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Benfeldt, E.; Hansen, S.H.; Vølund, A.; Menné, T.; Shah, V.P. Bioequivalence of Topical Formulations in Humans: Evaluation by Dermal Microdialysis Sampling and the Dermatopharmacokinetic Method. J. Investig. Dermatol. 2007, 127, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tettey-Amlalo, R.N.O.; Kanfer, I.; Skinner, M.F.; Benfeldt, E.; Verbeeck, R.K. Application of dermal microdialysis for the evaluation of bioequivalence of a ketoprofen topical gel. Eur. J. Pharm. Sci. 2009, 36, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benfeldt, E.; Serup, J.; Menné, T. Microdialysis vs. Suction Blister Technique for In vivo Sampling of Pharmacokinetics in the Human Dermis. Acta Derm. Venereol. 1999, 79, 338–342. [Google Scholar] [CrossRef]
| Biomarker | Dialysates 1 | Biopsies 1 |
|---|---|---|
| CCL27/CTACK | ↔ | ↔ |
| GM-CSF | ↑ | BLOQ |
| CXCL1/GROα | ↑ | ↑ |
| CXCL8/IL-8 | ↑ | ↑ |
| CXCL10/IP-10 | ↑ | BLOQ |
| IL-1α | ↑ | (↓) |
| IL-6 | ↑ | ↑ |
| MIF | ↑ | ↔ |
| TNF-α | ↑ | BLOQ |
| VEGF | ↑ | BLOQ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumann, K.; Knudsen, N.P.H.; Gadsbøll, A.-S.Ø.; Woetmann, A.; Skov, P.S. Investigating the Early Events after Skin-Barrier Disruption Using Microdialysis—A Human Ex Vivo Skin Model. Dermato 2021, 1, 47-58. https://doi.org/10.3390/dermato1020008
Baumann K, Knudsen NPH, Gadsbøll A-SØ, Woetmann A, Skov PS. Investigating the Early Events after Skin-Barrier Disruption Using Microdialysis—A Human Ex Vivo Skin Model. Dermato. 2021; 1(2):47-58. https://doi.org/10.3390/dermato1020008
Chicago/Turabian StyleBaumann, Katrine, Niels Peter Hell Knudsen, Anne-Sofie Østergaard Gadsbøll, Anders Woetmann, and Per Stahl Skov. 2021. "Investigating the Early Events after Skin-Barrier Disruption Using Microdialysis—A Human Ex Vivo Skin Model" Dermato 1, no. 2: 47-58. https://doi.org/10.3390/dermato1020008
APA StyleBaumann, K., Knudsen, N. P. H., Gadsbøll, A.-S. Ø., Woetmann, A., & Skov, P. S. (2021). Investigating the Early Events after Skin-Barrier Disruption Using Microdialysis—A Human Ex Vivo Skin Model. Dermato, 1(2), 47-58. https://doi.org/10.3390/dermato1020008







