Influence of Park Size and Noise Pollution on Avian Species Richness in Urban Green Spaces: A Case Study from Mexico City
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
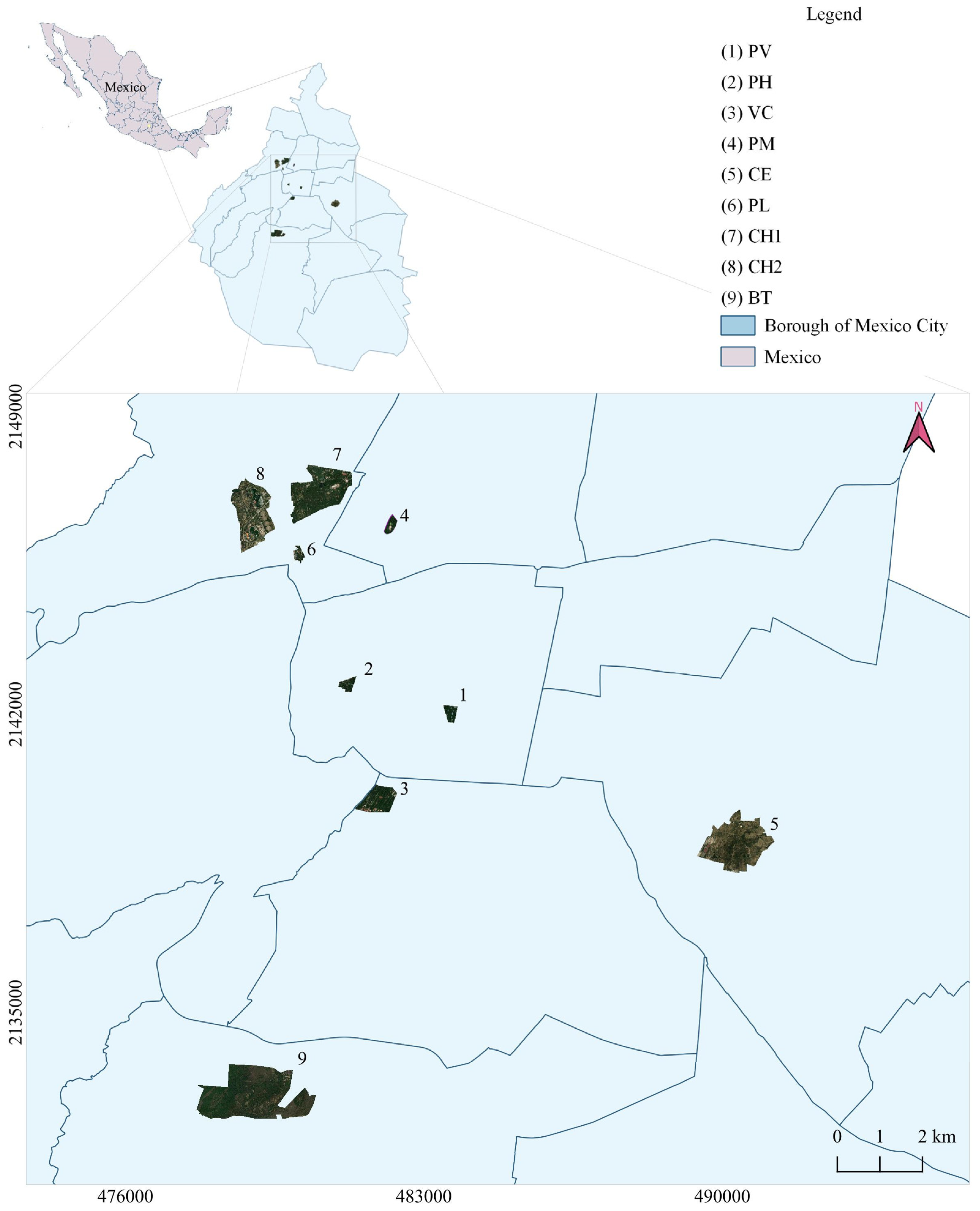
2.1. Study Site
2.2. Terrestrial Bird Monitoring
2.3. Environmental Variables
2.4. Data Analysis
3. Results
3.1. Environmental Variables
3.2. Avian Community Composition
3.3. Inventory Completeness
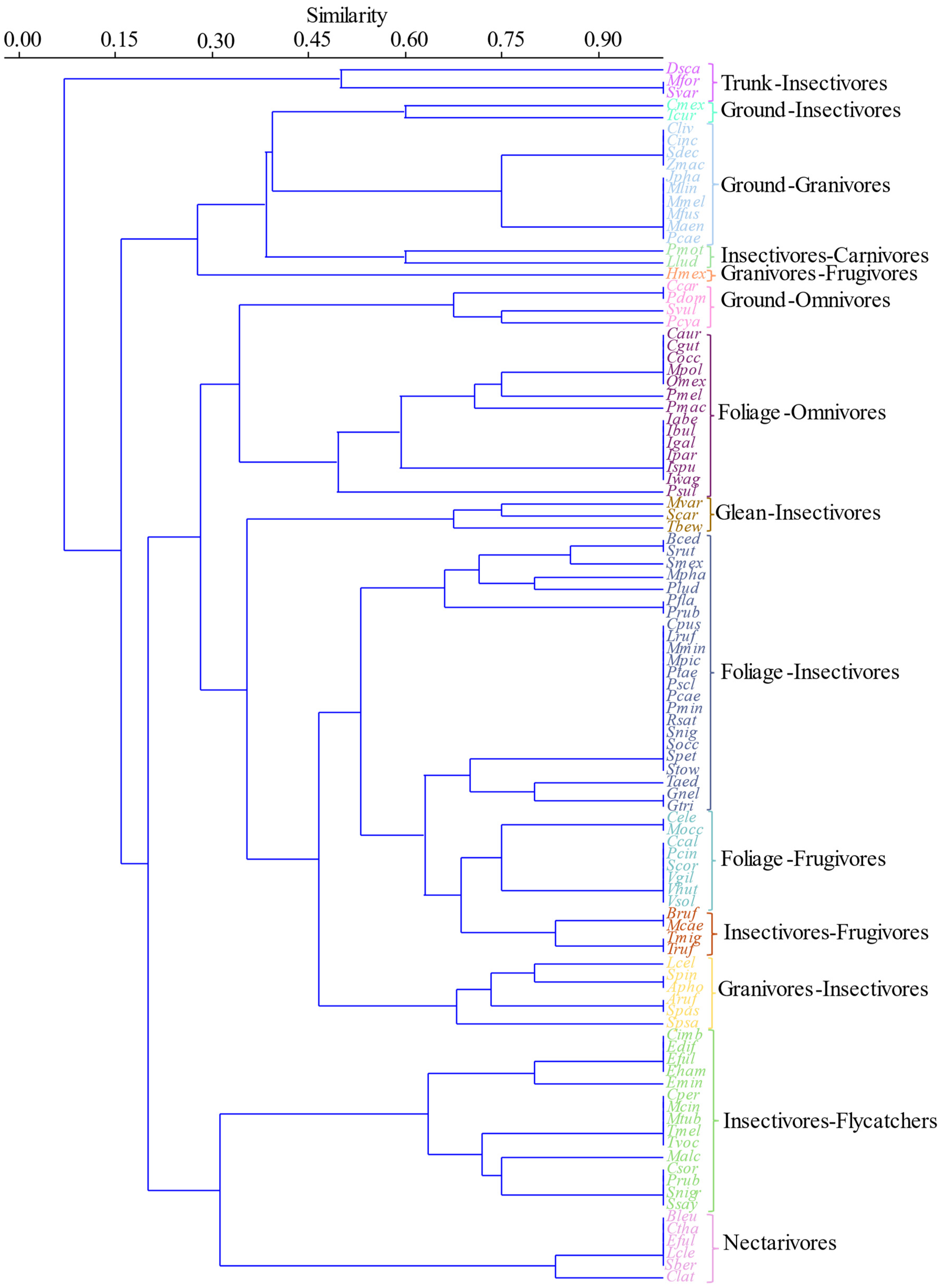
3.4. Trophic Guilds
3.5. Multivariate Analysis and Environmental Relationships per Species
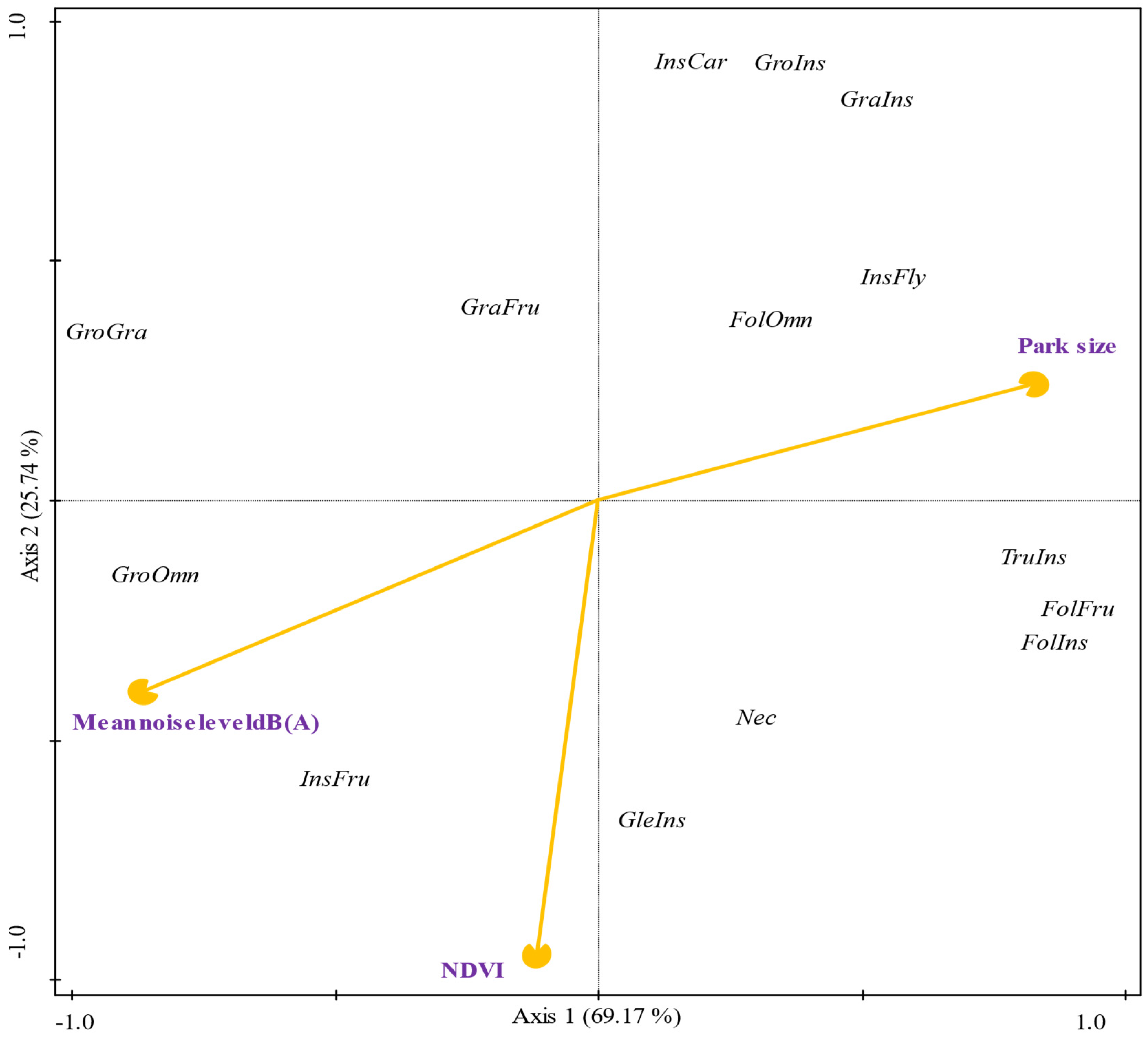
3.6. Multivariate Analysis and Environmental Relationships per Guilds
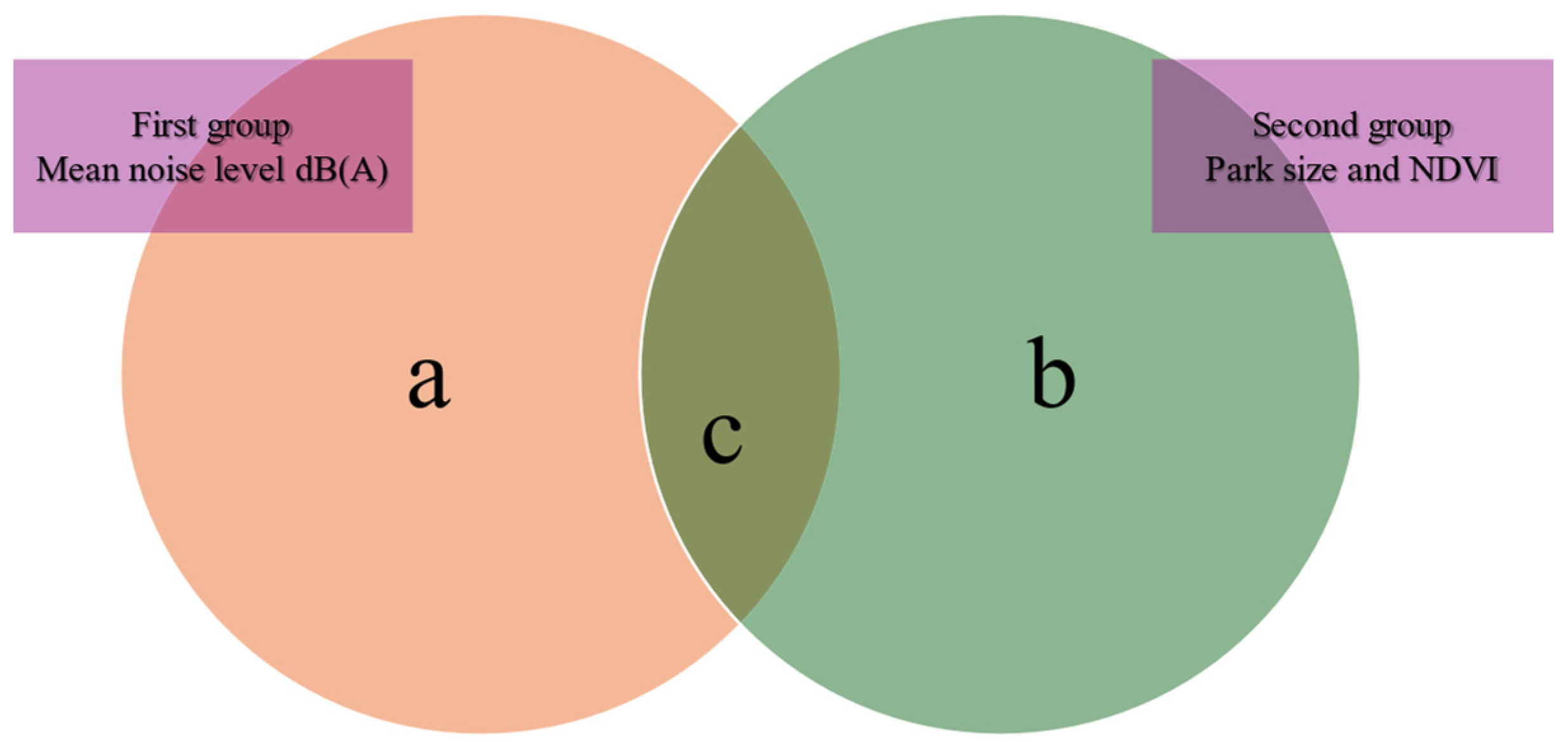
3.7. Variation Partitioning Analysis
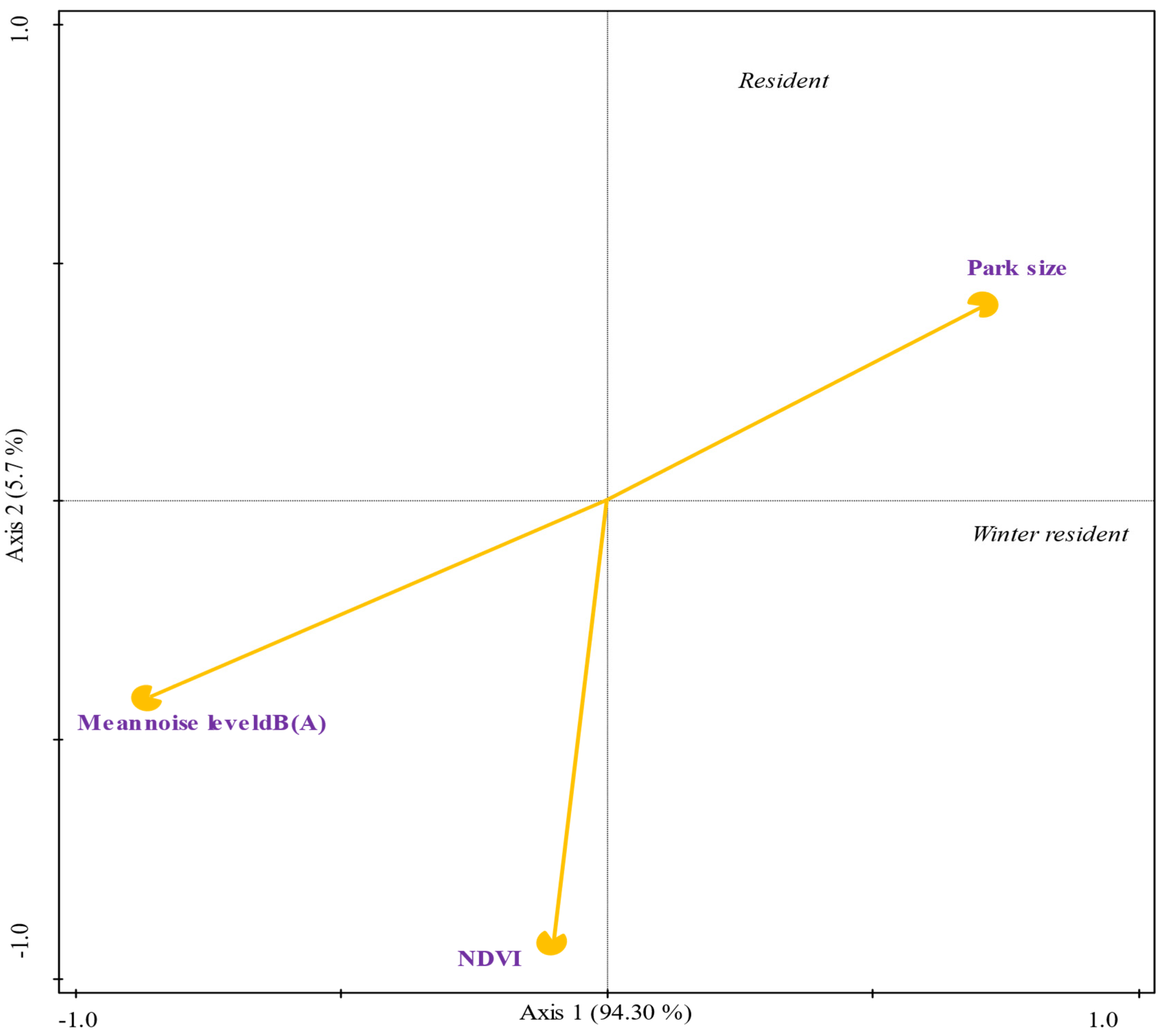
3.8. Correlation and Multivariate Analysis Between Environmental Variables and Residence in the CDMX
3.9. Correlation Between Environmental Variables and Conservation Status
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKinney, M.L. Urbanization, biodiversity, and conservation: The impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Grimm, N.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Aronson, M.F.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A Global Analysis of the Impacts of Urbanization on Bird and Plant Diversity Reveals Key Anthropogenic Drivers. Proc. Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson, M.F.J.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S. Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. BioScience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Jokimäki, J.; Kaisanlahti-Jokimäki, M.L.; Carbó-Ramírez, P. The importance of wooded urban green areas for breeding birds: A case study from Northern Finland. In Avian Urban Ecology: Behavioural and Physical Adaptations; Gil, D., Brumm, H., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 201–213. [Google Scholar] [CrossRef]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in Cities Needs Space: A Meta-Analysis of Factors Determining Intra-Urban Biodiversity Variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Ikin, K.; Knight, E.; Lindenmayer, D.B.; Fischer, J.; Fischer, J.; Manning, A.D. The Influence of Native versus Exotic Streetscape Vegetation on the Spatial Distribution of Birds in Suburbs and Reserves. Divers. Distrib. 2013, 19, 294–306. [Google Scholar] [CrossRef]
- Marzluff, J.M. A Decadal Review of Urban Ornithology and a Prospectus for the Future. Ibis 2017, 159, 1–13. [Google Scholar] [CrossRef]
- Hengeveld, R.; MacArthur, R.H.; Wilson, E.O.; MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography. Acta Biotheor. 2002, 50, 133–136. [Google Scholar] [CrossRef]
- Fernández-Juricic, E.; Jokimäki, J. A habitat island approach to conserving birds in urban landscapes: Case studies from southern and northern Europe. Biodivers. Conserv. 2001, 10, 2023–2043. [Google Scholar] [CrossRef]
- Garaffa, P.I.; Filloy, J.; Bellocq, M.I. Bird community responses along urban–rural gradients: Does the size of the urbanized area matter? Landsc. Urban Plan. 2009, 90, 33–41. [Google Scholar] [CrossRef]
- Charre, G.M.; Hurtado, J.A.Z.; Néve, G.; Ponce-Mendoza, A.; Corcuera, P. Relationship between habitat traits and bird diversity and composition in selected urban green areas of Mexico City. Ornitol. Neotrop. 2013, 24, 279–297. [Google Scholar]
- Jokimäki, J. Occurrence of breeding bird species in urban parks: Effects of park structure and broad-scale variables. Urban Ecosyst. 1999, 3, 21–34. [Google Scholar] [CrossRef]
- Laurance, W.F. Theory meets reality: How habitat fragmentation research has transcended island biogeographic theory. Biol. Conserv. 2008, 141, 1731–1744. [Google Scholar] [CrossRef]
- Nielsen, A.B.; van den Bosch, M.; Maruthaveeran, S.; van den Bosch, C.K. Species richness in urban parks and its drivers: A review of empirical evidence. Urban Ecosyst. 2013, 17, 305–327. [Google Scholar] [CrossRef]
- Matthies, S.; Rüter, S.; Prasse, R.; Schaarschmidt, F. Factors Driving the Vascular Plant Species Richness in Urban Green Spaces: Using a Multivariable Approach. Landsc. Urban Plan. 2015, 134, 177–187. [Google Scholar] [CrossRef]
- Bino, G.; Levin, N.; Darawshi, S.; Hal, N.V.D.; Reich-Solomon, A.; Kark, S. Accurate Prediction of Bird Species Richness Patterns in an Urban Environment Using Landsat-Derived NDVI and Spectral Unmixing. Int. J. Remote Sens. 2008, 29, 3675–3700. [Google Scholar] [CrossRef]
- Zambrano, L.; Aronson, M.F.J.; Fernández, T. The consequences of landscape fragmentation on socio-ecological patterns in a rapidly developing urban area: A case study of the National Autonomous University of Mexico. Front. Environ. Sci. 2019, 7, 152. [Google Scholar] [CrossRef]
- Evans, K.L.; Newson, S.E.; Gaston, K.J. Habitat influences on urban avian assemblages. Ibis 2009, 151, 19–39. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Ripmeester, E.A.P. Birdsong and anthropogenic noise: Implications and applications for conservation. Mol. Ecol. 2008, 17, 72–83. [Google Scholar] [CrossRef]
- Proppe, D.S.; Sturdy, C.B.; St. Clair, C.C. Anthropogenic noise decreases urban songbird diversity and may contribute to homogenization. Glob. Change Biol. 2013, 19, 1075–1084. [Google Scholar] [CrossRef]
- Halfwerk, W.; Holleman, L.J.M.; Lessells, C.M.; Slabbekoorn, H. Negative impact of traffic noise on avian reproductive success. J. Appl. Ecol. 2011, 48, 210–219. [Google Scholar] [CrossRef]
- Francis, C.D.; Ortega, C.P.; Cruz, A. Noise pollution changes avian communities and species interactions. Curr. Biol. 2009, 19, 1415–1419. [Google Scholar] [CrossRef]
- McClure, C.J.; Ware, H.E.; Carlisle, J.; Kaltenecker, G.; Barber, J.R. An experimental investigation into the effects of traffic noise on distributions of birds: Avoiding the phantom road. Proc. R. Soc. B 2013, 280, 20132290. [Google Scholar] [CrossRef]
- Warren, P.S.; Katti, M.; Ermann, M.; Brazel, A. Urban bioacoustics: It’s not just noise. Anim. Behav. 2006, 71, 491–502. [Google Scholar] [CrossRef]
- Liordos, V.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.L.; Valsamidis, E.; Kontsiotis, V.J. Patch, matrix and disturbance variables negatively influence bird community structure in small-sized managed green spaces located in urban core areas. Sci. Total Environ. 2021, 801, 149617. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Chelén, A.A.; Quirós-Guerrero, E.; Gil, D.; Macias Gracía, C. Dealing with urban noise: Vermilion flycatchers sing longer songs in noisier territories. Behav. Ecol. Sociobiol. 2013, 67, 145–152. [Google Scholar] [CrossRef]
- Croci, S.; Butet, A.; Clergeau, P. Does urbanization filter birds on the basis of their biological traits? Condor 2008, 110, 223–240. [Google Scholar] [CrossRef]
- Kark, S.; Iwaniuk, A.; Schalimtzek, A.; Banker, E. Living in the city: Can anyone become an ‘urban exploiter’? J. Biogeogr. 2007, 34, 638–651. [Google Scholar] [CrossRef]
- Fontana, S.; Sattler, T.; Bontadina, F.; Moretti, M. How to manage the urban green to improve bird diversity and community structure. Landsc. Urban Plan. 2011, 101, 278–285. [Google Scholar] [CrossRef]
- Luck, G.W.; Smallbone, L.T.; Sheffield, K.J. Environmental and socio-economic factors related to urban bird communities. Austral Ecol. 2013, 38, 111–120. [Google Scholar] [CrossRef]
- Sanchez, K.A.; Benedict, L.; Holt, E.A. Landscape Composition Is a Stronger Determinant Than Noise and Light of Avian Community Structure in an Urbanizing County. Front. Ecol. Evol. 2023, 11, 1254280. [Google Scholar] [CrossRef]
- Ciach, M.; Fröhlich, A. Habitat Type, Food Resources, Noise and Light Pollution Explain the Species Composition, Abundance and Stability of a Winter Bird Assemblage in an Urban Environment. Urban Ecosyst. 2017, 20, 547–559. [Google Scholar] [CrossRef]
- Chace, J.F.; Walsh, J.J. Urban effects on native avifauna: A review. Landsc. Urban Plan. 2006, 74, 46–69. [Google Scholar] [CrossRef]
- Marzluff, J.M.; Shulenberger, E.; Endlicher, W.; Alberti, M.; Bradley, G.; Ryan, C.; Simon, U.; ZumBrunnen, C. Urban Ecology: An International Perspective on the Interaction between Humans and Nature; Springer Science & Business Media: Boston, MA, USA, 2008. [Google Scholar] [CrossRef]
- United Nations (UN). The 2018 Revision of the World Urbanization Prospects. Available online: https://www.un.org/es/desa/2018-revision-world-urbanization-prospects (accessed on 17 July 2025).
- Secretaría de Gobernación (SEGOB). Unidad de Política Migratoria, Registro e Identidad de Personas, Secretaría de Gobernación. Boletín Mensual de Estadísticas Migratorias, 2025. Available online: https://portales.segob.gob.mx/work/models/PoliticaMigratoria/CEM/Estadisticas/Boletines_Estadisticos/2025/Boletin_2025.pdf (accessed on 17 July 2025).
- Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). Coordinación de Estrategias de Biodiversidad y Cooperación-CONABIO. In Resumen de La biodiversidad en la Ciudad de México. Estudio de Estado; CONABIO: Mexico City, Mexico, 2021. [Google Scholar]
- MacGregor-Fors, I.; Schondube, J.E. Gray vs. green urbanization: Relative importance of urban features for urban bird communities. Basic Appl. Ecol. 2011, 12, 372–381. [Google Scholar] [CrossRef]
- Ortega-Álvarez, R.; MacGregor-Fors, I. Dusting-off the file: A review of knowledge on urban ornithology in Latin America. Landsc. Urban Plan. 2011, 101, 1–10. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana nom-001-SEDATU-2021, Espacios Públicos en los Asentamientos Humanos. Ciudad de México, México. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5643417&fecha=22/02/2022#gsc.tab=0 (accessed on 19 July 2025).
- Instituto Nacional de Estadística y Geografía (INEGI). Superficie Estatal por tipo de Clima. Ciudad de México. Available online: https://www.inegi.org.mx/app/cuadroentidad/CDMX/2019/01/1_6 (accessed on 18 July 2025).
- Estrada, F.; Martínez-Arroyo, A.; Fernández-Eguiarte, A.; Luyando, E.; Gay, C. Defining climate zones in México City using multivariate analysis. Atmosfera 2009, 22, 175–193. [Google Scholar]
- Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). Coordination of Biodiversity Strategies and Cooperation. Summary of “Biodiversity in Mexico City: State of Knowledge”; CONABIO: Mexico City, Mexico; Available online: https://www.biodiversidad.gob.mx/media/1/region/eeb/files/CDMX_resumen.pdf (accessed on 13 December 2024).
- Ralph, J.; Geupel, G.R.; Pyle, P.; Martin, T.E.; DeSante, D.F. Handbook of Field Methods for Monitoring Landbirds; Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture: Albany, CA, USA, 1993; p. 41. [Google Scholar] [CrossRef]
- Hutto, R.L.; Pletschet, S.M.; Hendricks, P. A fixed-radius point count method for nonbreeding and breeding season use. Auk 1986, 103, 593–602. [Google Scholar] [CrossRef]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A.; Mustoe, S.H. Bird Census Techniques, 2nd ed.; Academic Press: London, UK, 2000; p. 302. [Google Scholar]
- Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). NOM-059-SEMARNAT-2010: Listing of Species of Wild Flora and Fauna at Risk, Terrestrial and Aquatic, 1st ed.; Diario Oficial de la Federación: Mexico City, Mexico, 2010. [Google Scholar]
- Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). AvesMX: Database on the Birds of Mexico. Available online: https://avesmx.conabio.gob.mx/ (accessed on 18 June 2024).
- International Union for Conservation of Nature (IUCN). The IUCN Red List of Threatened Species, Version 2024-1. Available online: https://www.iucnredlist.org (accessed on 22 June 2024).
- Soberón, J.; Llorente, J. The use of species accumulation functions for the prediction of species richness. Conserv. Biol. 1993, 7, 480–488. [Google Scholar] [CrossRef]
- Heltshe, J.F.; Forrester, N.E. Estimating species richness using the Jackknife procedure. Biometrics 1983, 39, 1–11. [Google Scholar] [CrossRef]
- Chao, A.; Lee, S.-M. Estimating the number of classes via sample coverage. J. Am. Stat. Assoc. 1992, 87, 210–217. [Google Scholar] [CrossRef]
- Hulbert, S.H. The nonconcept of species diversity: A critique and alternative parameters. Ecology 1971, 52, 577–586. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Harper & Row: New York, NY, USA, 1999; p. 765. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed. In Developments in Environmental Modeling; Elsevier: Amsterdam, The Netherlands, 2012; p. 419. [Google Scholar]
- González-Salazar, C.; Martínez-Meyer, E.; López-Santiago, G. A hierarchical classification of trophic guilds for North American birds and mammals. Rev. Mex. De Biodivers. 2014, 85, 931–941. [Google Scholar] [CrossRef]
- Greenacre, M.J. Theory and Applications of Correspondence Analysis; Academic Press: London, UK, 1984; p. 364. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002; p. 496. [Google Scholar]
- Navarro-Sigüenza, A.G.; Rebón-Gallardo, M.F.; Gordillo-Martínez, A.; Peterson, A.T.; Berlanga-García, H.; Sánchez-González, L.A. Biodiversidad de aves en México. Rev Mex Biodiv 2014, 85, S476–S495. [Google Scholar] [CrossRef]
- Filloy, J.; Zurita, G.A.; Bellocq, M.I. Bird diversity in urban ecosystems: The role of the biome and land use along urbanization gradients. Ecosystems 2019, 22, 213–227. [Google Scholar] [CrossRef]
- Villegas, M.; Garitano-Zavala, Á. Bird community responses to different urban conditions in La Paz, Bolivia. Urban Ecosyst. 2010, 13, 375–391. [Google Scholar] [CrossRef]
- González-García, F.; Straub, R.; Lobato García, J.A.; MacGregor-Fors, I. Birds of a neotropical green city: An up-to-date review of the avifauna of the city of Xalapa with additional unpublished records. Urban Ecosyst. 2014, 17, 991–1012. [Google Scholar] [CrossRef]
- Carbó-Ramírez, P.; Zuria, I. The value of small urban greenspaces for birds in a Mexican city. Landsc. Urban Plan. 2011, 100, 213–222. [Google Scholar] [CrossRef]
- González-Oreja, J.A.; Barillas-Gómez, A.L.; Bonache-Regidor, C.; Buzo-Franco, D.; Garcia-Suárez, M.D.; Hernández-Santín, L. Does habitat heterogeneity affect bird community structure in urban parks? Stud. Avian Biol. 2018, 45, 1–32. [Google Scholar]
- Casas, G.; Darski, B.; Ferreira, P.M.A.; Kindel, A.; Müller, S.C. Habitat structure influences the diversity, richness and composition of bird assemblages in successional Atlantic rainforests. Trop. Conserv. Sci. 2016, 9, 503–524. [Google Scholar] [CrossRef]
- Davison, C.W.; Assmann, J.J.; Normand, S.; Rahbek, C.; Morueta-Holme, N. Vegetation structure from LiDAR explains the local richness of birds across Denmark. J. Anim. Ecol. 2023, 92, 1332–1344. [Google Scholar] [CrossRef]
- Francis, C.D.; Newman, P.; Taff, B.D.; White, C.; Monz, C.A.; Levenhagen, M.; Petrelli, A.R.; Abbott, L.C.; Newton, J.; Burson, S.; et al. Acoustic environments matter: Synergistic benefits to humans and ecological communities. J. Environ. Manag. 2017, 203, 245–254. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Morales-Pérez, L.; Quesada, J.; Schondube, J.E. Relationship between the presence of House Sparrows (Passer domesticus) and Neotropical bird community structure and diversity. Biol. Invasions 2010, 12, 87–96. [Google Scholar] [CrossRef]
- Ramírez-Albores, J.E.; Sánchez-González, L.A.; Pérez-Suárez, M.; Navarro-Sigüenza, A.G.; Franco-Maass, S. Greenspaces as Shelters for the Conservation of Bird Diversity in a Big City. Urban Ecosyst. 2024, 27, 2047–2059. [Google Scholar] [CrossRef]
- Jokimäki, J.; Ramos-Chernenko, A. Innovative Foraging Behavior of Urban Birds: Use of Insect Food Provided by Cars. Birds 2024, 5, 469–486. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Arévalo, C.; Amaya-Espinel, J.D.; Henríquez, C.; Ibarra, J.T.; Bonacic, C. Urban noise and surrounding city morphology influence green space occupancy by native birds in a Mediterranean-type South American metropolis. Sci. Rep. 2022, 12, 4471. [Google Scholar] [CrossRef]
- Oropeza-Sánchez, M.T.; Solano-Zavaleta, I.; Cuandón-Hernández, W.L.; Martínez-Villegas, J.A.; Palomera-Hernández, V.; Zúñiga-Vega, J.J. Urban green spaces with high connectivity and complex vegetation promote occupancy and richness of birds in a tropical megacity. Urban Ecosyst. 2025, 28, 50. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef]
- Korňan, M.; Holmes, R.T.; Recher, H.F.; Adamík, P.; Kropil, R. Convergence in foraging guild structure of forest breeding bird assemblages across three continents is related to habitat structure and foraging opportunities. Community Ecol. 2013, 14, 89–100. [Google Scholar] [CrossRef]
- Hanz, D.M.; Böhning-Gaese, K.; Ferger, S.W.; Fritz, S.A.; Neuschulz, E.L.; Quitián, M.; Santillán, V.; Töpfer, T.; Schleuning, M. Functional and phylogenetic diversity of bird assemblages are filtered by different biotic factors on tropical mountains. J. Biogeogr. 2018, 46, 291–303. [Google Scholar] [CrossRef]
- Bender, I.M.A.; Kissling, W.D.; Böhning-Gaese, K.; Hensen, I.; Kühn, I.; Wiegand, T.; Dehling, D.M.; Schleuning, M. Functionally specialised birds respond flexibly to seasonal changes in fruit availability. J. Anim. Ecol. 2017, 86, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Loiselle, B.A.; Blake, J.G. Temporal variation in birds and fruits along an elevational gradient in Costa Rica. Ecology 1991, 72, 180–193. [Google Scholar] [CrossRef]
- Stiles, F.G. Temporal organization of flowering among the hummingbird foodplants of a tropical wet forest. Biotropica 1978, 10, 194–210. [Google Scholar] [CrossRef]
- Bleiweiss, R. Origin of Hummingbird Faunas. Biol. J. Linn. Soc. 1998, 65, 77–97. [Google Scholar] [CrossRef]
- Curzel, F.E.; Leveau, L.M. Bird Taxonomic and Functional Diversity in Three Habitats in Buenos Aires City, Argentina. Birds 2021, 2, 217–229. [Google Scholar] [CrossRef]
- Clergeau, P.; Jokimäki, J.; Savard, J.-P.L. Are urban bird communities influenced by the bird diversity of adjacent landscapes. J. Appl. Ecol. 2001, 38, 1122–1134. [Google Scholar] [CrossRef]
- Blair, R.B. Land use and avian species diversity along an urban gradient. Ecol. Appl. 1996, 6, 506–519. [Google Scholar] [CrossRef]
- Bellocq, M.I.; Filloy, J.; Zurita, G.A.; Apellaniz, M.F. Responses in the abundance of generalist birds to environmental gradients: The rufous-collared sparrow (Zonotrichia capensis) in the southern Neotropics. Ecoscience 2011, 18, 354–362. [Google Scholar] [CrossRef]
- Galiano, L.; Leveau, C.M.; Leveau, L.M. Long-Term Changes in Bird Communities in the Urban Parks of Mar del Plata City, Argentina. Birds 2024, 5, 814–831. [Google Scholar] [CrossRef]
- Remeš, V.; Remešová, E.; Friedman, N.R.; Matysioková, B.; Rubáčová, L. Functional diversity of avian communities increases with canopy height: From individual behavior to continental-scale patterns. Ecol. Evol. 2021, 11, 11839–11851. [Google Scholar] [CrossRef]
- Arriaga-Weiss, S.L.; Calmé, S.; Kampichler, C. Bird communities in rainforest fragments: Guild responses to habitat variables in Tabasco, Mexico. Biodivers. Conserv. 2008, 17, 173–190. [Google Scholar] [CrossRef]
- Hawkinson, A.J.; Montgomery, R.A.; Roy, C.L.; Shartell, L.M.; Andersen, D.E.; Stevens, T.K.; Knosalla, L.J.; Frelich, L.E. Bird-habitat associations and local-scale vegetation structure in lowland brushlands. J. Wildl. Manag. 2024, 88, e22568. [Google Scholar] [CrossRef]
- Benedetti, Y.; Callaghan, C.T.; Ulbrichová, I.; Galanaki, A.; Kominos, T.; Abou Zeid, F.; Ibáñez-Álamo, J.D.; Suhonen, J.; Díaz, M.; Markó, G.; et al. EVI and NDVI as Proxies for Multifaceted Avian Diversity in Urban Areas. Ecol. Appl. 2023, 33, e2808. [Google Scholar] [CrossRef]
- Arroyo-Solís, A.; Castillo, J.M.; Figueroa, E.; López-Sánchez, J.L.; Slabbekoorn, H. Experimental Evidence for an Impact of Anthropogenic Noise on Dawn Chorus Timing in Urban Birds. J. Avian Biol. 2013, 44, 288–296. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Peet, M. Birds sing at a higher pitch in urban noise. Nature 2003, 424, 267. [Google Scholar] [CrossRef]
- Leveau, L.M. Urban Parks Are Related to Functional and Phylogenetic Filtering of Raptor Assemblages in the Austral Pampas, Argentina. Birds 2024, 5, 38–47. [Google Scholar] [CrossRef]
- Benítez-López, A.; Alkemade, R.; Verweij, P.A. The impacts of roads and other infrastructure on mammal and bird populations: A meta-analysis. Biol. Conserv. 2010, 143, 1307–1316. [Google Scholar] [CrossRef]
- Fernández Juricic, E. Avifaunal use of wooded streets in an urban landscape. Conserv. Biol. 2000, 14, 513–521. [Google Scholar] [CrossRef]
- Lerman, S.B.; Warren, P.S. The conservation value of residential yards: Linking birds and people. Ecol. Appl. 2011, 21, 1327–1339. [Google Scholar] [CrossRef]
- Ortega Álvarez, R.; MacGregor Fors, I. Living in the big city: Effects of urban land use on bird community structure, diversity, and composition. Landsc. Urban Plan. 2009, 90, 189–195. [Google Scholar] [CrossRef]
- Leveau, L.M.; Leveau, C.M. Does urbanization affect the seasonal dynamics of bird communities in urban parks? Urban Ecosyst. 2016, 19, 631–647. [Google Scholar] [CrossRef]
- Leveau, L.M. Long-term directional changes in urban bird communities of Mar del Plata City, Argentina. Front. Ecol. Evol. 2024, 12, 1457476. [Google Scholar] [CrossRef]
- Ramírez-Albores, J.E. Avian Community Structureand Spatial Distribution in Anthropogenic Landscapesin Central Mexico. Birds 2025, 6, 18. [Google Scholar] [CrossRef]

| Urban Parks | Number of Count Points | Mean MIN dB(A) ± SE | Mean MAX dB(A) ± SE | Mean Noise Level dB(A) ± SE | Park Size (ha) | NDVI |
|---|---|---|---|---|---|---|
| VC | 12 | 44.68 ± 0.240 | 51.70 ± 0.235 | 48.19 ± 0.290 | 39.95 | 0.59 |
| PL | 5 | 50.49 ± 0.365 | 55.34 ± 0.525 | 52.91 ± 0.525 | 6.15 | 0.5 |
| CH2 | 16 | 46.93 ± 0.229 | 53.41 ± 0.242 | 50.17 ± 0.291 | 107.79 | 0.47 |
| PM | 4 | 53.24 ± 0.420 | 58.58 ± 0.473 | 55.91 ± 0.532 | 6.83 | 0.64 |
| CE | 15 | 41.58 ± 0.177 | 48.24 ± 0.199 | 44.91 ± 0.194 | 145.15 | 0.24 |
| PH | 5 | 51.59 ± 0.438 | 57.14 ± 0.510 | 54.36 ± 0.529 | 8.5 | 0.52 |
| PV | 4 | 51.91 ± 0.424 | 58.94 ± 0.571 | 55.42 ± 0.531 | 9.01 | 0.62 |
| BT | 15 | 41.11 ± 0.187 | 47.10 ± 0.179 | 44.11 ± 0.194 | 239.49 | 0.57 |
| CH1 | 15 | 48.91 ± 0.280 | 54.92 ± 0.320 | 51.91 ± 0.390 | 116.47 | 0.49 |
| Urban Parks | Observed Richness | Rarefied Richness | Chao1 (%) | Jackknife 1 (%) |
|---|---|---|---|---|
| VC | 54 | 53.93 | 96.36 | 82.81 |
| PL | 30 | 29.95 | 95.23 | 86.75 |
| CH2 | 62 | 60.95 | 93.02 | 80.35 |
| PM | 27 | 26.96 | 90 | 88.03 |
| CE | 76 | 75.95 | 86.11 | 77.55 |
| PH | 36 | 35.93 | 85.47 | 79.69 |
| PV | 35 | 34.90 | 85.11 | 80.92 |
| BT | 74 | 73.88 | 84.04 | 80.14 |
| CH1 | 51 | 50.93 | 83.61 | 81.05 |
| Model | Variable | Coefficient (β) | Standard Error | z-Value | p-Value | AIC |
|---|---|---|---|---|---|---|
| Foliage Insectivores | 47.54 | |||||
| Intercept | 2.354 | 0.478 | 4.927 | <0.001 | ||
| Park size | 0.005 | 0.001 | 3.582 | <0.001 | ||
| NDVI | −1.004 | 0.852 | −1.180 | 0.238 | ||
| Ground Omnivores | 34.49 | |||||
| Intercept | 1.072 | 1.047 | 1.024 | 0.306 | ||
| Park size | −0.00007 | 0.003 | −0.028 | 0.978 | ||
| NDVI | 0.199 | 1.816 | 0.110 | 0.913 | ||
| Total Richness | 66.78 | |||||
| Intercept | 4.053 | 0.222 | 18.262 | <0.001 | ||
| Park size | 0.003 | 0.0005 | 5.628 | <0.001 | ||
| NDVI | −0.885 | 0.392 | −2.258 | 0.024 | ||
| Protected Species | 31.74 | |||||
| Intercept | 1.080 | 0.917 | 1.179 | 0.238 | ||
| Park size | 0.005 | 0.003 | 2.046 | 0.041 | ||
| NDVI | −1.397 | 1.643 | −0.850 | 0.395 |
| Fraction | Variation (adj) | % of Explained Variance | % of All Variance |
|---|---|---|---|
| A | 0.071 | 30.8 | 7.2 |
| B | 0.092 | 39.9 | 9.3 |
| C | 0.068 | 29.3 | 6.8 |
| Total explained | 0.23286 | 100.00 | 23.3 |
| Test Components | F | p |
|---|---|---|
| a + b + c | 1.8 | 0.044 |
| a + c | 2.3 | 0.044 |
| b + c | 1.8 | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Rodríguez, C.Y.; Lara, C.; Sánchez-González, L.A.; Corcuera, P. Influence of Park Size and Noise Pollution on Avian Species Richness in Urban Green Spaces: A Case Study from Mexico City. Birds 2025, 6, 46. https://doi.org/10.3390/birds6030046
Salas-Rodríguez CY, Lara C, Sánchez-González LA, Corcuera P. Influence of Park Size and Noise Pollution on Avian Species Richness in Urban Green Spaces: A Case Study from Mexico City. Birds. 2025; 6(3):46. https://doi.org/10.3390/birds6030046
Chicago/Turabian StyleSalas-Rodríguez, Claudia Yeyetzi, Carlos Lara, Luis A. Sánchez-González, and Pablo Corcuera. 2025. "Influence of Park Size and Noise Pollution on Avian Species Richness in Urban Green Spaces: A Case Study from Mexico City" Birds 6, no. 3: 46. https://doi.org/10.3390/birds6030046
APA StyleSalas-Rodríguez, C. Y., Lara, C., Sánchez-González, L. A., & Corcuera, P. (2025). Influence of Park Size and Noise Pollution on Avian Species Richness in Urban Green Spaces: A Case Study from Mexico City. Birds, 6(3), 46. https://doi.org/10.3390/birds6030046





