Is Winter Feeder Visitation by Songbirds Risk-Dependent? An Experimental Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Analysis of Recorded Material
2.3. Statistical Analysis
3. Results
3.1. Number of Visits per 30 Min
3.2. Foraging Duration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Brittingham, M.C.; Temple, A.A. A survey of avian mortality at winter feeders. Wildl. Soc. Bull. 1986, 14, 445–450. [Google Scholar]
- Orros, M.E.; Fellowes, M.D.E. Wild bird feeding in an urban area: Intensity, economics and numbers of individuals supported. Acta Ornithol. 2015, 50, 43–58. [Google Scholar] [CrossRef]
- Houston, A.I.; McNamara, J.M. A theoretical investigation of the fat reserves and mortality levels of small birds in winter. Ornis Scand. 1993, 24, 205–219. [Google Scholar] [CrossRef]
- Pravosudov, V.V.; Grubb, T.C., Jr. Energy management in passerine birds during the nonbreeding season: A review. Curr. Ornithol. 1997, 14, 189–234. [Google Scholar] [CrossRef]
- Brittingham, M.C.; Temple, S.A. Impacts of supplemental feeding on survival rates of black-capped chickadees. Ecology 1988, 69, 581–589. [Google Scholar] [CrossRef]
- Grubb, T.C.; Cimprich, D.A. Supplementary Food Improves the Nutritional Condition of Wintering Woodland Birds: Evidence from Ptilochronology. Ornis Scand. 1990, 21, 277–281. [Google Scholar] [CrossRef]
- Robb, G.N.; McDonald, R.A.; Chamberlain, D.E.; Bearhop, S. Food for thought: Supplementary feeding as a driver of ecological change in avian populations. Front. Ecol. Environ. 2008, 6, 476–484. [Google Scholar] [CrossRef]
- Pierret, P.; Jiguet, F. The potential virtue of garden bird feeders: More birds in citizen backyards close to intensive agricultural landscapes. Biol. Conserv. 2018, 222, 14–20. [Google Scholar] [CrossRef]
- Wilcoxen, T.E.; Horn, D.J.; Hogan, B.M.; Hubble, C.N.; Huber, S.J.; Flamm, J.; Knott, M.; Lundstrom, L.; Salik, F.; Wassenhove, S.J.; et al. Effects of bird-feeding activities on the health of wild birds conservation. Physiology 2015, 3, cov058. [Google Scholar]
- Broggi, J.; Hohtola, E.; Koivula, K. Winter feeding influences the cost of living in boreal passerines. Ibis 2021, 163, 260–267. [Google Scholar] [CrossRef]
- Krama, T.; Krams, R.; Popovs, S.; Trakimas, G.; Rantala, M.J.; Freeberg, T.M.; Krams, I.A. Permanent Ad-lib Feeders Decrease the Survival of Wintering Great Tits (Parus major). Birds 2023, 4, 225–235. [Google Scholar] [CrossRef]
- Goławski, A.; Polakowski, M.; Filimowski, P.; Stępniewska, K.; Stępniewski, K.; Kiljan, G.; Kilon, D. Factors influencing the fat load variation in three wintering bird species under the stable food. J. Ethol. 2015, 33, 205–211. [Google Scholar] [CrossRef]
- Bonter, D.N.; Zuckerberg, B.; Sedgwick, C.W.; Hochachka, W.M. Daily foraging patterns in free-living birds: Exploring the predation–starvation trade-off. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123087. [Google Scholar] [CrossRef]
- Mónus, F.; Barta, Z. Is Foraging Time Limited During Winter?—A Feeding Experiment with Tree Sparrows Under Different Predation Risk. Ethology 2016, 122, 20–29. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Mikula, P.; Morelli, F. Dynamic interactions at birdfeeders: Attracting both prey and predators across urban and rural habitats. Basic Appl. Ecol. 2024, 79, 84–89. [Google Scholar] [CrossRef]
- Kettel, E.F.; Gentle, L.K.; Quinn, J.L.; Yarnell, R.W. The breeding performance of raptors in urban landscapes: A review and meta-analysis. J. Ornithol. 2018, 159, 1–18. [Google Scholar] [CrossRef]
- Guthery, F.S.; Hiller, T.L.; Puckett, W.H.; Baker, R.A.; Smith, S.G.; Rybak, A.R. Effects of feeders on dispersion and mortality of bobwhites. Wildl. Soc. Bull. 2004, 32, 1248–1254. [Google Scholar] [CrossRef]
- Devereux, C.L.; Whittingham, M.J.; Fernández-Juricic, E.; Vickery, J.A.; Krebs, J.R. Predator detection and avoidance by starlings under differing scenarios of predation risk. Behav. Ecol. 2006, 17, 303–309. [Google Scholar] [CrossRef]
- Abrahams, M.V.; Dill, L.M. A determination of the energetic equivalence of the risk of predation. Ecology 1989, 4, 999–1007. [Google Scholar] [CrossRef]
- Bednekoff, P.A.; Lima, S.L. Re-examining in numbers: Interaction between risk dilution and collective detection depend upon predator targeting behaviour. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1998, 265, 2021–2026. [Google Scholar] [CrossRef]
- Curio, E.; Klump, G.; Regelmann, K. An anti-predator response in the great tit (Parus major): Is it turned to predator risk? Oecologia 1983, 60, 83–88. [Google Scholar] [CrossRef]
- Tvardíková, K.; Fuchs, R. Do birds behave according to dynamic risk assessment theory? A feeder experiment. Behav. Ecol. Sociobiol. 2011, 65, 727–733. [Google Scholar] [CrossRef]
- MacLeod, R.; Gosler, A.G.; Cresswell, W. Diurnal mass gain strategies and perceived predation risk in the great tit Parus major. J. Anim. Ecol. 2005, 74, 956–964. [Google Scholar] [CrossRef]
- Moody, A.L.; Houston, A.I.; McNamara, J.M. Ideal free distributions under predation risk. Behav. Ecol. Sociobiol. 1996, 38, 131–143. [Google Scholar] [CrossRef]
- Keddy, P.A. Competition, 2nd ed.; Kluwer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Tvardíková, K.; Fuchs, R. Tits recognize the potential dangers of predators and harmless birds in feeder experiments. J. Ethol. 2012, 30, 157–165. [Google Scholar] [CrossRef]
- Desrochers, A.; Belisle, M.; Bourque, J. Do mobbing calls affect the perception of predation risk by forest birds? Anim. Behav. 2002, 64, 709–714. [Google Scholar] [CrossRef]
- Veselý, P.; Buršíková, M.; Fuchs, R. Birds at the Winter Feeder do not Recognize an Artificially Coloured Predator. Ethology 2016, 122, 937–944. [Google Scholar] [CrossRef]
- Marsh, R.E.; Erickson, W.A.; Salmon, T.P. Scarecrows and predator models for frightening birds from specific areas. In Proceedings of the Vertebrate Pest Conference (15, 15), Newport Beach, CA, USA, 3–5 March 1992. [Google Scholar]
- Wojczulanis-Jakubas, K.; Kulpińska, M.; Minias, P. Who bullies whom at a garden feeder? Interspecific agonistic interactions of small passerines during a cold winter. J. Ethol. 2015, 33, 159–163. [Google Scholar] [CrossRef]
- Erritzoe, J.; Mazgajski, T.; Rejt, L. Bird casualties on European roads—A review. Acta Ornithol. 2003, 38, 77–93. [Google Scholar] [CrossRef]
- Orłowski, G. Factors affecting road mortality of the Barn Swallows Hirundo rustica in farmland. Acta Ornithol. 2005, 40, 117–125. [Google Scholar] [CrossRef]
- Gosler, A. Strategy and constraint in the winter fattening response to temperature in the great tit Parus major. J. Anim. Ecol. 2002, 71, 771–779. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Ball, G.F.; Dufty, A.M., Jr.; Hegner, R.E.; Ramenofsky, M. Testosterone and Aggression in Birds. Am. Sci. 1987, 75, 602–608. [Google Scholar]
- Marchetti, C.; Drent, P.J. Individual differences in the use of social information in foraging by captive great tits. Anim. Behav. 2000, 60, 131–140. [Google Scholar] [CrossRef]
- Verbeek, M.E.; Boone, A.; Drent, P.J. Exploration, agonistic behaviour and dominance in juvenile male great tits. Behaviour 1996, 133, 945–963. [Google Scholar] [CrossRef]
- Azevedo, C.S.D.; Young, R.J. Shyness and boldness in greater rheas Rhea americana Linnaeus (Rheiformes, Rheidae): The effects of antipredator training on the personality of the birds. Rev. Bras. De Zool. 2006, 23, 202–210. [Google Scholar] [CrossRef]
- Hollander, F.A.; Overveld, T.V.; Tokka, I.; Matthysen, E. Personality and Nest Defence in the Great Tit (Parus major). Ethology 2008, 114, 405–412. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Morelli, F.; Skórka, P.; Goławski, A.; Indykiewicz, P.; Møller, A.P.; Mitrus, C.; Wysocki, D.; Zduniak, P. Who started first? Bird species visiting novel birdfeeders. Sci. Rep. 2015, 5, 11858. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Møller, A.P.; Morelli, F.; Indykiewicz, P.; Zduniak, P.; Myczkot, Ł. Food preferences by birds using bird-feeders in winter: A large-scale experiment. Avian Res. 2018, 9, 16. [Google Scholar] [CrossRef]
- Wilson, W.E., Jr. The effects of supplemental feeding on wintering Black-capped Chickadees (Poecile atricapilla) in central Maine: Population and individual responses. Wilson Bull. 2001, 113, 65–72. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Møller, A.P.; Morelli, F.; Biaduń, W.; Brauze, T.; Ciach, M.; Czechowski, P.; Czyż, S.; Dulisz, B.; Goławski, A.; et al. Urbanization affects neophilia and risk-taking at bird-feeders. Sci. Rep. 2016, 6, 28575. [Google Scholar] [CrossRef]
- Kitowski, I. Winter diet of the barn owl (Tyto alba) and the long-eared owl (Asio otus) in Eastern Poland. N.-West. J. Zool. 2013, 9, 16–22. [Google Scholar]
- Koivula, K.; Orell, M.; Lahti, K. Plastic daily fattening routines in willow tits. J. Anim. Ecol. 2002, 71, 816–823. [Google Scholar] [CrossRef]
- Watanabe, S.; Furuya, I. Video display for study of avian cognition: From psychophysics to sign language. Int. J. Comp. Psychol. 1997, 10, 111–127. [Google Scholar] [CrossRef]
- TuTiempo.net. Available online: https://en.tutiempo.net/climate/poland.html (accessed on 10 January 2016).
- Demongin, L. Identification Guide to Birds in the Hand; Privately published by Laurent Demongin; 2016; p. 392. [Google Scholar]
- Fokkema, M.; Smits, N.; Zeileis, A.; Hothorn, T.; Kelderman, H. Detecting treatment-subgroup interactions in clustered data with generalized linear mixed-effects model trees. Behav. Res. Methods 2018, 50, 2016–2034. [Google Scholar] [CrossRef]
- Zeileis, A.; Hothorn, T.; Hornik, K. Model-Based Recursive Partitioning. J. Comput. Graph. Stat. 2008, 17, 492–514. [Google Scholar] [CrossRef]
- De’ath, G.; Fabricius, K.E. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased recursive partitioning: A conditional inference framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 15 August 2022).
- Tvardíková, K.; Fuchs, R. Tits use amodal completion in predator recognition: A field experiment. Anim. Cogn. 2010, 13, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Rensel, L.J.; Wilder, J.D. The Effects of Owl Decoys and Non-threatening Objects on Bird Feeding Behavior. Quercus Linfield J. Undergrad. Res. 2011, 1, 4. [Google Scholar]
- Nováková, N.; Veselý, P.; Fuchs, R. Object categorization by wild ranging birds—Winter feeder experiments. Behav. Process. 2017, 143, 7–12. [Google Scholar] [CrossRef]
- Ekman, J. Tree use and predator vulnerability of wintering passerines. Ornis Scand. 1986, 17, 261–267. [Google Scholar] [CrossRef]
- Carrascall, M.; Moreno, E. Proximal costs and benefits of heterospecific social foraging in the Great Tit, Parus major. Can. J. Zool. 1992, 70, 1947–1952. [Google Scholar] [CrossRef]
- Suhonen, J. Predation risk influences the use of foraging sites by tits. Ecology 1993, 74, 1197–1203. [Google Scholar] [CrossRef]
- Chamberlain, D.E.; Vickery, J.A.; Glue, D.E.; Robinson, R.A.; Conway, G.J.; Woodburn, R.J.W.; Cannon, A.R. Annual and seasonal trends in the use of garden feeders by birds in winter. Ibis 2005, 147, 563–575. [Google Scholar] [CrossRef]
- Bednekoff, P.A.; Houston, A.I. Avian daily foraging patterns: Effects of digestive constraints and variability. Evol. Ecol. 1994, 8, 36–52. [Google Scholar] [CrossRef]
- Lajoie, J.L.; Ganio, L.M.; Rivers, J.W. Individual variation and seasonality drive bird feeder use during winter in a Mediterranean climate. Ecol. Evol. 2019, 9, 2535–2549. [Google Scholar] [CrossRef] [PubMed]
- Blem, C.R. Avian energy storage. Curr. Ornithol. 1990, 7, 59–113. [Google Scholar]
- Koivula, K.; Orell, M.; Rytkönen, S.; Lahti, K. Fatness, sex and dominance; seasonal and daily body mass changes in Willow Tits. J. Avian Biol. 1995, 26, 209–216. [Google Scholar] [CrossRef]
- Byrkjedal, I.; Lislevand, T.; Vogler, S. Do passerine birds utilise artificial light to prolong their diurnal activity during winter at northern latitudes. Ornis Nor. 2012, 35, 37–42. [Google Scholar] [CrossRef]
- Goławski, A.; Polakowski, M.; Filimowski, P.; Stępniewski, K.; Stępniewska, K.; Kiljan, G.; Kilon, D.; Pietkiewicz, M.; Sztwiertnia, H.; Cichocka, A.; et al. Does the sex and age of birds and the size of human settlements affect recapturing of the Great Tit (Parus major) at bird feeders? Behav. Process. 2019, 162, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Krams, I. Length of feeding day and body weight of great tits in a single and a two-predator environment. Behav. Ecol. Sociobiol. 2000, 48, 147–153. [Google Scholar] [CrossRef]
- Senar, J.C.; Conroy, M.J.; Carrascal, L.M.; Domènech, J.; Mozetich, I.; Uribe, F. Identifying sources of heterogeneity in capture probabilities: An example using the Great Tit Parus major. Bird Study 1999, 46, S248–S252. [Google Scholar] [CrossRef]
- Regelmann, K.; Curio, E. Why do great tit (Parus major) males defend their brood more than females do? Anim. Behav. 1986, 34, 1206–1214. [Google Scholar] [CrossRef]
- Laet, J.F.D. Dominance and anti-predator behaviour of Great Tits Parus major: A field study. Ibis 1985, 127, 372–377. [Google Scholar] [CrossRef]
- Hegner, R.E. Dominance and anti-predator behaviour in blue tits (Parus caeruleus). Anim. Behav. 1985, 33, 762–768. [Google Scholar] [CrossRef]
- Villén-Pérez, S.; Carrascal, L.M.; Seoane, J. Foraging patch selection in winter: A balance between predation risk and thermoregulation benefit. PLoS ONE 2013, 8, e68448. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, C.P.; Lill, A.; Reina, R.D. Habitat loss, fragmentation and degradation effects on small mammals: Analysis with conditional inference tree statistical modelling. Biol. Conserv. 2014, 176, 80–98. [Google Scholar] [CrossRef]

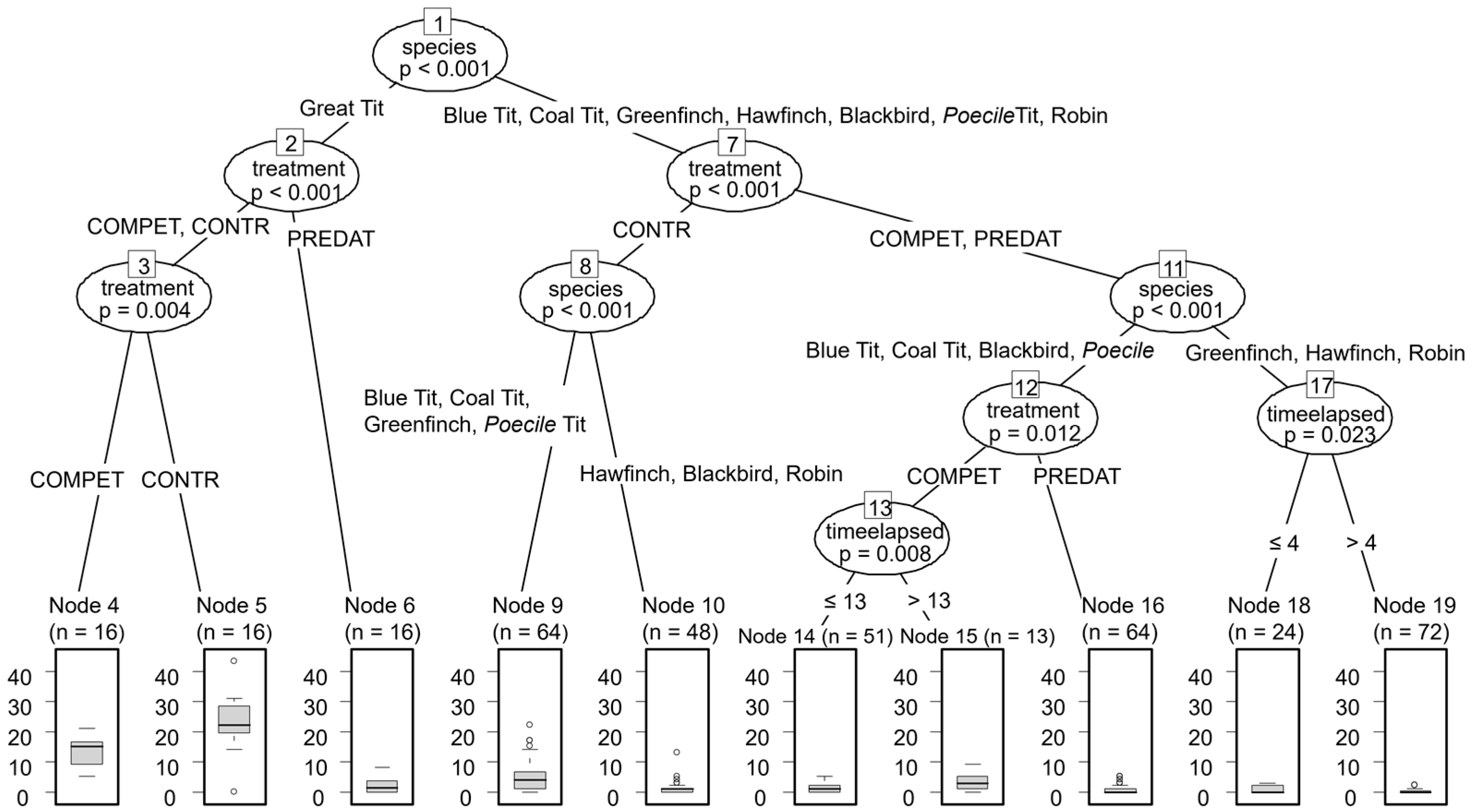

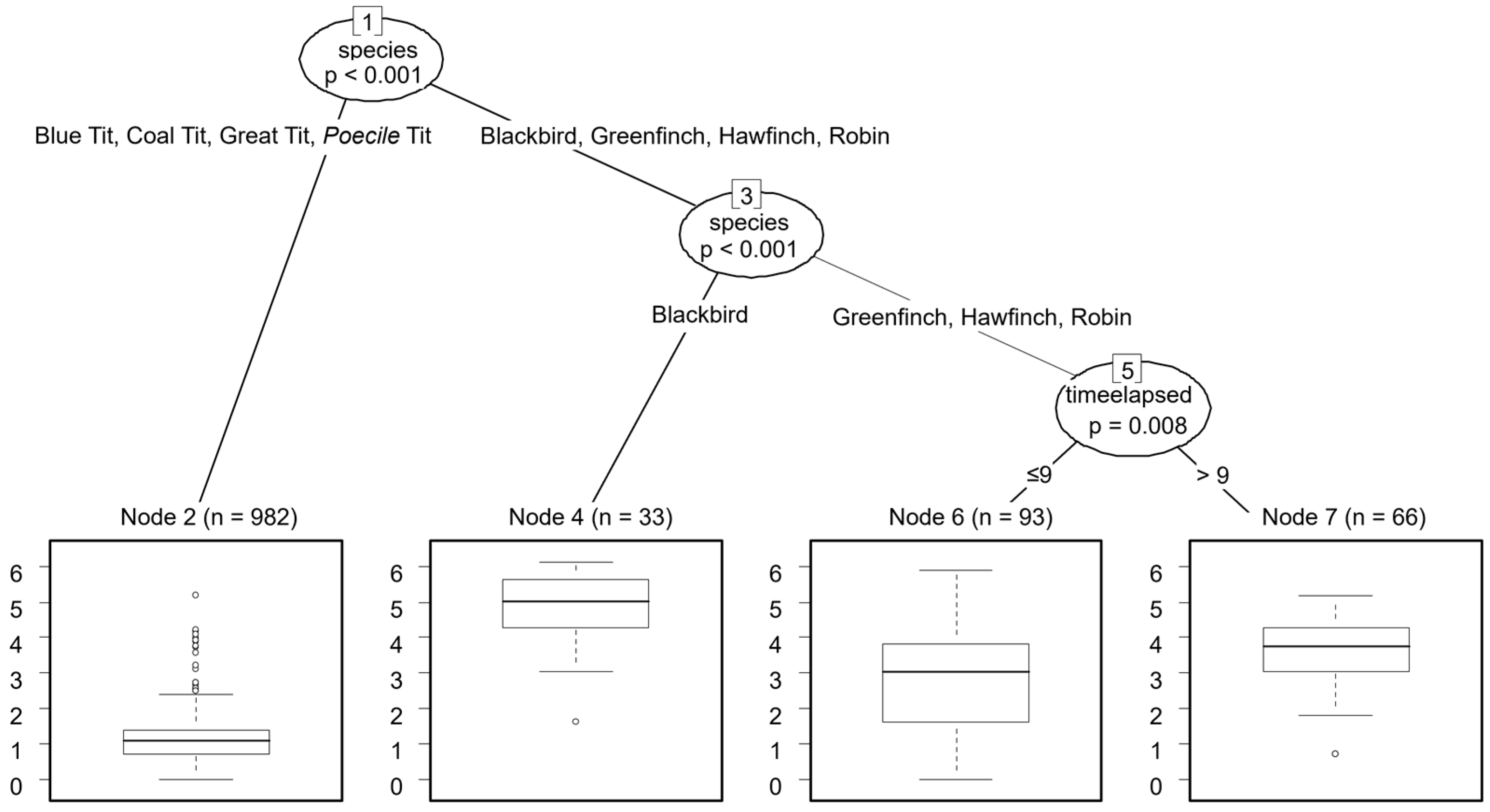
| Species | PREDAT [N; %] | COMPET [N; %] | CONTROL [N; %] | Total [N; %] |
|---|---|---|---|---|
| Great Tit (Parus major) * | 35; 25.74 | 209; 60.40 | 344; 43.88 | 588; 46.45 |
| Crested Tit (Lophophanes cristatus) | - | - | 13; 1.66 | 13; 1.03 |
| Eurasian Siskin (Spinus spinus) | 13; 9.56 | 3; 0.87 | - | 16; 1.26 |
| European Greenfinch (Chloris chloris) * | 5; 3.68 | 3; 0.87 | 76; 9.69 | 84; 6.64 |
| Hawfinch (Coccothraustes coccothraustes) * | 6; 4.41 | 2; 0.58 | 21; 2.68 | 29; 2.29 |
| Blackbird (Turdus merula) * | 7; 5.15 | 22; 6.36 | 4; 0.51 | 33; 2.61 |
| Eurasian Tree Sparrow (Passer montanus) | - | - | 24; 3.06 | 24; 1.90 |
| Eurasian Blue Tit (Cyanistes caeruleus) * | 26; 19.12 | 45; 13.01 | 113; 14.41 | 184; 14.53 |
| European Robin (Erithacus rubecula) * | 11; 8.09 | 10; 2.89 | 25; 3.19 | 46; 3.63 |
| Coal Tit (Periparus ater) * | 9; 6.62 | 29; 8.38 | 59; 7.53 | 97; 7.66 |
| Eurasian Jay (Garrulus glandarius) | 1; 0.74 | 2; 0.58 | 10; 1.28 | 13; 1.03 |
| Yellowhammer (Emberiza citrinella) | 4; 2.94 | 1; 0.29 | 21; 2.68 | 26; 2.05 |
| Poecile Tit (Poecile palustris and P. montanus) * | 19; 13.97 | 20; 5.78 | 74; 9.44 | 113; 8.93 |
| Total | 136 | 346 | 784 | 1266 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manikowska-Ślepowrońska, B.; Ślepowroński, K. Is Winter Feeder Visitation by Songbirds Risk-Dependent? An Experimental Study. Birds 2025, 6, 45. https://doi.org/10.3390/birds6030045
Manikowska-Ślepowrońska B, Ślepowroński K. Is Winter Feeder Visitation by Songbirds Risk-Dependent? An Experimental Study. Birds. 2025; 6(3):45. https://doi.org/10.3390/birds6030045
Chicago/Turabian StyleManikowska-Ślepowrońska, Brygida, and Krzysztof Ślepowroński. 2025. "Is Winter Feeder Visitation by Songbirds Risk-Dependent? An Experimental Study" Birds 6, no. 3: 45. https://doi.org/10.3390/birds6030045
APA StyleManikowska-Ślepowrońska, B., & Ślepowroński, K. (2025). Is Winter Feeder Visitation by Songbirds Risk-Dependent? An Experimental Study. Birds, 6(3), 45. https://doi.org/10.3390/birds6030045





