Bird Community Structure Changes as Araucaria Forest Cover Increases in the Highlands of Southeastern Brazil
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Bird Sampling
2.3. Birds Functional Traits
2.4. Data Analysis
2.4.1. Overall Diversity
2.4.2. Functional Diversity
3. Results
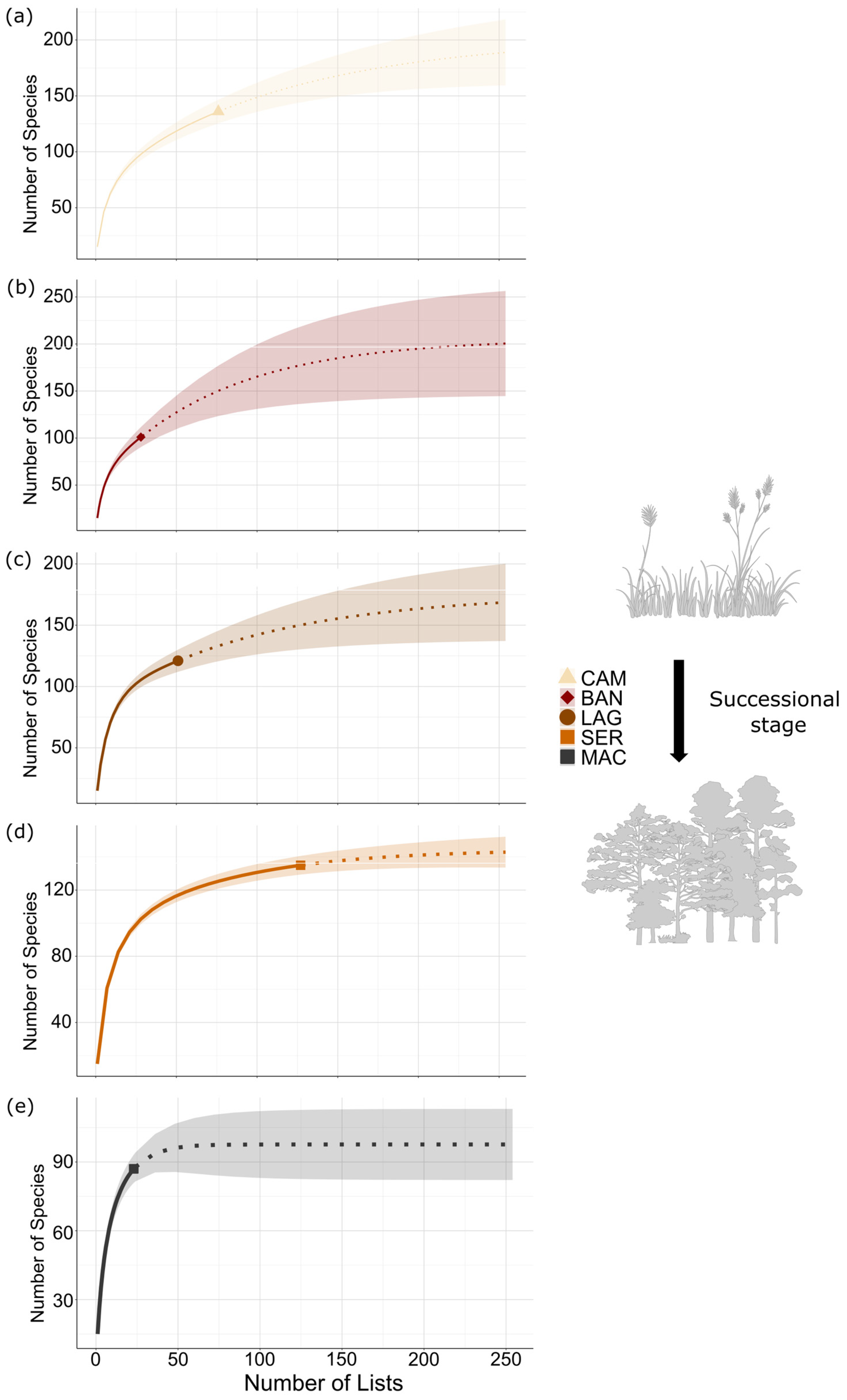
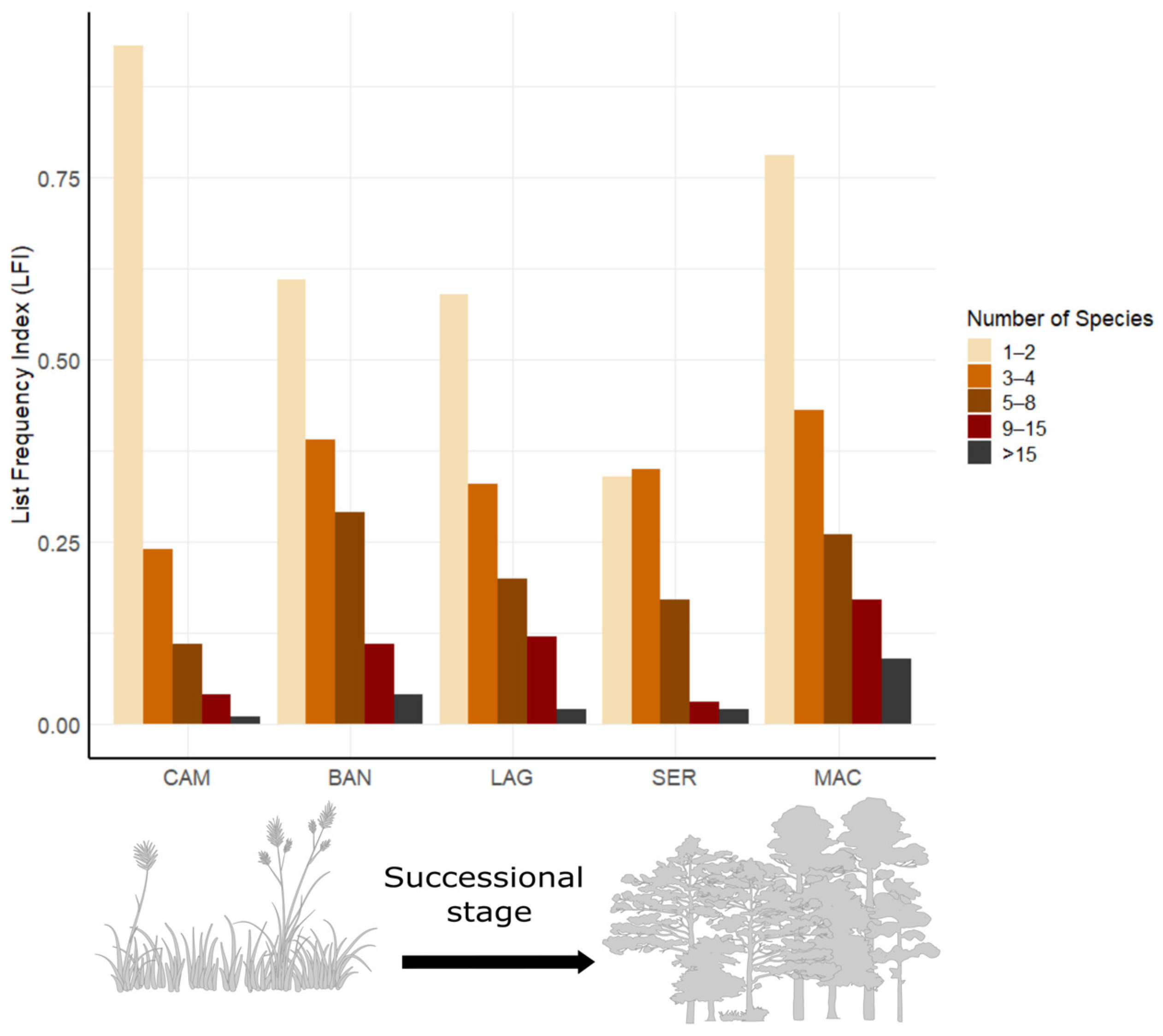
3.1. Overall Diversity
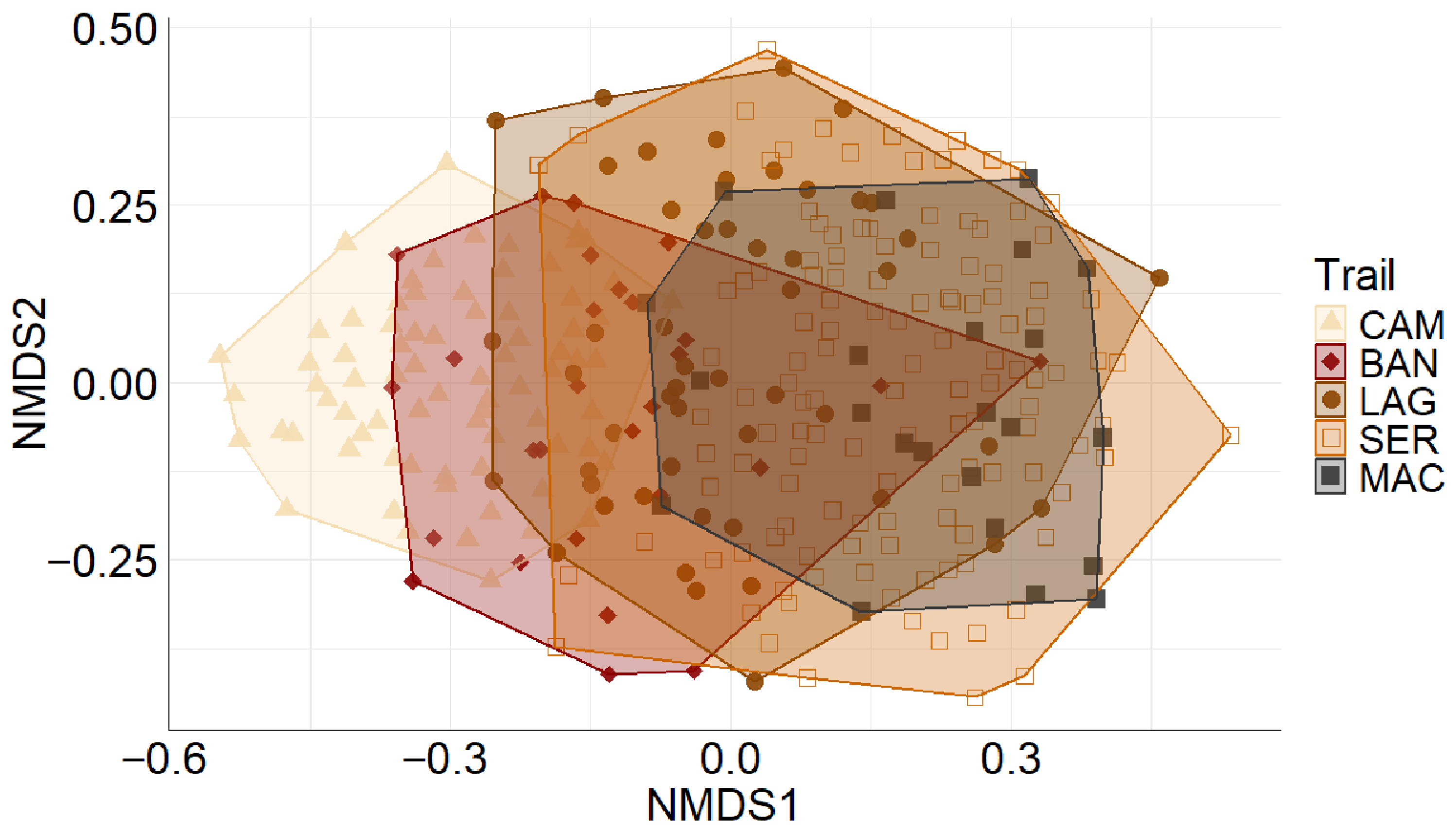
3.2. Composition
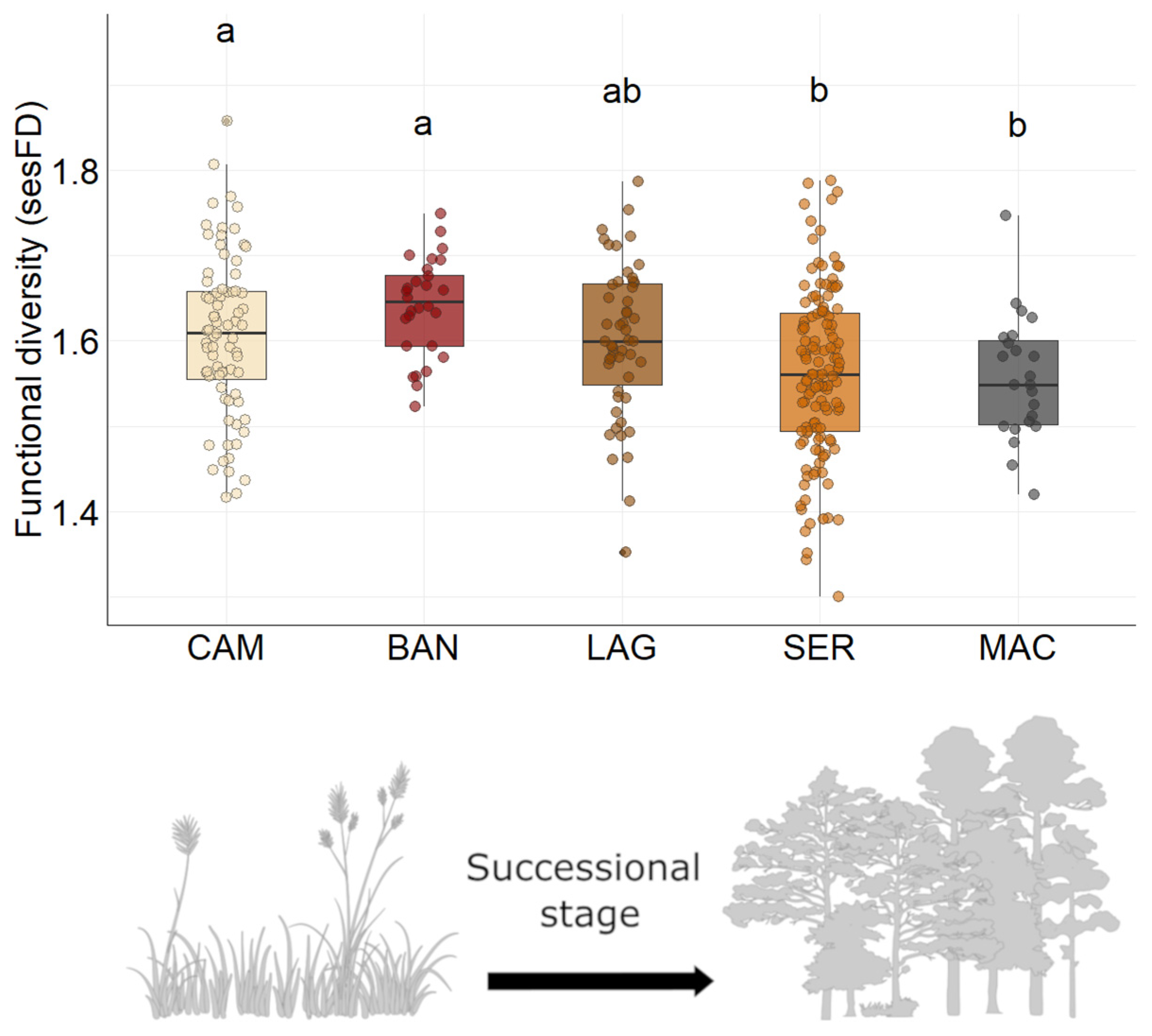
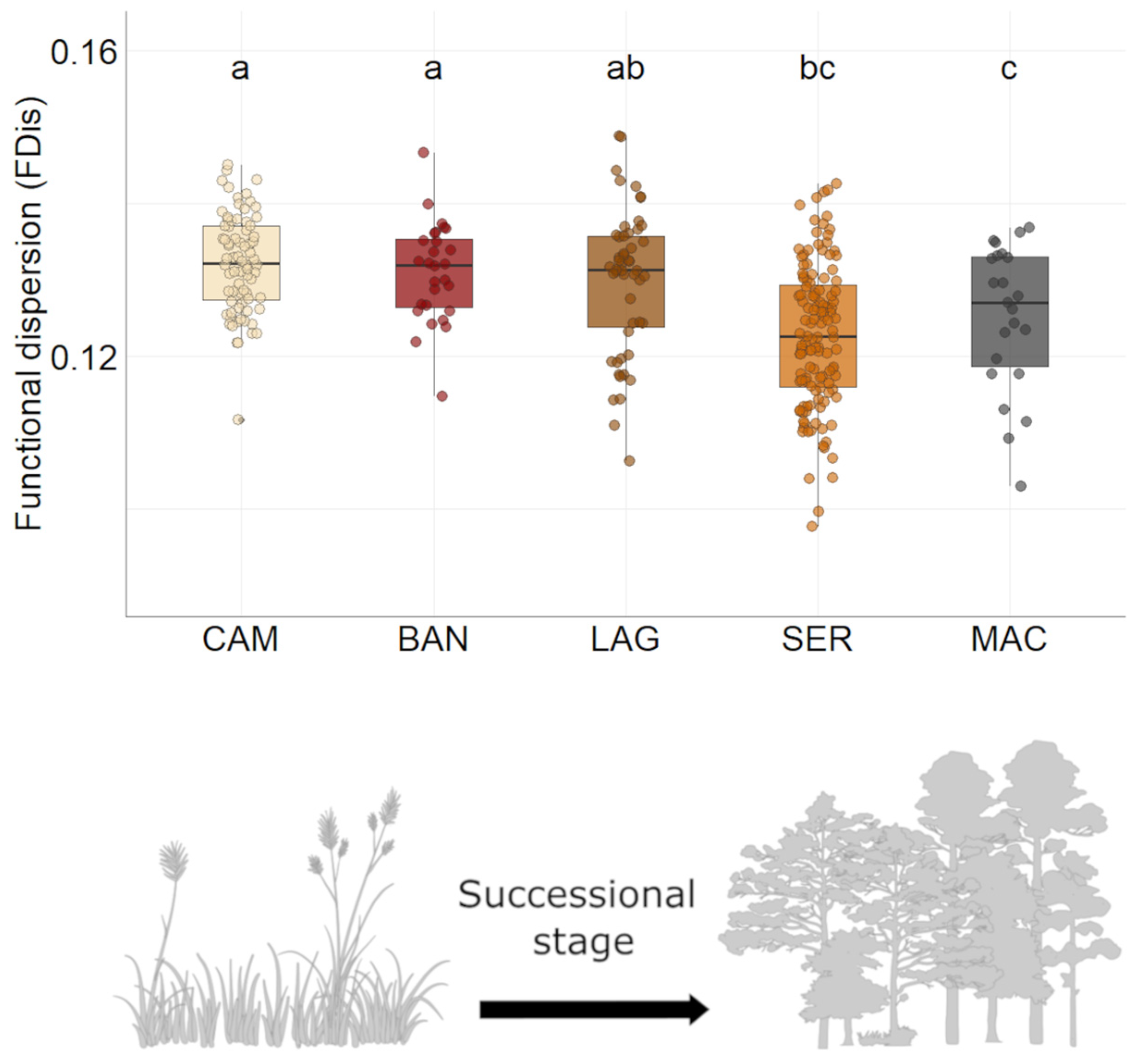
3.3. Bird Functional Diversity
4. Discussion
4.1. Bird Species Composition and Richness
4.2. Functional Diversity
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Wilson, O.J.; Mayle, F.E. A conservation assessment of Brazil’s iconic and threatened Araucaria Forest-Campos mosaic. Biol. Conserv. 2024, 296, 110650. [Google Scholar] [CrossRef]
- Fundação SOS Mata Atlântica. Atlas dos Remanescentes Florestais da Mata Atlântica: Período 2019/2020; Relatório técnico; Fundação SOS Mata Atlântica; Instituto Nacional de Pesquisas Espaciais–INPE: São Paulo, Brazil, 2021; 73p. [Google Scholar]
- Overbeck, G.E.; Vélez-Martin, E.; Menezes, L.S. Placing Brazil’s grasslands and savanas on the map of science and conservation. Perspect. Plant Ecol. Evol. Syst. 2022, 56, 125687. [Google Scholar] [CrossRef]
- Thomas, P. Araucaria angustifolia. The IUCN Red List of Threatened Species 2013, e.T32975A2829141. Available online: https://www.iucnredlist.org/species/32975/2829141 (accessed on 14 April 2025).
- Carlucci, M.B.; Jarenkow, J.A.; Duarte, L.S.; Pillar, V.D.P. Conservação da Floresta com Araucária no Extremo Sul do Brasil. Nat. Conserv. 2011, 9, 111–114. [Google Scholar] [CrossRef]
- BirdLife International. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 28 April 2025).
- Rio Grande do Sul. Decreto Nº. 51.797, de 8 de setembro 2014. Diário Oficial do Estado do Rio Grande do Sul, Porto Alegre, Brazil. Available online: https://www.diariooficial.rs.gov.br/ (accessed on 24 April 2025).
- Suhs, R.B.; Giehl, E.L.H.; Peroni, N. Preventing traditional management can cause grassland loss within 30 years in southern Brazil. Sci. Rep. 2020, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Linzmeier, A.M.; Ribeiro-Costa, C.S.; Marinoni, R.C. Fauna de Alticini (Newman) (Coleoptera, Chrysomelidae, Galerucinae) em diferentes estágios na Floresta com Araucária do Paraná, Brasil: Diversidade e estimativa de riqueza de espécies. Rev. Bras. Entomol. 2006, 50, 101–109. [Google Scholar] [CrossRef]
- Conte, C.E.; Rossa-Feres, D.C. Riqueza e distribuição espaço-temporal de anuros em um remanescente de Floresta de Araucária no sudeste do Paraná. Rev. Bras. Zool. 2007, 24, 1025–1037. [Google Scholar] [CrossRef]
- Kaminski, N.; Angelo, A.C.; Nicola, P.A. A influência do gradiente sucessional e da frutificação de Merostachys aff. multiramea em uma comunidade de aves da Floresta com Araucária. Iheringia Sér. Zool. 2016, 106, e2016002. [Google Scholar] [CrossRef]
- Siqueira, P.R. Influência da cobertura florestal nas comunidades de aves e funções ecossistêmicas. Ph.D. Thesis, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil, 3 February 2023. Available online: http://hdl.handle.net/1843/55608 (accessed on 7 May 2025).
- Sekercioglu, C.H. Increasing awareness of avian ecological function. Trends Ecol. Evol. 2006, 21, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Luck, G.W.; Smallbone, L.T.; Sheffield, K.J. Enviromental and socio-economic factors related to urban bird communities. Austral Ecol. 2013, 38, 111–120. [Google Scholar] [CrossRef]
- Mouillot, D.; Graham, N.A.J.; Villéger, S. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.M.; Tobias, J.A.; Edwards, D.P.; Davies, R.G. Contrasting impacts of land-use change on phylogenetic and functional diversity of tropical forest birds. J. Appl. Ecol. 2017, 55, 1604–1614. [Google Scholar] [CrossRef]
- Smith, D.A.E.; Si, X.; Smith, Y.C.E.; Kalle, R.; Ramesh, T.; Downs, C.T. Patterns of avian diversity across a decreasing patch-size gradient in a critically endangered subtropical forest system. J. Biogeogr. 2018, 45, 2118–2132. [Google Scholar] [CrossRef]
- Casas, G.; Darski, B.; Ferreira, P.M.A.; Kindel, A.; Müller, S.C. Habitat Structure Influences the Diversity, Richness and Composition of Bird Assemblages in Successional Atlantic Rain Forests. Trop. Conserv. Sci. 2016, 9, 503–524. [Google Scholar] [CrossRef]
- Shen, Y.; Estrada-Villegas, S.; Umaña, M.N.; Goodale, E.; Robinson, S.; Quan, Q.; Zhang, Q. Differences in mixed-species bird flocks across forest succession: Combining network analysis and trait-based ecology related to the fast-slow continuum. Funct. Ecol. 2024, 38, 1236–1249. [Google Scholar] [CrossRef]
- Reif, J.; Marhoul, P.; Koptík, J. Bird communities in habitats along a successional gradient: Divergent patterns of species richness, specialization and threat. Basic Appl. Ecol. 2013, 14, 423–431. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Martínez-Núñes, C.; Martínez-Prentice, R.; García-Navas, V. Protected area coverage of vulnerable regions to conserve functional diversity of birds. Conserv. Biol. 2023, 37, e14131. [Google Scholar] [CrossRef] [PubMed]
- Mikolaiczik, N.M.; Barreto, M.S.; Hartmann, M.T.; Hartmann, P.A. Bird fauna in secondary forest stages: A study in a southern Brazilian protected area. Oecologia Aust. 2019, 23, 261–279. [Google Scholar] [CrossRef]
- Melo, M.A.; Silva, M.A.G.; Piratelli, A.J. Improvement of vegetation structure enhances bird functional traits and habitat resilience in an area of ongoing restoration in the Atlantic Forest. Ann. Braz. Acad. Sci. 2020, 92, e20191241. [Google Scholar] [CrossRef] [PubMed]
- Carello, C.A.; Yanco, S.W. Early successional habitat supports unique avian communities dominated by wintering migrants in a premontane tropical forest. J. Trop. Ecol. 2023, 39, e22. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Cleary, D.F.R.; Boyle, T.J.B.; Setyawati, T.; Anggraeni, C.D.; Van Loon, E.E.; Menken, S.B.J. Bird species and traits associated with logged and unlogged forest in Borneo. Ecol. Appl. 2007, 17, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Jacoboski, L.I.; Debastiani, V.J.; Mendonça-Lima, A.; Hartz, S.M. How do diversity and functional nestedness of bird communities respond to changes in the landscape caused by eucalyptus plantations? Commun. Ecol. 2016, 17, 107–113. [Google Scholar] [CrossRef]
- Souza, J.L.; Côrte, D.A.A.; Ferreira, L.M. Perguntas e Respostas sobre Reserva Particular do Patrimônio Natural; Ministério do Meio Ambiente, Instituto Chico Mendes de Conservação da Biodiversidade: Brasília, Brazil, 2012; 75p. Available online: https://www.gov.br/icmbio/pt-br/servicos/servicos-do-icmbio-no-gov.br/crie-sua-rppn/perguntaserespostasrppn1.pdf (accessed on 24 April 2025).
- Backes, A.; Fernandes, A.V.; Zeni, D.J. Produção de folhedo em uma floresta com Araucaria angustifolia no sul do Brasil. Pesq. (Bot.) 2000, 50, 97–117. [Google Scholar]
- Marchiori, J.N.C. Fitogeografia do Rio Grande do Sul, Enfoque Histórico e Sistemas de Classificação, 1st ed.; EST Edições: Porto Alegre, Brasil, 2002; 118p, ISBN 9788575170143. [Google Scholar]
- Pró-Mata: Pesquisa. Available online: https://www.pucrs.br/ima/pro-mata/pesquisa/ (accessed on 26 June 2025).
- Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS)-Instituto do Meio Ambiente. Plano de Manejo do Centro de Pesquisas e Conservação da Natureza PRÓ-MATA. 2011. 258p. Available online: https://www.pucrs.br/ima/wp-content/uploads/sites/116/2019/07/plano-de-manejo-2011.pdf (accessed on 15 April 2025).
- QGIS Development Team. QGIS Geographic Information System (Version 3.36). Windows. 2024. Available online: https://qgis.org (accessed on 24 April 2025).
- Karasiak, N. Dzetsaka QGIS Classification Plugin. 2016. Available online: https://plugins.qgis.org/plugins/dzetsaka/ (accessed on 24 April 2025).
- Baaske, R. Vegetationskartierung des Forschungsgebietes Pró-Mata, Rio Grande do Sul, Brasilien, unter Verwendung von CIR-Luftbildern; Fachhochschule Rottenburg: Rottenburg, Germany, 2001; 129p. [Google Scholar]
- MacKinnon, J.; Phillipps, K. The Birds of Borneo, Sumatra, Java and Bali: The Greater Sunda Islands, 1st ed.; Oxford University Press: Oxford, UK, 1993; 491p. [Google Scholar] [CrossRef]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A.; Mustoe, S.H. Bird Census Techniques; Academic Press: London, UK, 2000; 132p. [Google Scholar]
- Von Matter, S.; Straube, F.; Accordi, I.; Piacentini, V.Q.; Cândido, J.F., Jr.; Ribon, R. Amostragem de aves pelo método de listas de Mackinnon. In Ornitologia e Conservação: Ciência Aplicada, Técnicas de Pesquisa e Levantamento, 1st ed.; Technical Books Editora: Rio de Janeiro, Brazil, 2010; Volume 1, pp. 33–44. ISBN 978-85-61368-04-3. [Google Scholar]
- MacLeod, R.; Herzog, S.K.; Maccormick, A.; Ewing, S.R.; Bryce, R.; Evans, K.L. Rapid monitoring of species abundance for biodiversity conservation: Consistency and reliability of the MacKinnon lists technique. Biol. Conserv. 2011, 144, 1374–1381. [Google Scholar] [CrossRef]
- Tobias, J.A.; Sheard, C.S.; Pigot, A.L.; Devenish, A.J.M.; Yang, J.; Sayol, F.; Neate-Clegg, M.H.C.; Alioravainen, N.; Weeks, T.L.; Barber, R.A.; et al. AVONET: Morphological, ecological and geographical data for all birds. Ecol. Lett. 2022, 25, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Wilman, H.; Belmaker, J.; Simpson, J.; de la Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef]
- Fitzpatrick, S. Patterns of morphometric variation in birds’ tails: Length, shape and variability. Biol. J. Linn. Soc. 1997, 62, 145–162. [Google Scholar] [CrossRef]
- Felice, R.N.; Tobias, J.A.; Pigot, A.L.; Goswami, A. Dietary niche and the evolution of cranial morphology in birds. Proc. R. Soc. B 2019, 286, 20182677. [Google Scholar] [CrossRef] [PubMed]
- Quitián, M.; Santillán, V.; Bender, I.M.A.; Espinosa, C.I.; Homeier, J.; Böhning-Gaese, K.; Schleuning, M.; Neuschulz, E.L. Functional responses of avian frugivores to variation in fruit resources between natural and fragmented forests. Funct Ecol. 2019, 33, 399–410. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: iNterpolation and EXTrapolation for Species Diversity. 2024. Available online: https://cran.r-project.org/web/packages/iNEXT/iNEXT.pdf (accessed on 15 April 2025).
- McGill, B.J.; Etienne, R.S.; Gray, J.S.; Alson, D.; Anderson, M.J.; Benecha, H.K.; Dornelas, M.; Enquist, B.J.; Green, J.L.; He, F.; et al. Species abundance distributions: Moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 2007, 10, 995–1015. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2008, 26, 32–46. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2024. Available online: https://cran.r-project.org/package=vegan (accessed on 25 April 2025).
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Swenson, N.G. Functional and Phylogenetic Ecology in R, 1st ed.; Springer Science & Business Media: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Villéger, S.; Manson, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in funcitional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R Software Version 4.4.0; R Development Core Team: Vienna, Austria, 2024. [Google Scholar]
- Mason, D.J.; Thiollay, J.-M. Tropical Forestry and the Conservation of Neotropical Birds: Conserving Wildlife in Logged Tropical Forests; Fimbel, R.A., Robinson, J., Grajal, A., Eds.; Columbia University Press: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Oliveira, J.; Almeida, S.M.; Florêncio, F.P.; Pinho, J.B.; Oliveira, D.M.M.; Ligeiro, R.; Rodrigues, D.J. Environmental structure affects taxonomic diversity but not functional structure of understory birds in the southwestern Brazilian Amazon. Acta Amaz. 2019, 49, 232–241. [Google Scholar] [CrossRef]
- Matthews, T.J.; Whittaker, R.K. On the species abundance distribution in applied ecology and biodiversity management. J. Appl. Ecol. 2014, 52, 443–454. [Google Scholar] [CrossRef]
- O’Dea, N.; Watson, J.E.M.; Whittaker, R.J. Rapid assessment in conservation research: A critique of avifaunal assessment techniques illustrated by Ecuadorian and Madagascan case study data. Divers. Distrib. 2004, 10, 55–63. [Google Scholar] [CrossRef]
- Hartz, S.M.; Pinheiro, G.C.; Mendonça-Lima, A.; Duarte, L.S. The Potential Role of Migratory Birds in the Expansion of Araucaria Forest. Nat. Conserv. 2012, 10, 52–56. [Google Scholar] [CrossRef]
- Gonzalez-Gomez, P.L.; Estades, C.F.; Simonetti, J.A. Strengthened insectivory in a temperate fragmented forest. Oecologia 2006, 148, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, L.; Giffard, B.; Charbonnier, Y.; van Halder, I.; Brockerhoff, E.G. Bird functional diversity enhances insectivory at forest edges: A transcontinental experiment. Divers. Distrib. 2014, 20, 149–159. [Google Scholar] [CrossRef]
- Karpińska, O.; Kamionka-Kanclerska, K.; Czortek, P.; Dyderski, M.K.; Czeszczewik, D. Mechanisms shaping the functional diversity of birds’ composition in the primeval forest ecosystem of the Białowieża National Park. Eur. J. For. Res. 2024, 143, 1015–1033. [Google Scholar] [CrossRef]
- Borges, S.H.; Tavares, T.R.S.; Crouch, N.M.A.; Baccaro, F. Sucessional trajetories of bird assemblages in amazonian secondary forests: Perspectives from complementary biodiversity dimensions. For. Ecol. Manag. 2021, 483, 118731. [Google Scholar] [CrossRef]
- Matuoka, M.A.; Benchimol, M.; Morante-Filho, J.C. Tropical forest loss drives divergent patterns in functional diversity of forest and non-forest birds. Biotropica 2020, 52, 738–748. [Google Scholar] [CrossRef]
- Schleuter, D.; Daufresne, M.; Massol, F.; Argillier, C. A user’s guide to functional diversity indices. Ecol. Monogr. 2010, 80, 469–484. [Google Scholar] [CrossRef]
- Vicente-Silva, J.; Bergamin, R.S.; Zanini, K.J.; Pillar, V.D.; Müller, S.C. Assembly patterns and functional diversity of tree species in a successional gradient of Araucaria forest in Southern Brazil. Nat. Conserv. 2016, 14, 67–73. [Google Scholar] [CrossRef]

| Trail Code | Trail Name | Vegetation and Habitat Characteristics | Successional Stage | Forest Cover (%) |
|---|---|---|---|---|
| CAM | Campo Trail | Dominated by grassland with Baccharis shrubs, rocky outcrops, poorly drained herbaceous-shrub zones, and occasional forest patches with Araucaria. Some forest strips border the area. | Initial (IN) | 15% |
| BAN | Bananeiras Trail | Grassland with dominant Baccharis, poorly drained herbaceous-shrub areas, patches of early successional trees, and cloud forest. Includes mature forest patches with bamboo along hill slopes. | Early to Intermediate (IN) | 45% |
| LAG | Lago Trail | Mixed Araucaria Forest and bamboo. Begins in advanced stage, transitions to intermediate along slopes with shallow soils, and ends in early successional vegetation near a large artificial lake with wetlands. | Intermediate to Late (IT) | 82% |
| SER | Serraria Trail | Predominantly Araucaria Forest with areas of intermediate succession due to past timber extraction. Includes exotic species (Citrus, Hydrangea) and Pinus. Advanced segments with epiphytes and wet habitats. | Intermediate to Advanced (AS) | 90% |
| MAC | Macuco Trail | Mature Araucaria Forest with near-primary characteristics: tall old trees (e.g., Cedro, large Araucaria), abundant epiphytes (e.g., Bromeliaceae), sparse understory, and few younger bamboo patches. | Advanced (AS) | ~100% |
| Trail ID | Trail Length (m) | Buffer Area 100 (m2) | Forest Cover (m2) |
|---|---|---|---|
| CAM/IN | 6046 | 983,808 | 148,096 |
| BAN/IN | 2202 | 417,088 | 187,136 |
| LAG/IT | 2439 | 455,68 | 371,90 |
| SER/AS | 5431 | 1,062,272 | 930,688 |
| MAC/AS | 1139 | 245,888 | 238,912 |
| Trail Code | # MacKinnon Lists (n) | Richness Observed | Richness Extrapolated | # Exclusive spp. | Sp/Total spp. (%) |
|---|---|---|---|---|---|
| CAM (7) | 76 | 136 (CI: 124.4–146.6) | 189 (CI: 159.4–218.3) | 27 | 68.6 |
| BAN (7) | 28 | 101 (CI: 90.4–111.6) | 200 (CI: 144.6–256.3) | 5 | 62.4 |
| LAG (7) | 51 | 121 (CI: 112.0–130.0) | 169 (CI: 137.1–200.1) | 6 | 63.4 |
| SER (7) | 127 | 135 (CI: 129.4–140.6) | 143 (CI: 133.6–152.2) | 16 | 70.2 |
| MAC (7) | 23 | 87 (CI: 80.9–93.1) | 98 (CI: 82.1–113.1) | 3 | 45.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, C.S.; Jacoboski, L.I.; Rosoni, J.R.R.; da Silva, J.L.; Bernardi, F.A.P.; Malmoria, P.E.; Beier, C.; Hartz, S.M. Bird Community Structure Changes as Araucaria Forest Cover Increases in the Highlands of Southeastern Brazil. Birds 2025, 6, 37. https://doi.org/10.3390/birds6030037
Fontana CS, Jacoboski LI, Rosoni JRR, da Silva JL, Bernardi FAP, Malmoria PE, Beier C, Hartz SM. Bird Community Structure Changes as Araucaria Forest Cover Increases in the Highlands of Southeastern Brazil. Birds. 2025; 6(3):37. https://doi.org/10.3390/birds6030037
Chicago/Turabian StyleFontana, Carla Suertegaray, Lucilene Inês Jacoboski, Jonas Rafael Rodrigues Rosoni, Juliana Lopes da Silva, Filipe Augusto Pasa Bernardi, Pamela Eliana Malmoria, Christian Beier, and Sandra Maria Hartz. 2025. "Bird Community Structure Changes as Araucaria Forest Cover Increases in the Highlands of Southeastern Brazil" Birds 6, no. 3: 37. https://doi.org/10.3390/birds6030037
APA StyleFontana, C. S., Jacoboski, L. I., Rosoni, J. R. R., da Silva, J. L., Bernardi, F. A. P., Malmoria, P. E., Beier, C., & Hartz, S. M. (2025). Bird Community Structure Changes as Araucaria Forest Cover Increases in the Highlands of Southeastern Brazil. Birds, 6(3), 37. https://doi.org/10.3390/birds6030037






