Habitat Requirements of the Grey-Headed Woodpecker in Lowland Areas of NE Poland: Evidence from the Playback Experiment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
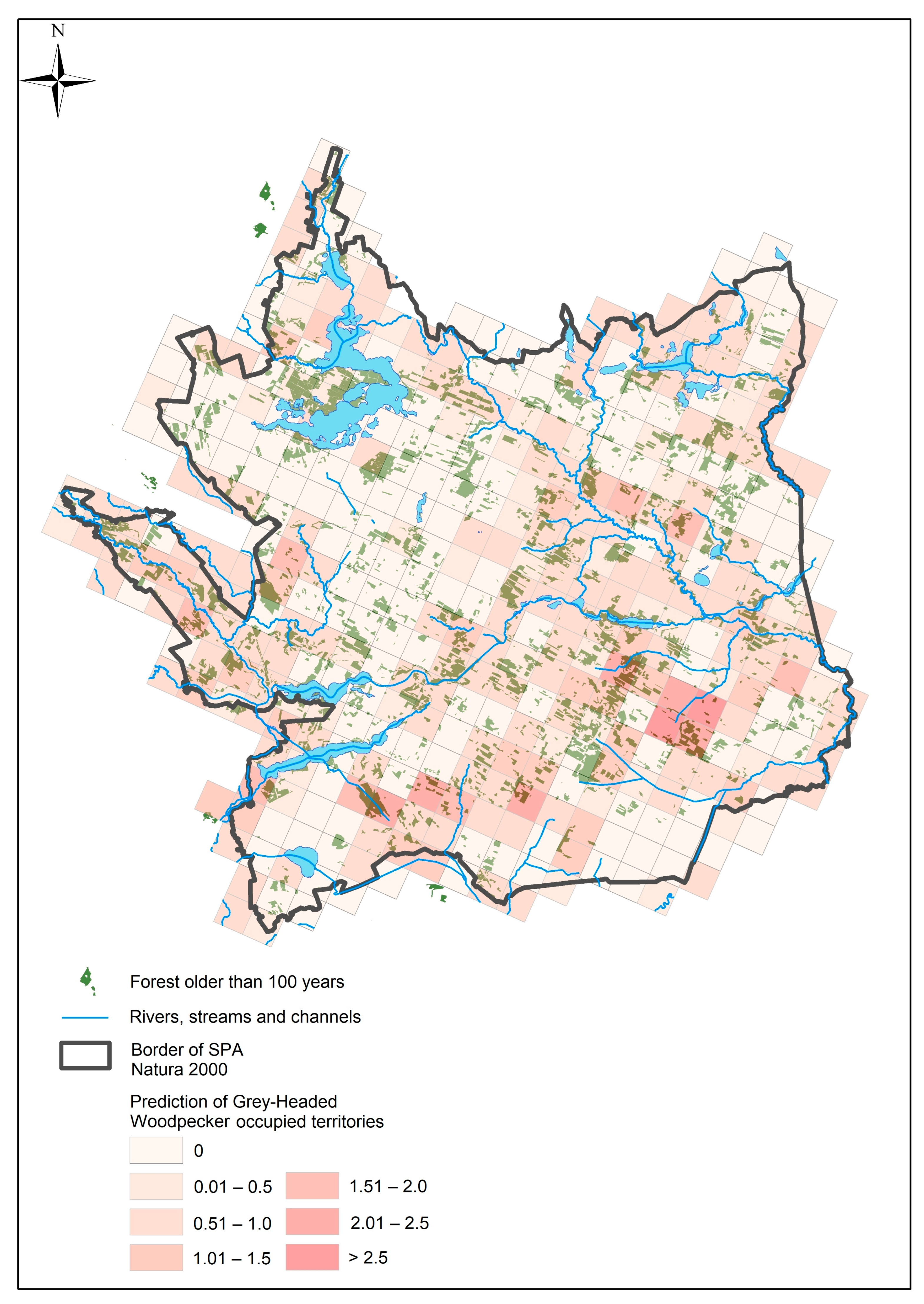
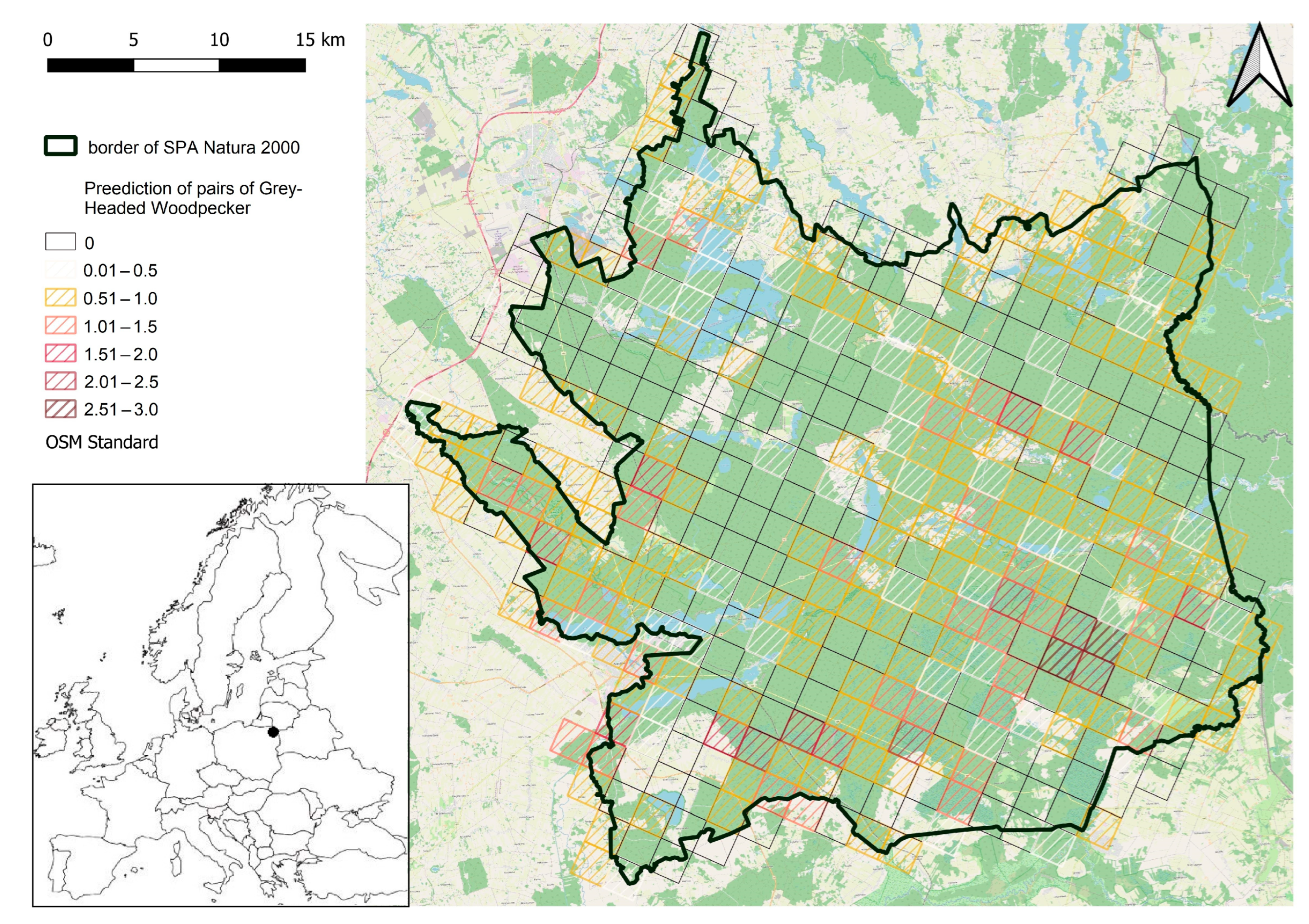
2.1. Study Area
2.2. Field Work
2.3. Data Collection
2.4. Statistical Analysis
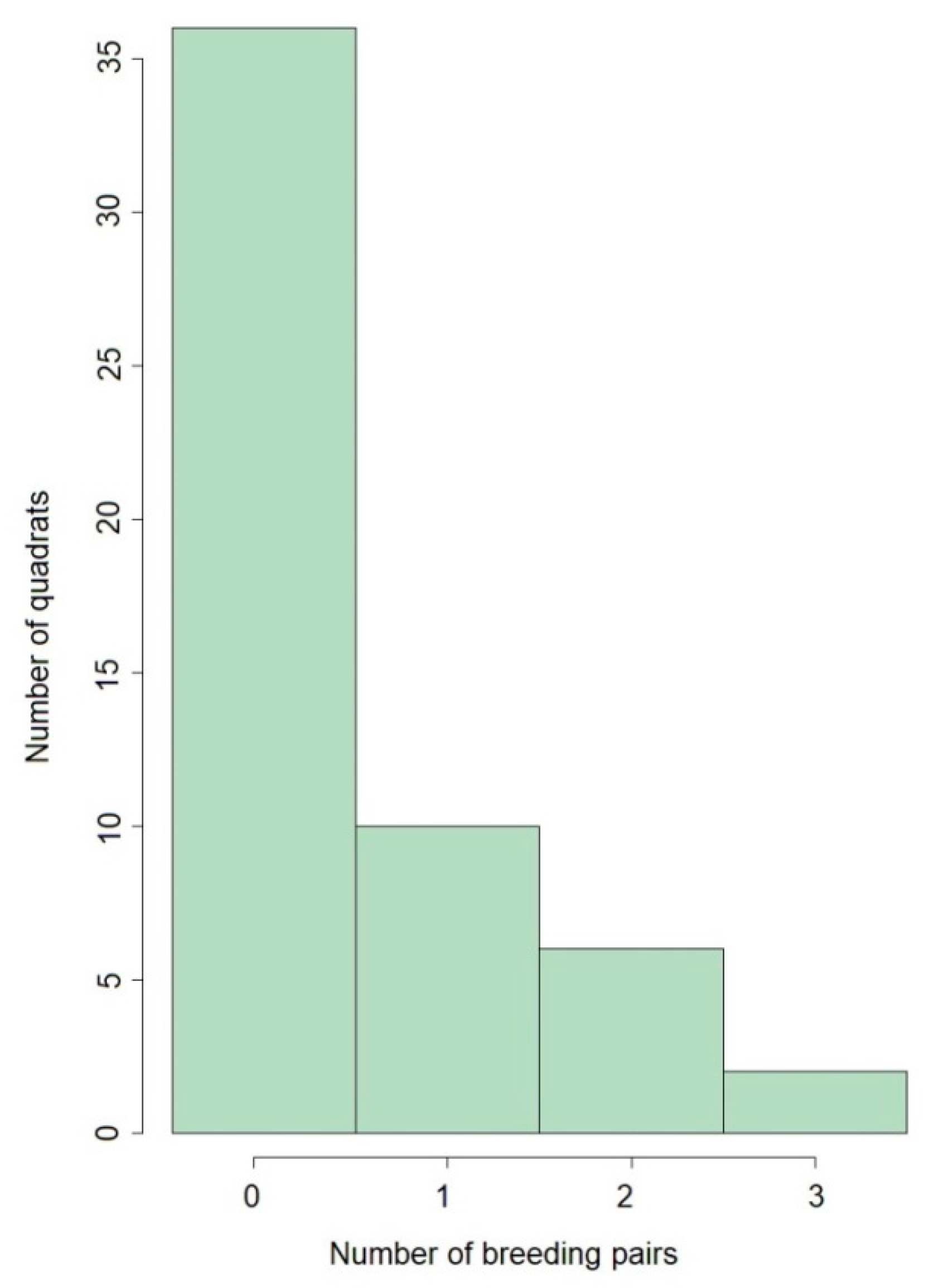
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Number | Intercept | Watercourses | >120 Share | Alder Share | Oak Share | Mixed Forest Share | R2 | df | Log Link | AICc | Delta | Weight |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 32 | −3.184 | 2.044 | 0.0571 | 0.0340 | −1.7870 | 0.0820 | 0.504 | 6 | −35.22 | 84.2 | 0.00 | 0.363 |
| 24 | −2.907 | 2.083 | 0.0469 | x | −1.1140 | 0.1001 | 0.466 | 5 | −37.19 | 85.6 | 1.40 | 0.180 |
| 16 | −2.690 | 1.840 | 0.0639 | 0.0435 | −1.0830 | x | 0.464 | 5 | −37.30 | 85.9 | 1.63 | 0.161 |
| 30 | −2.354 | 1.528 | x | 0.0262 | −1.3110 | 0.0887 | 0.461 | 5 | −37.47 | 86.2 | 1.96 | 0.136 |
| 22 | −2.313 | 1.715 | x | x | −0.9533 | 0.1041 | 0.432 | 4 | −38.88 | 86.6 | 2.35 | 0.112 |
| 14 | −1.816 | 1.387 | x | 0.0387 | −0.7502 | x | 0.399 | 4 | −40.41 | 89.6 | 5.41 | 0.024 |
| 12 | −2.525 | 1.432 | 0.0521 | 0.0372 | x | x | 0.375 | 4 | −41.46 | 91.7 | 7.50 | 0.01 |
| 8 | −2.331 | 2.094 | 0.0571 | x | −0.5531 | x | 0.367 | 4 | −41.79 | 92.4 | 8.17 | 0.01 |
| 29 | −1.511 | x | x | 0.0441 | −0.9127 | 0.0708 | 0.354 | 4 | −42.35 | 93.5 | 9.29 | 0.003 |
| 10 | −1.879 | 1.158 | x | 0.0359 | x | x | 0.323 | 3 | −43.59 | 93.7 | 9.44 | 0.003 |
Appendix B
References
- Passinelli, G. Population biology of European woodpeckers: A review. Ann. Zool. Fenn. 2006, 43, 96–111. [Google Scholar]
- Gorman, G. Characteristics of Grey-headed Woodpecker (Picus canus) cavities in Hungary. Aquila 2019, 126, 33–39. [Google Scholar]
- Gorman, G. Woodpeckers of Europe: A Study of the European Picidae; Bruce Coleman: London, UK, 2004. [Google Scholar]
- Keller, V.; Herrando, S.; Voříšek, P.; Franch, M.; Kipson, M.; Milanesi, P.; Marti, D.; Klvanowa, A.; Kalyakin, M.V.; Bauer, H.G.; et al. European Breeding Bird Atlas 2: Distribution, Abundance and Change; Lynx Editions: Barcelona, Spain, 2020. [Google Scholar]
- Grey-faced Woodpecker Picus Canus Species. BirdLife DataZone. Available online: https://datazone.birdlife.org/species/factsheet/grey-faced-woodpecker-picus-canus (accessed on 21 January 2025).
- Lehikoinen, A.; Virkkala, R. North by northwest: Climate change and directions of density shifts in birds. Glob. Change Biol. 2016, 22, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Rolstad, J.; Rolstad, E. Seasonal patterns in home range and habitat use of the grey-headed woodpecker Picus canus as influenced by the availability of food. Ornis Fenn. 1995, 72, 1–13. [Google Scholar]
- Mikusiński, G.; Roberge, J.M.; Fuller, R.J. (Eds.) Ecology and Conservation of forest Birds; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Kosiński, Z.; Ciach, M. Dzięcioł zielonosiwy Picus canus. In Materiały do Wyznaczania i Określania Stanu Zachowania Siedlisk Ptasich w Obszarach Specjalnej Ochrony Ptaków Natura 2000; Zawadzka, D., Ciach, M., Figarski, T., Kajtoch, Ł., Rejt, Ł., Eds.; GDOŚ: Warsaw, Poland, 2013; pp. 94–102. [Google Scholar]
- Chodkiewicz, T.; Kuczyński, L.; Sikora, A.; Chylarecki, P.; Neubauer, G.; Ławicki, Ł.; Stawarczyk, T. Ocena liczebności populacji ptaków lęgowych w Polsce w latach 2008–2012. Ornis Pol. 2015, 56, 149–189. [Google Scholar]
- Chylarecki, P.; Chodkiewicz, T.; Neubauer, G.; Sikora, A.; Meissner, W.; Woźniak, B.; Wylegała, P.; Ławicki, Ł.; Marchowski, D.; Betleja, J.; et al. Trendy Liczebności Ptaków w Polsce; GIOŚ: Warszaw, Poland, 2018.
- Kempa, M.; Kosiński, Z. Ekspansja i pierwsze przypadki gniazdowania dzięcioła zielonosiwego Picus canus w Wielkopolsce. Not. Ornitol. 2003, 44, 131–135. [Google Scholar]
- Sikora, A. Rozmieszczenie i liczebność dzięcioła zielonosiwego Picus canus na Wysoczyźnie Elbląskiej i jego ekspansja na Warmii i Mazurach. Not. Ornitol. 2006, 47, 32–42. [Google Scholar]
- Directive 2009/147/EC on the Conservation of Wild Birds. Available online: https://eur-lex.europa.eu/eli/dir/2009/147/oj/eng (accessed on 21 January 2025).
- Angelstam, P.; Breuss, M.; Mikusiński, G.; Stenström, M.; Stighäll, K.; Thorell, D. Effects of forest structure on the presence of woodpeckers with different specialisation in a landscape history gradient in NE Poland. In Proceedings of the Eleventh Annual IALE (UK) Conference, Norwich, UK, 10–13 September 2002; Avian Landscape Ecology, International Association for Landscape Ecology, Chamberlain, D., Wilson, A., Eds.; University of East Anglia: Stowmarket, UK, 2002; pp. 25–38. [Google Scholar]
- Gjerde, I.; Sætresdal, M.; Nilsen, T. Abundance of two threatened woodpecker species in relation to the proportion of spruce plantations in native pine forests of western Norway. Biodivers. Conserv. 2005, 14, 377–393. [Google Scholar] [CrossRef]
- Sikora, A.; Kosiński, Z. Dzięcioł zielonosiwy Picus canus. In Monitoring Ptaków Lęgowych. Poradnik Metodyczny; Chylarecki, P., Sikora, A., Cenian, Z., Chodkiewicz, T., Eds.; GIOŚ: Warsaw, Poland, 2015; pp. 485–490. [Google Scholar]
- Pakkala, T.; Tiainen, J.; Pakkala, H.; Piha, M.; Kouki, J. Nest tree characteristics of Grey-headed Woodpeckers (Picus canus) in boreal forests. Ornis Fenn. 2020, 97, 89–100. [Google Scholar] [CrossRef]
- Zawadzka, D.; Zawadzki, G. Nest Trees Selected by the Grey-Headed Woodpecker in Northeastern Poland. Sylwan 2022, 166, 566–578. [Google Scholar] [CrossRef]
- Kosiński, Z.; Kempa, M. Density, distribution and nest-sites of woodpeckers Picidae, in a managed forest of western Poland. Pol. J. Ecol. 2007, 55, 519–533. [Google Scholar]
- Pakkala, T.; Tiainen, J.; Pakkala, H.; Piha, M.; Kouki, J. Dynamics of the cavities of Grey-headed Woodpeckers Picus canus reveal their long- and short-term ecological roles in boreal forest. Acta Ornithol. 2021, 56, 199–208. [Google Scholar] [CrossRef]
- Sokołowski, A.W. Puszcza Augustowska; CILP: Warszawa, Poland, 2010. [Google Scholar]
- Bank Danych o Lasach. 2024. Available online: https://dane.gov.pl/en/dataset/629,bank-danych-o-lasach (accessed on 21 September 2024).
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Biecek, P.; Burzykowski, T. Explanatory Model Analysis: Explore, Explain, and Examine Predictive Models; Chapman and Hall/CRC: New York, NY, USA, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed−effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Tumiel, T.; Białomyzy, P.; Grygoruk, G.; Korniluk, M.; Świętochowski, P.; Wereszczuk, M.; Skierczyński, M. Cenne i nieliczne ptaki lęgowe na Obszarze Specjalnej Ochrony Puszcza Knyszyńska. Ornis Pol. 2013, 54, 170–186. [Google Scholar]
- Cramp, S. The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1985. [Google Scholar]
- Domokos, E.; Cristea, V. Effects of managed forest structure on woodpeckers (Picidae) in the Niraj valley (Romania): Woodpecker populations in managed forests. North-West J. Zool. 2014, 10, 110–117. [Google Scholar]
- Shurulinkov, P.; Stoyanov, G.; Komitov, E.; Daskalova, G.; Ralev, A. Contribution to the Knowledge on Distribution, Number and Habitat Preferences of Rare and Endangered Birds in Western Rhodopes Mts, Southern Bulgaria. Strigiformes and Piciformes. Acta Zool. Bulg. 2012, 64, 43–56. [Google Scholar]
- Südbeck, P. Beitrag zur Höhlenökologie des Grauspechts Picus canus. Osnabrücker Naturwissenschaftliche Mitteilungen 2009, 35, 263–274. [Google Scholar]
- Valkama, J.; Vepsäläinen, V.; Lehikoinnen, A. The Third Finnish Breeding Bird Atlas; Finnish Museum of Natural History and Ministry of Environment: Helsinki, Finland, 2011. [Google Scholar]
- Wojton, A.; Krasoń, K. Wykorzystanie płatów drzewostanów liściastych przez stenotopowe gatunki dzięciołów w lasach z dominacją sosny w południowo−wschodniej Polsce. Sylwan 2017, 161, 940–948. [Google Scholar] [CrossRef]
- Kebrle, D.; Zasadil, P.; Hošek, J.; Barták, V.; Šťastný, K. Large trees as a key factor for bird diversity in spruce-dominated production forests: Implications for conservation management. For. Ecol. Manag. 2021, 496, 119460. [Google Scholar] [CrossRef]
- Zawadzka, D.; Zawadzki, J.; Zawadzki, G.; Zawadzki, S. Wyniki inwentaryzacji ornitologicznej na terenie OSO PLB 200002 Puszcza Augustowska w 2010 r. Stud. I Mat. CEPL 2011, 27, 89–104. [Google Scholar]
- Krajewski, Ł.; Chodkiewicz, T.; Czernek, T.; Grajewska, A.; Henel, K.; Korniluk, M.; Maciorowski, G.; Marczakiewicz, P.; Mirski, P.; Neubauer, G.; et al. Awifauna lęgowa Doliny Biebrzy—Stan aktualny i zmiany. Ornis Pol. 2023, 64, 161–189. [Google Scholar]
- Wilk, T.; Jujka, M.; Krogulec, J.; Chylarecki, P. (Eds.) Important Bird Areas of International Importance in Poland; OTOP: Marki, Poland, 2010. [Google Scholar]

| Parameters | Description |
|---|---|
| Watercourse | The presence of a river, stream, or canal |
| Field | The presence of open areas, like fields or grasslands |
| Lake | The presence of lakes or ponds |
| Transport | The presence of a railway, an asphalt road, or a high-tension line |
| Forest | Share of forest area |
| Fresh Pine Forest | Share of pine-dominated forest at fresh, humid site types (percentage of deciduous trees 20–40%) |
| Wet/Bog Pine Forest | Share of pine-dominated forest at wet and bog-humid site types (percentage of deciduous trees 20–40%) |
| Fresh Deciduous Forest | Share of mixed and deciduous forest growing at fresh and wet site types (percentage of coniferous trees 20–40%) |
| Alder Forests | Share of alder forest at bog site types |
| Clear Cut | Share of clear cuts |
| Decay | Share of stands over 140 years old with clear cuts, plus stands younger than 10 years old |
| Mixed-Aged Forests | Share of stands between 41 and 100 years old |
| Over 100 years | Share of stands older than 100 years |
| Over 120 years | Share of stands older than 120 years |
| Old Stand Composition | Species composition of old stands (pine/mixed coniferous/mixed coniferous and deciduous |
| Pine Area | Share of stands with pine domination |
| Mixed Forest Area | Share of stands with spruce domination, with a significant share of aspen (Populus tremula), alder, and birch (percentage of deciduous trees 30–60%) |
| Birch Area | Share of stands with birch domination |
| Alder Area | Share of stands with alder domination |
| Aspen Area | Share of stands with aspen domination |
| Oak Area | Share of stands with oak domination |
| Deciduous Area | Share of stands with deciduous tree species domination |
| Fixed Effect Parameter | Estimate | Std. Error | z-Value | p |
|---|---|---|---|---|
| Intercept | −3.245 | 1.02 | −3.187 | 0.001 |
| Watercourses | 3.003 | 1.00 | 3.001 | 0.003 |
| Oak share | −1.365 | 0.99 | −1.374 | 0.17 |
| Mixed forest share | 0.339 | 0.12 | 2.829 | 0.005 |
| Fresh deciduous forests share | −0.124 | 0.07 | −1.699 | 0.089 |
| AIC | 48.459 |
| Fixed Effect Parameter | Estimate | Std. Error | t-Value | p |
|---|---|---|---|---|
| Intercept | −3.184 | 0.786 | −4.050 | <0.001 |
| Watercourses | 2.044 | 0.637 | 3.208 | 0.001 |
| Oak share | −1.787 | 0.951 | −1.878 | 0.06 |
| Mixed forest share | 0.082 | 0.038 | 2.139 | 0.03 |
| Alder share | 0.034 | 0.017 | 2.023 | 0.043 |
| Over 120-year forests | 0.057 | 0.027 | 2.132 | 0.03 |
| AIC | 82.437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzki, G.; Zawadzka, D. Habitat Requirements of the Grey-Headed Woodpecker in Lowland Areas of NE Poland: Evidence from the Playback Experiment. Birds 2025, 6, 32. https://doi.org/10.3390/birds6030032
Zawadzki G, Zawadzka D. Habitat Requirements of the Grey-Headed Woodpecker in Lowland Areas of NE Poland: Evidence from the Playback Experiment. Birds. 2025; 6(3):32. https://doi.org/10.3390/birds6030032
Chicago/Turabian StyleZawadzki, Grzegorz, and Dorota Zawadzka. 2025. "Habitat Requirements of the Grey-Headed Woodpecker in Lowland Areas of NE Poland: Evidence from the Playback Experiment" Birds 6, no. 3: 32. https://doi.org/10.3390/birds6030032
APA StyleZawadzki, G., & Zawadzka, D. (2025). Habitat Requirements of the Grey-Headed Woodpecker in Lowland Areas of NE Poland: Evidence from the Playback Experiment. Birds, 6(3), 32. https://doi.org/10.3390/birds6030032






