Simple Summary
Migratory bird species face numerous threats during their annual journeys, including habitat loss and degradation in breeding and non-breeding areas. Ecological traps, which occur when a habitat appears attractive based on environmental cues but are actually harmful for raising offspring, may be a significant factor in reduced breeding success for both migratory and resident birds. These traps may arise from human land-use changes or even well-intentioned conservation actions, such as providing nest boxes. In our study, we found that pest outbreaks occurring after the arrival of European Pied Flycatchers, a migratory species, severely degraded forest habitat quality. Because degraded pine (Pinus sylvestris) forests were visually indistinguishable from healthy ones early in the season, the birds selected nest sites in affected areas and subsequently suffered reduced reproductive success. Compared to birds nesting in unaffected forests, offspring from pest-affected areas had a lower body mass and tarsus length, and were less likely to fledge successfully. Our findings suggest that conservation tools, like nest boxes, must be used with caution in forests experiencing pest outbreaks, as they may unintentionally attract birds to unsuitable breeding habitats.
Abstract
When selecting a habitat, it is optimal for organisms to choose one that maximizes reproductive success through access to high-quality resources, particularly in species that engage in parental care. However, organisms may inadvertently select a habitat for breeding that would initially appear preferential and undamaged, but may, in reality, be detrimental to parent and/or offspring fitness. In this study, we tested whether migratory European Pied Flycatchers (Ficedula hypoleuca) nesting in forest patches affected by outbreaks of the great web-spinning sawfly (Acantholyda posticalis) experienced fitness reductions indicative of an ecological trap, compared to those nesting in unaffected forest patches. After installing nest boxes to attract breeding pairs and potentially combat the outbreak, we found that Flycatchers inhabiting areas with sawfly outbreaks had similar clutch sizes to pairs breeding in unaffected forest patches. Contrarily, the fledgling number and body condition were significantly lower for those nesting in the damaged forests. In providing nest boxes for migrating Flycatcher pairs in forest patches that were subsequently impacted by a pest insect outbreak, an ecological trap arose for those pairs choosing to nest in what appeared to be an unaffected forest at first. Given the inability of breeding pairs to distinguish habitat quality on initial inspection, we suggest that nest boxes be used with caution in areas with unfavorable habitat conditions when attracting migratory birds, given the trends of their declining global numbers.
1. Introduction
In a dynamic biosphere increasingly shaped by human activity, rapid environmental change imposes strong selective pressures on organisms to adapt swiftly. Individuals depend on their ability to accurately interpret environmental cues and assess resource availability when selecting habitats [1,2], and habitat selection is known to be a hierarchical process in many species (e.g., birds [3,4,5]). While adaptive habitat selection is crucial to avoid fitness costs or the need for constant dispersal, some habitats may appear attractive based on these cues but are in fact of poor quality. Such scenarios are known as ecological traps, defined as cases where organisms preferentially select habitats that appear suitable but are ultimately less appropriate than other available options [1,6,7]. These traps typically arise when rapid environmental change disrupts the historical reliability of habitat cues, leading individuals to make maladaptive choices [1,8]. There are three key conditions that define an ecological trap: (1) individuals exhibit no preference or show a preference for the lower-quality habitat, (2) habitat choice leads to differential fitness among individuals, and (3) fitness is reduced when individuals occupy the lower-quality habitat [1,9,10]. Importantly, conservation interventions, such as habitat restoration or the provision of artificial nesting structures, can unintentionally contribute to ecological traps if the surrounding environment becomes unsuitable due to unforeseen changes [11].
Forest habitats support biodiversity and ecosystem health while also providing critical services to humans when managed sustainably [12]. However, they are increasingly impacted by pest insect outbreaks, which can lead to the overconsumption of vegetation and disrupt forest structure and species interactions [13,14]. While many insects are essential for ecosystem functioning, including pollination, decomposition, and food web dynamics, pest outbreaks can cause cascading ecological effects [15]. In response, forest managers sometimes use insecticides, but these chemicals can harm non-target organisms, including insectivorous birds, and degrade environmental quality [16]. As a more sustainable alternative, conservation efforts increasingly rely on attracting natural insectivores, such as cavity-nesting birds, to suppress pest populations and maintain ecosystem balance [17,18].
In the context of pest outbreaks, biological control efforts often rely on insectivorous birds [19,20]. In Europe, nest boxes are commonly used to attract cavity-nesting species to pest-affected areas [21,22,23], and their structural advantages—such as enhanced climate control and predator protection—make them highly attractive compared to natural cavities [24,25]. While nest boxes can increase perceived habitat quality, some studies suggest that birds select these sites independently of actual food resource availability [26,27]. This decoupling between cue and habitat quality raises the potential for ecological traps, where birds settle in visually appealing but functionally degraded environments. A previous study found that Great Tits (Parus major) breeding in pest-affected patches with nest boxes produced fewer fledglings with a lower body mass and shorter tarsi compared to those in unaffected forest [28], supporting the possibility that such conditions may act as ecological traps under specific circumstances.
European Pied Flycatchers (Ficedula hypoleuca) are migratory passerine birds that breed in pairs across most of Europe during the spring and summer seasons before returning to Africa for overwintering [29]. Moreover, they are well-known cavity nesters and will routinely favor breeding in artificial nest boxes over naturally occurring cavities [30]. Pied Flycatchers are a common, insectivorous species [31], and this, in combination with their attraction to artificial nest boxes, makes them an inherently viable choice as a biological control for insect pests. In Latvia, the great web-spinning sawfly (Acantholyda posticalis) has been observed each spring and summer outbreaking in forests near the city of Daugavpils since 2013. Their larvae greatly defoliate and damage Scots pine trees (Pinus sylvestris) [32], which dominate the surrounding forest and serve as the core basis of many ecosystem functions for countless species [33]. As a result, total pine canopy cover and volume, in addition to the proportion of dead and fully defoliated pine trees, can be measured to make assumptions about forest health and resource availability, and to estimate larval biomass [28,34].
In this study, we examined whether nest boxes installed in forest patches degraded by sawfly outbreaks acted as ecological traps for European Pied Flycatchers. Nest boxes offer consistent cues for habitat selection, but the surrounding habitat quality declined due to long-term defoliation by the great web-spinning sawfly. Drawing from the previous findings on Great Tits [28], which showed that reduced canopy cover and high defoliation negatively affect larval abundance and fledgling success, we predicted that Pied Flycatchers nesting in degraded patches would have lower fledgling success, body mass, and tarsus length than those in unaffected forests. We also predicted that these same measures of offspring condition would be positively associated with larval biomass, which correlates with canopy cover and volume, and negatively with defoliation [35]. Since egg-laying in Flycatchers occurs before the outbreak phase begins, we predicted that clutch size would not be influenced by pest activity.
2. Methods
2.1. Study Location and Experimental Setup
The study area was located in southeastern Latvia near the city of Daugavpils (55.55° N, 26.34° E). Scots pines dominate the forest landscape of this urban forest area, in which there were clearly distinguishable patches of trees that were either affected or unaffected by the sawfly outbreak. Including the current event, there have been two recorded outbreaks of this pest species in Latvia: the first in 1966–1982 (roughly 40 km east of this study area) and the second in 2013–present day. It is usual for outbreaks to span years, and there can be fluctuating levels of pine damage each year that correlates with which phase of development the sawflies are in. Active larval stages result in more pine defoliation, while the years with more sawflies in their larval diapause stages allow pines to regrow needles [36].
The active larval outbreak and Flycatcher breeding season overlapped in 2020. Sawfly larvae emerge in late May and feed heavily on pine needles until early June. Flycatchers typically arrive in late April, lay eggs by mid-May, incubate until early June, and fledge their young by mid-June. We observed both extensive pine defoliation caused by the larvae and Flycatchers actively hunting them. Although Scots pines are evergreen trees, those affected by the pests were visibly degraded. When the Flycatchers returned from Africa and began establishing their breeding territories, the degraded pines resembled neighboring deciduous trees, which had not yet leafed out—leaves in southeastern Latvia typically emerge only by the second week of May (https://www.silava.lv/images/Petijumi/2015-LVM-Priezu-tikllapsene/2016-LVM-Priezu-tikllapsene-Parskats.pdf (accessed on 13 May 2025)). Cavity availability is often the most critical limiting factor for Pied Flycatchers, particularly in younger forests where natural nesting sites are rare [37,38]. It is important to note that Flycatchers arrived, occupied nest boxes, and initiated egg-laying in both affected and unaffected areas almost simultaneously, within a one-week window. We conducted observations from late April to late June 2020, when larval sawflies served as a reliable food source. Although pest outbreaks typically follow a multi-year cycle—beginning with population buildup, peaking in larval abundance and defoliation, and then gradually declining—our study took place when pest insect availability remained high.
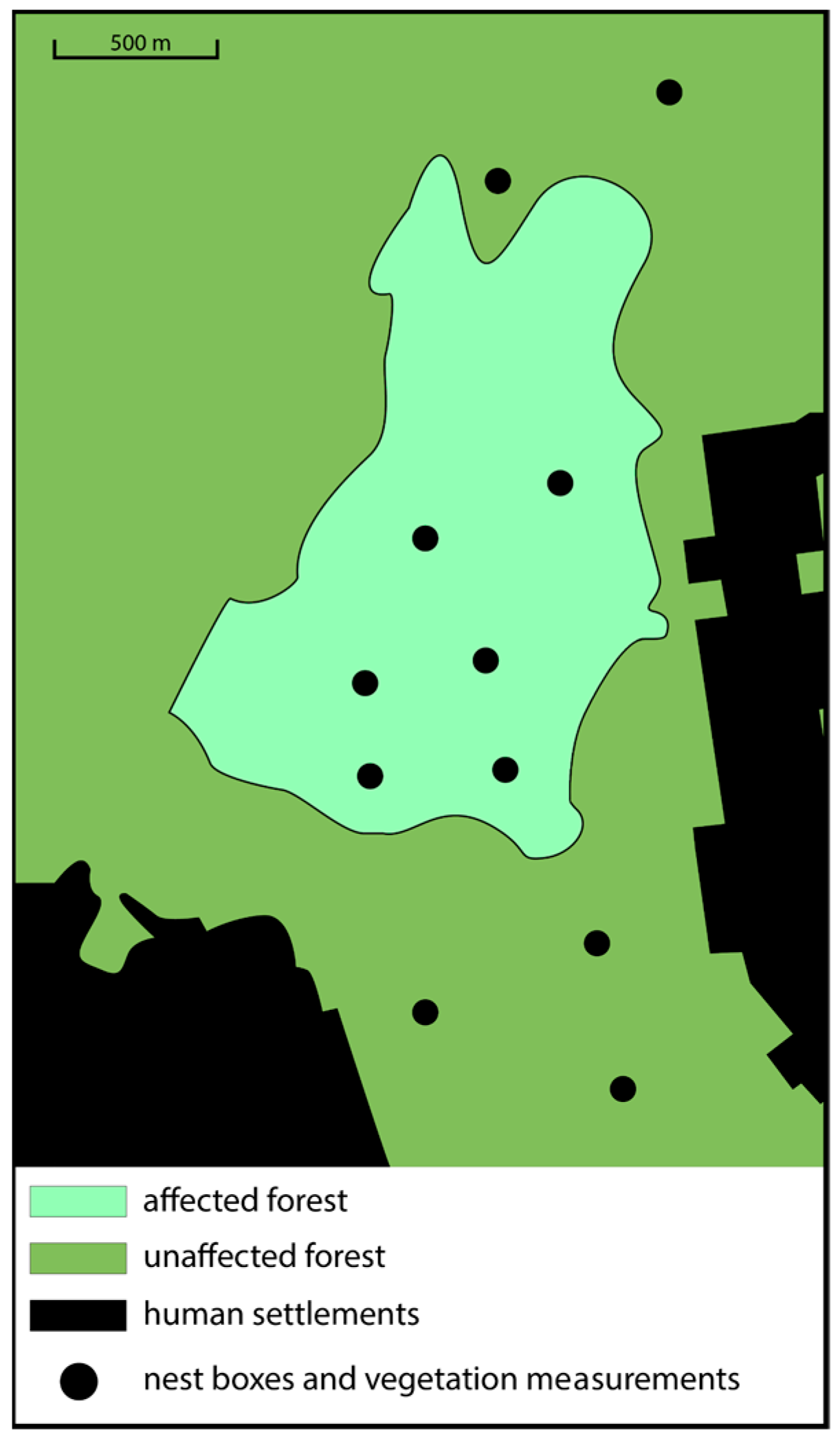
Data were collected in two patches of pine forest differentially impacted by the sawfly outbreak: (1) stands of unaffected pines (i.e., undamaged); and (2) an approximately 120 ha stand of affected pines (i.e., damaged) located within the greater unaffected forest. We placed nest boxes at a height of roughly 3 m on the trunks of pine trees to attract Pied Flycatcher pairs one-to-two weeks before their arrival. The dimensions of the nest boxes were 0.13 × 0.13 × 0.25 m, and the entrance was 3.6 cm in diameter. We kept the entrance of all nest boxes closed until late April, when the first migrating Pied Flycatchers arrived. This prevented the nest boxes from being occupied by Great Tits, a competing cavity-nesting species, which are resident and typically begin nesting in mid-April. Seven-to-eight days after the first Flycatchers arrived and began occupying nest boxes, we closed the entrances of unoccupied boxes and removed them from the forest. There were six areas in the affected forest and five areas in the unaffected forest that were selected for mounting nest boxes (Figure 1), and these were the same exact areas as in [28]. Each of these areas was about 3.8 ha, and they were separated by at least 490 m [39]. A total of 12 nest boxes were placed in each area (affected patch: 72 total; unaffected patch: 62 total). Each of the 12 boxes within each of the 11 study areas was separated by at least 50 m to avoid competition with neighbors [39]. The Pied Flycatcher is a facultatively polygynous species, where males may breed with more than one female [25]. However, polygynous males typically provide parental care only to their first mate’s offspring. Although we could not directly control for or exclude polygyny in this study, all observed nests had an attending male providing food and antipredator protection.
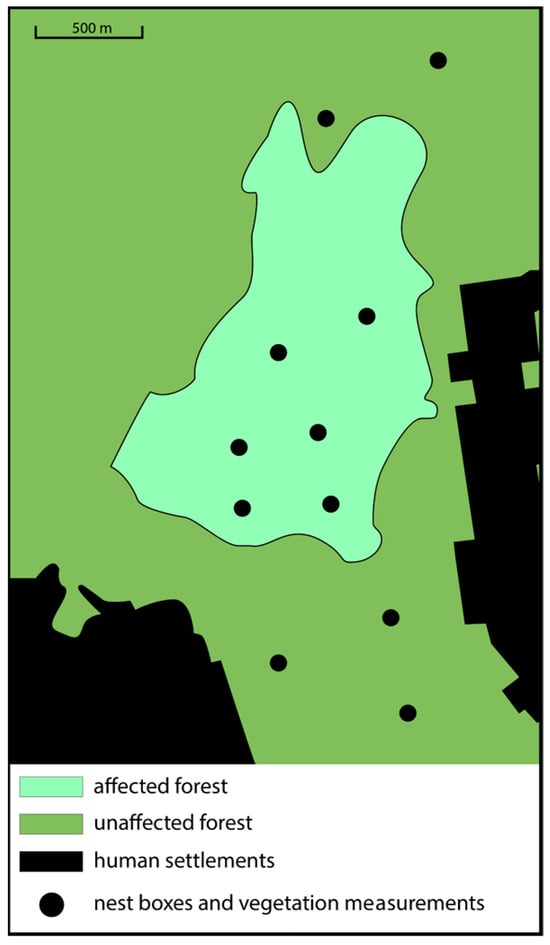
Figure 1.
Map of our study area denoting sites for where nest boxes were placed, and vegetation measurements were taken (dots). Black color represents human-made structures, darker-green shade represents forest not impacted by the pest outbreak, and lighter-green shade represents forest impacted by pest outbreak.
Once the boxes were occupied (i.e., male singing and female building the nest) by Flycatcher pairs, we checked them once every three days and recorded the following for each site: clutch size, number of successful fledglings (i.e., those that had successfully fledged the nest), and the body mass and tarsus length of successful fledglings. We calculated the proportion of young fledged per clutch (%) to compare the reproductive success of Pied Flycatchers between the forest affected by A. posticalis and the unaffected forest. A Pesola spring balance was used to weigh individuals to a precision of 0.1 g, and tarsi (mm) were measured with sliding calipers to the nearest 0.1 mm on day 14 after hatching [40].
2.2. Food Resource Estimation
The frass-fall method was employed to estimate food resource availability for insectivorous birds in the forest patches [28,41]. Using coffee filters (size 1 × 4) attached to plastic funnels (35 cm diameter), larval frass production was collected. Rainwater could pass through the filter while fallen frass was retained. Three funnels, each 60 m apart, were attached to randomly chosen trees in each study area (33 total; all started on the same day). Upon nestlings reaching seven-days old, funnels were set up for four consecutive days (late May 2020) to collect frass before being transferred to a freezer for frass preservation. To determine frass production for each collecting filter, we counted a filter’s individual frass and measured the diameter of randomly selected frass with an ocular micrometer. By quantifying the allometric relationship between frass dry mass and diameter [42] and the equation used by [43], we calculated larval biomass from the dry mass of frass and expressed it as g per m2 (gm−2). We noted that frass production was not discriminated among larvae of different species (e.g., various moths and sawflies). This methodology is an accurate estimation of the food resource availability across forest patches [44].
2.3. Tree Canopy Estimation
The observation period for the tree canopy vegetation parameter data used in our estimations was mid-June 2020. To study the vegetation, 11 forest stands were selected, and in each stand, circular plots of 100 m2 were set up. We assessed trees as the following: dead (<25% foliage remaining), damaged (25–75% foliage loss), and undamaged (<25% foliage loss) [34]. After this initial assessment, we measured the following variables: (1) total pine canopy cover (%), (2) relative number of dead/dying pines with 75–100% loss of needles from sawfly disturbance (%), and (3) total pine crown volume (m3 ha−1). The method for tree canopy estimation was based on field measurements in plots, as described in ref. [34]. Suggested in ref. [34] and used by ref. [28], tree crown parameters can reliably be used to estimate the availability of food resources. If substantial amounts of needle damage lead to the eventual death of trees and the proportion of dead and fully defoliated trees are reflective of pests’ damage rates, then the total pine crown volume would be indicative of the total amount of space that insectivorous birds, like Flycatchers, can use when foraging for their prey.
Canopy cover (i.e., the layer formed by tree leaves and branches) is represented by lower values when discontinuous (affected) and higher values when continuous (unaffected). From a centralized location within each of the 11 forest study areas, four circular plots (10 m2 in size) were established in azimuth directions at a distance of 50 m from the central point. Canopy cover was estimated by canopy closure using a forest densitometer (concave gridded mirror) (Forestry Suppliers Spherical Crown Densitometer) in each plot, where we measured in the four azimuth directions from the central point. We ensured that we were at least 2 m from the nearest tree when measuring. Calculated as canopy cover percentage, the grid on the mirror was used to count points at line crossings that were reflections of the tree canopy in the mirror.
Additionally, we measured the diameter at breast height (DBH, from 1.3 m) for all trees within each plot. Moreover, for 2–4 trees per plot with varying sizes and degrees of damage, we assessed two crown parameters: (1) width of crown from edge to edge in two perpendicular directions (using a GRS densitometer); and (2) height to the base (i.e., lowest living branch) and to the top of the live tree crown (using a Haglof VL5 vertex). A total of 66 affected (>25% defoliated) and 16 unaffected trees were included in the crown volume calculations, which were estimated as an ellipsoid in the case of the Scots pine [45]. The allometric relationship between crown volume and DBH from the sampled trees for crown parameters was used to approximate the volume for all pines in patches using an exponential regression model: [undamaged pine volume = 10.529588 × EXP(0.068715 × DBH)] and [damaged pine volume = 3.85498 × EXP(0.09189 × DBH)]. The exponential model was found to best explain the relationship between crown volume and DBH (R2 = 0.525 and R2 = 0.605 for unaffected and affected trees, respectively). A total of 82 pine tree measurements from the ref. [34] study were included in our calculations. We then estimated the total pine crown volume per ha in the stands.
2.4. Data Analyses
We utilized Bayesian generalized linear mixed-effects models (GLMMs) and Bayesian linear mixed-effects models (LMERs) in R 4.4.1. [46] library brms [47] to analyze the effects of vegetation parameters (total pine canopy cover, proportion of dead and fully defoliated pine trees, and total pine canopy volume) and sawfly larval biomass on Flycatcher parameters (clutch size, proportion of fledglings, and body mass and tarsus length of fledglings). Separate models with one fixed factor and one dependent variable were implemented for each combination of vegetation parameters/larval biomass: canopy cover, dead and fully defoliated trees, canopy volume, and larval biomass; and Flycatcher parameters: clutch size (Poisson GLMM), proportion of fledglings (binary logistic GLMM), body mass (LMER), and tarsus length (LMER). To account for pseudoreplication in all models, the ‘plot ID’ was set as a random factor. ‘Nest ID’ was added as a nested random factor within ‘plot ID’ for models, including body mass and tarsus length. For each of the four chains, the number of iterations was set to 2500. We used Rhat values (all close to 1) to evaluate the convergence of the models. p values for the models were calculated with R library bayestestR [48] function p_map. Spearman correlation analysis was used to assess relationships between vegetation parameters (canopy cover, dead and fully defoliated trees, and canopy volume) and larval biomass.
2.5. Use of Artificial Intelligence
We used ChatGPT via https://openai.com/ to aid in the generation of our Graphical Abstract. The image is original and unpublished elsewhere.
3. Results
Flycatchers occupied 25 boxes out of 62 (40.3%) in the undamaged forest and 30 boxes out of 72 (41.7%) in the damaged forest (Fisher’s exact test, p > 0.05). Offspring fledged in all these occupied nest boxes (Fisher’s exact test, p > 0.05).
3.1. Larval Biomass and Vegetation Parameters
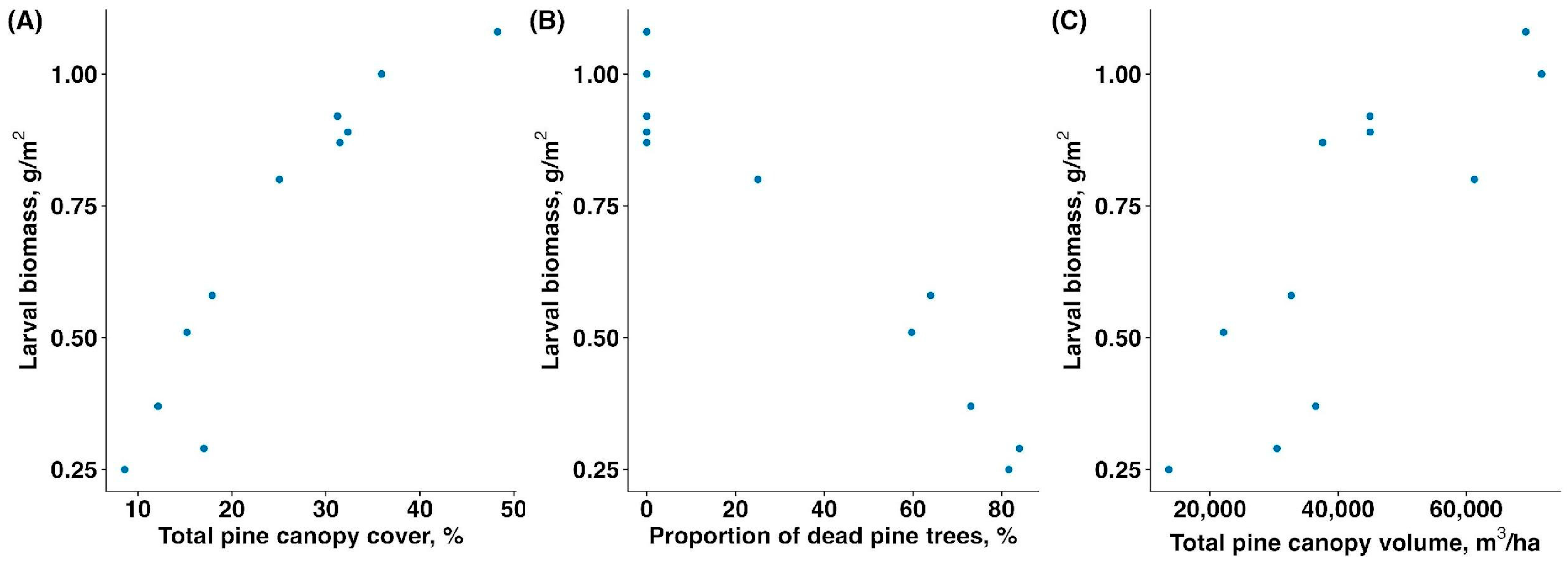
The biomass of sawfly larvae was significantly associated with all three vegetation parameters, and, thus, the sawfly outbreak. The mass of larvae increased with total pine canopy cover (rs = 0.945, p < 0.001 Figure 2A), decreased with proportion of dead and fully defoliated pine trees (rs = −0.934, p < 0.001, Figure 2B), and increased with total pine canopy volume (rs = 0.882, p = 0.001, Figure 2C).
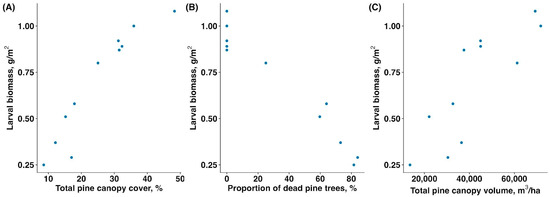
Figure 2.
Relationship between larval sawfly biomass (g/m2) and (A) total pine canopy cover (%), (B) proportion of dead and fully defoliated pine trees (%), and (C) total pine canopy volume (m3/ha).
3.2. Clutch Size
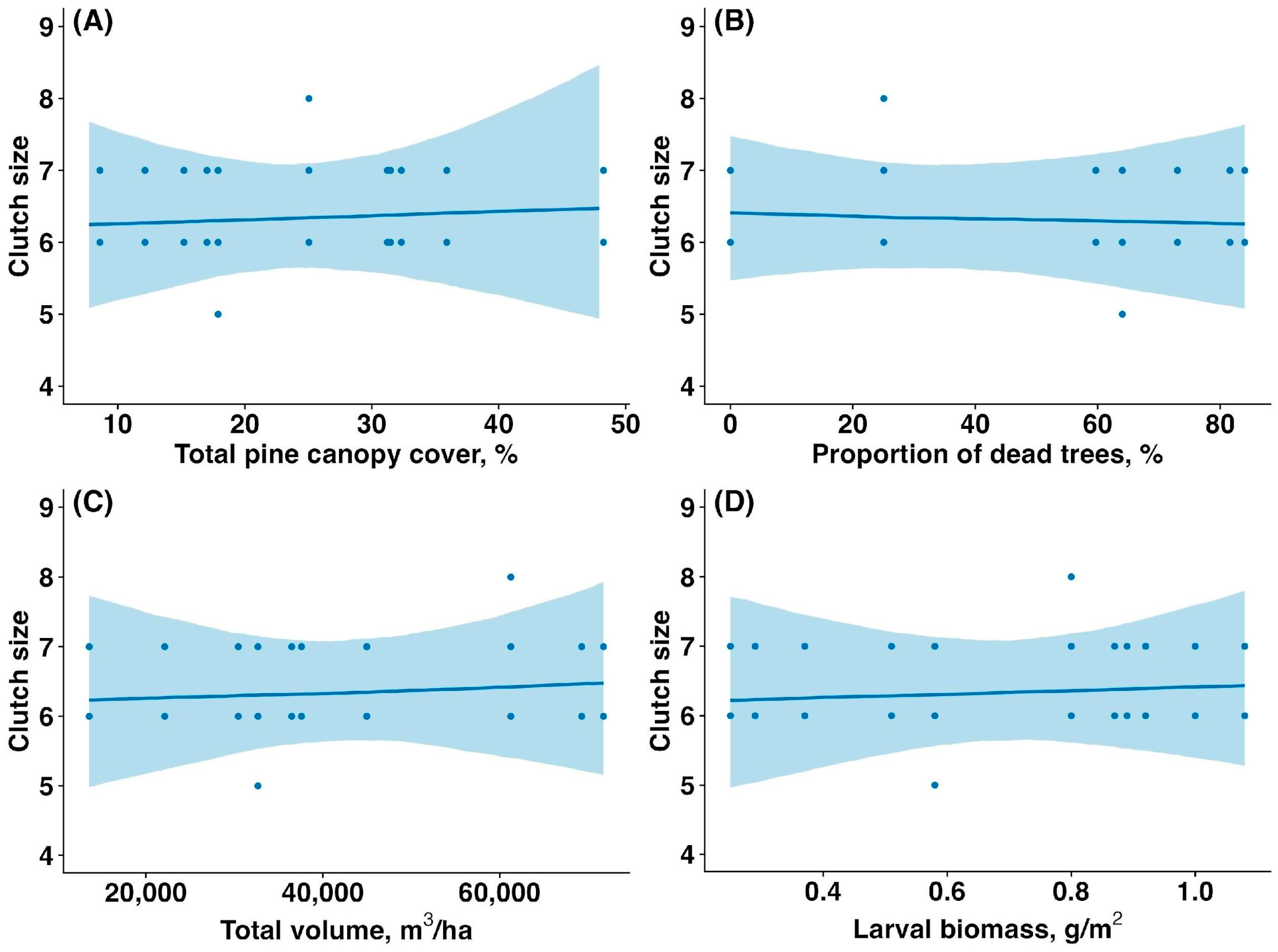
The average clutch size (the number of eggs) did not differ significantly between the unaffected and affected forest patches (6.33 ± 0.62 vs. 6.4 ± 0.5, mean ± SD; t = 0.44, p = 0.66). The clutch size of Pied Flycatchers was not significantly associated with the sawfly outbreak. Clutch size was not influenced by total pine canopy cover (slope estimate: 0, confidence interval (CI): (−0.01,0.01), p = 0.962, Figure 3A), proportion of dead and fully defoliated pine trees (estimate: 0, CI: (0,0), p = 0.984, Figure 3B), total pine canopy volume (estimate: 0.01, CI: (−0.11,0.13), p = 0.997, Figure 3C), or sawfly larval biomass (estimate: 0.01, CI: (−0.10,0.12), p = 0.977, Figure 3D).
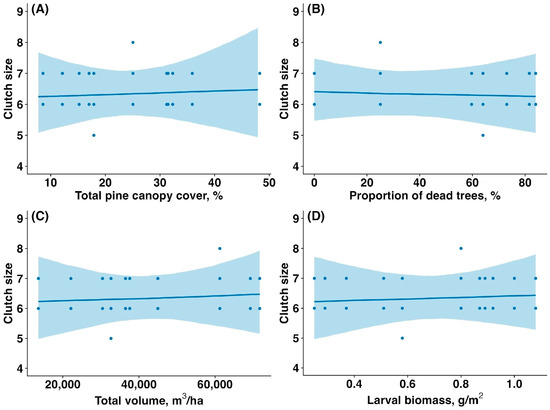
Figure 3.
Relationship between the clutch size of Pied Flycatchers and (A) total pine canopy cover (%), (B) proportion of dead and fully defoliated pine trees (%), (C) total pine canopy volume (m3/ha), and (D) sawfly larval biomass (g/m2). Solid lines represent model-predicted trends based on Poisson generalized linear mixed models (GLMMs), which are appropriate for count data, such as clutch size. The curved nature of some trend lines reflects the link function used in GLMMs. Shaded areas indicate 95% confidence intervals.
3.3. Fledglings
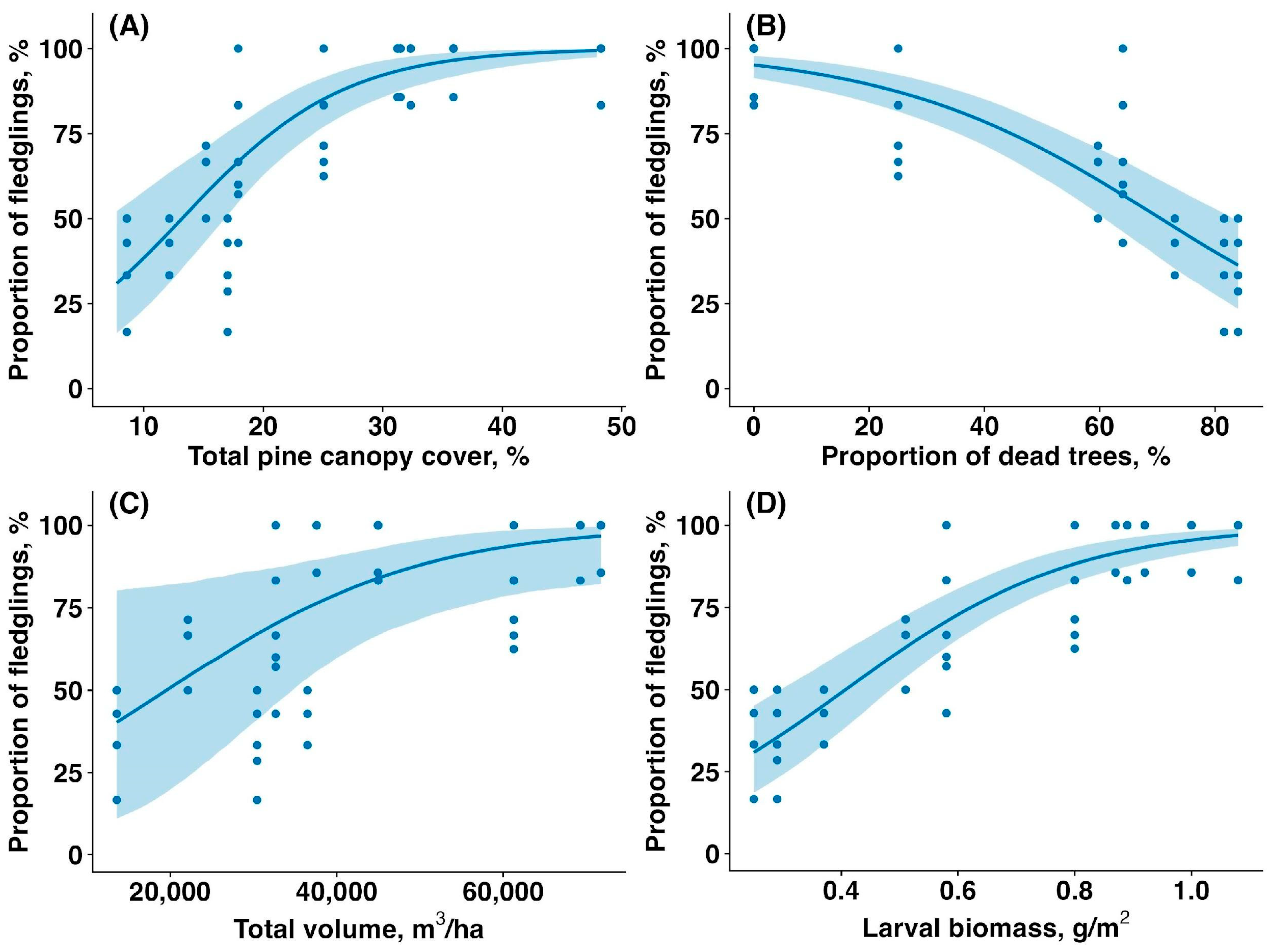
The average number of fledglings per nest was significantly higher in the unaffected forest compared to the affected forest (6.16 ± 0.62 vs. 3.57 ± 1.36, mean ± SD; 42% reduction in the affected patches; t = 8.80, p < 0.00001), indicating a strong negative impact of the sawfly outbreak on reproductive success. The proportion of fledglings increased with total canopy cover (estimate: 0.15, CI: (0.10,0.20), p < 0.001, Figure 4A), decreased with proportion of dead, fully defoliated trees (estimate: −0.04, CI: (−0.06,−0.03), p < 0.001, Figure 4B), tended to increase with total canopy volume (estimate: 1.19, CI: (0.20,2.16), p = 0.060, Figure 4C), and increased with larval biomass (estimate: 1.44, CI: (1.05,1.90), p < 0.001, Figure 4D).
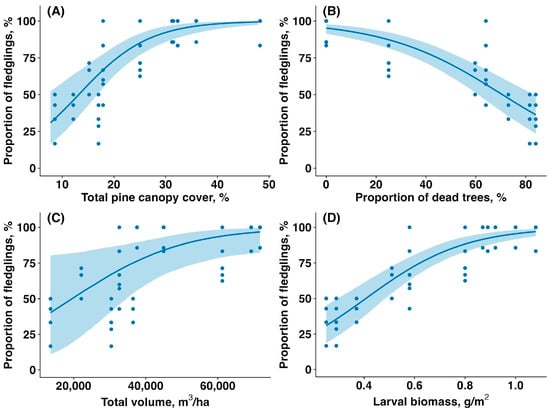
Figure 4.
Relationship between the proportion of young fledglings per Pied Flycatcher clutch (%) and (A) total pine canopy cover (%), (B) proportion of and fully defoliated pine trees (%), (C) total pine canopy volume (m3/ha), and (D) sawfly larval biomass (g/m2). Solid lines are trendlines estimated by the model, and shaded areas show 95% confidence intervals.
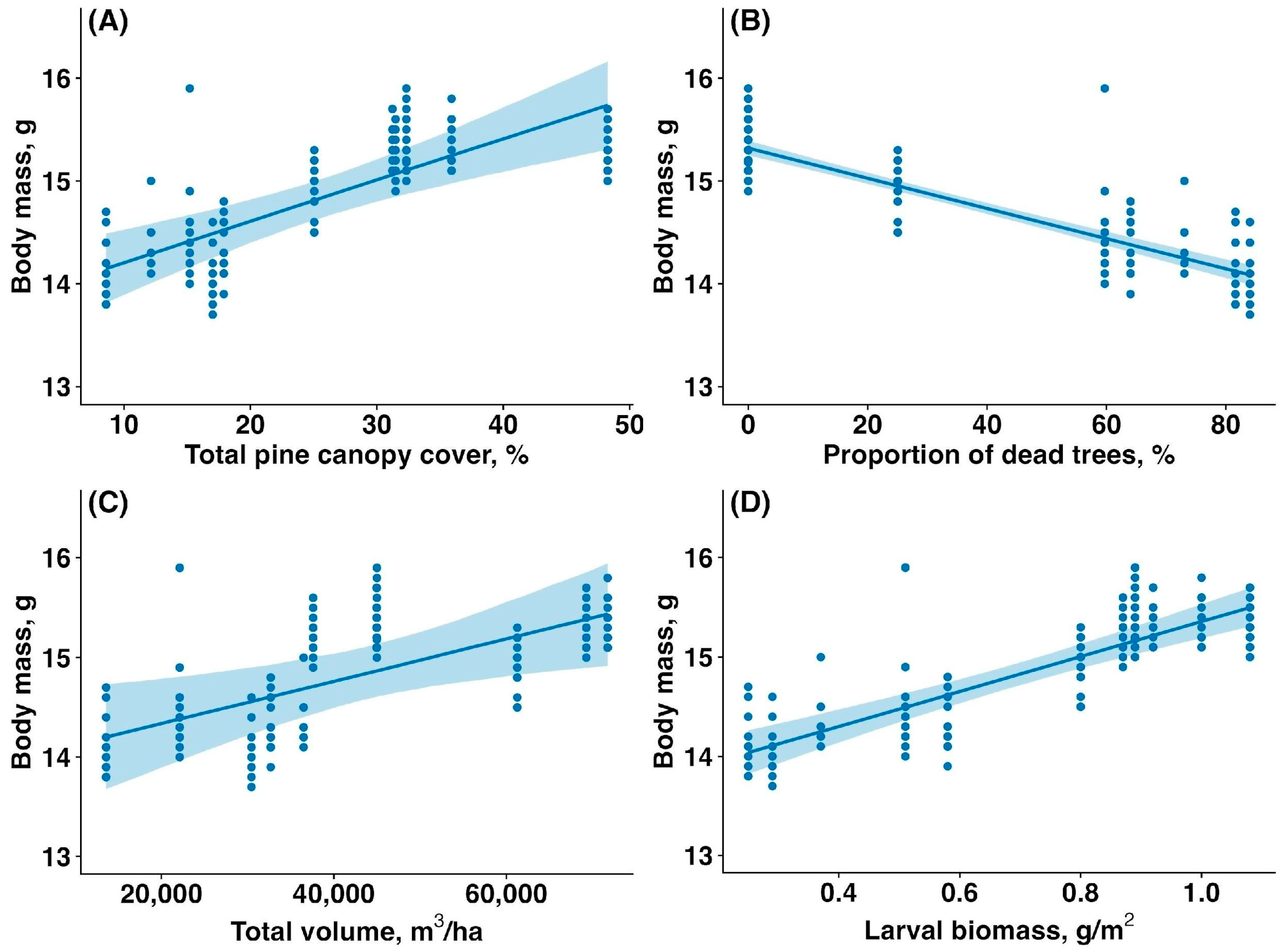
3.4. Fledgling Body Mass
The average body mass of fledglings was significantly higher in the unaffected forest compared to the affected forest (15.32 ± 0.22 g vs. 14.42 ± 0.43 g, mean ± SD; t = 17.77, p < 0.00001). The body mass of fledglings was reduced by the sawfly outbreak. The body mass of fledglings increased with total canopy cover (estimate: 0.04, CI: (0.02, 0.06), p < 0.001, Figure 5A), decreased with the proportion of dead, fully defoliated trees (estimate: −0.01, CI: (−0.02,−0.01), p < 0.001, Figure 5B), increased with total canopy volume (estimate: 0.38, CI: (0.10,0.66), p = 0.019, Figure 5C), and increased with larval biomass (estimate: 0.47, CI: (0.36,0.58), p < 0.001, Figure 5D).
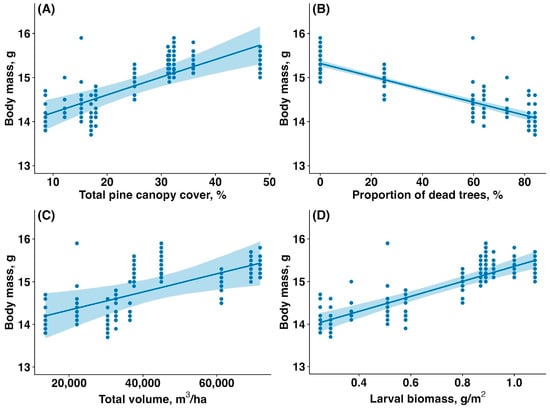
Figure 5.
Relationship between the body mass of Pied Flycatcher fledglings (g) and (A) total pine canopy cover (%), (B) proportion of dead and fully defoliated pine trees (%), (C) total pine canopy volume (m3/ha), and (D) sawfly larval biomass (g/m2). Solid lines are trendlines estimated by the model, and shaded areas show 95% confidence intervals.
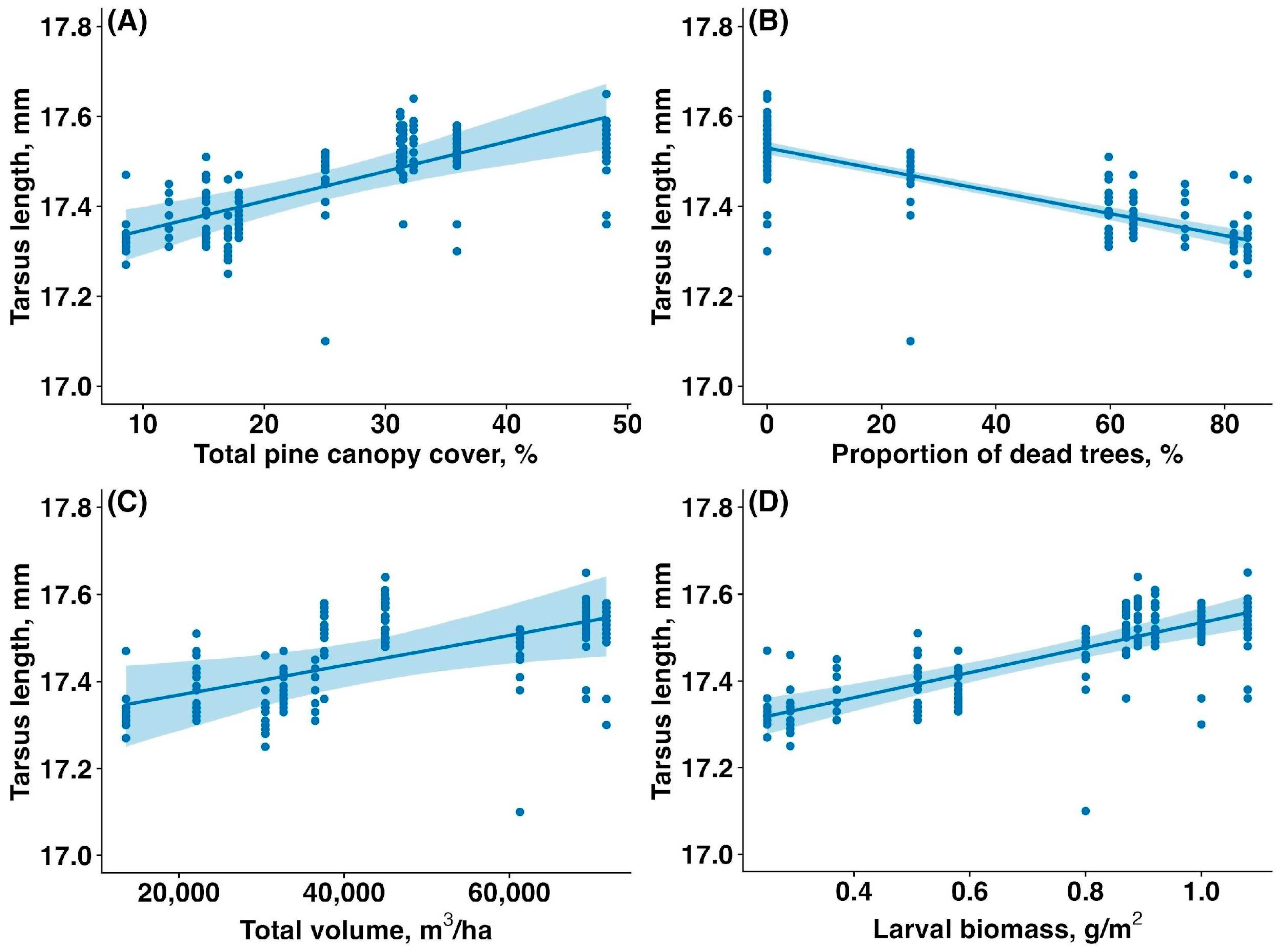
3.5. Fledgling Tarsus Length
The average tarsus length of fledglings was significantly higher in the unaffected forest compared to the affected forest (17.53 ± 0.06 mm vs. 17.38 ± 0.08 mm, mean ± SD; t = 14.94, p < 0.00001). The tarsus length of fledglings was reduced by the sawfly outbreak. The tarsus length of fledglings increased with total canopy cover (estimate: 0.01, CI: (0.00,0.01), p < 0.001, Figure 6A), decreased with the proportion of dead and fully defoliated trees (estimate: −0.002, CI: (−0.003,−0.002), p < 0.001, Figure 6B), increased with total canopy volume (estimate: 0.06, CI: (0.01,0.11), p = 0.041, Figure 6C), and increased with larval biomass (estimate: 0.08, CI: (0.05,0.10), p < 0.001, Figure 6D).
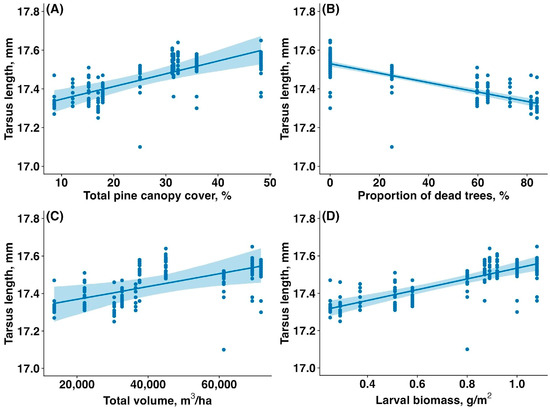
Figure 6.
Relationship between the tarsus length of Pied Flycatcher fledglings (mm) and (A) total pine canopy cover (%), (B) proportion of dead and fully defoliated pine trees (%), (C) total pine canopy volume (m3/ha), and (D) sawfly larval biomass (g/m2). Solid lines are trendlines estimated by the model, and shaded areas show 95% confidence intervals.
4. Discussion
Our findings show that European Pied Flycatchers selected nest boxes equally in both degraded and unaffected forest patches, indicating that nest box availability—rather than habitat quality—was the primary cue guiding settlement decisions. Importantly, it is not the nest boxes themselves that created the ecological trap, but the surrounding degraded habitat, which appeared superficially suitable, yet led to reduced fledgling success. This mismatch between apparent and actual habitat quality fulfills the criteria of an ecological trap, where maladaptive habitat selection occurs despite consistent cue reliability [1,9]. Our study highlights the need to assess the ecological context of conservation interventions, such as nest box provisioning, particularly in landscapes affected by long-term disturbances. These findings also underscore how extended pest outbreaks can alter both the perceived and functional quality of breeding habitats. Although larval abundance could be lower in the later phase of the outbreak we studied, cumulative defoliation likely impaired forest productivity and microclimatic conditions, reducing food availability and affecting nest success. While both Great Tits and European Pied Flycatchers primarily rely on Lepidoptera larvae to feed their nestlings [41], defoliation by sawflies likely suppressed the availability of these preferred prey. Although sawflies were still available to Flycatchers in our study, they may represent a lower-quality or less-preferred food source. This dietary shift could explain why fledglings from damaged patches still performed worse, despite apparent food availability. Thus, indirect effects of pest outbreaks—via reduced abundance of high-quality prey—may significantly impair reproductive success in insectivorous birds. Future studies should consider both temporal outbreak dynamics and structural forest degradation when evaluating habitat suitability and avian reproductive outcomes.
Overall, we found that providing nest boxes to attract migrating Flycatchers as a means of biological control resulted in an ecological trap for pairs choosing nest boxes in patches of pest-disturbed forest. The rapid environmental change (i.e., the outbreak) resulted in the severe deterioration of forest habitat quality that, in turn, negatively impacted the offspring of Flycatchers that had chosen to nest in pest-affected patches. Compared to the pairs that had nested in undamaged forest, ecologically trapped offspring were less likely to have successfully fledged, and they had a lower body mass and tarsus length. As predicted, clutch size was not associated with any of the estimated vegetation parameters or larval biomass, suggesting that nesting site selection is largely based on availability and breeding suitability. It is evident that pairs were not able to differentiate forest quality upon arrival, as all nest boxes were filled regardless of if the box was in the undamaged or damaged part of the forest. The cascading effect of the sawfly larvae deteriorating the quality of the forest, which would disrupt the established ecosystem processes of the area, decreased the fitness of Flycatchers residing in the outbreak-affected habitat.
One possible explanation for this lack of discrimination between the habitats is that degraded Scots pines, having lost most of their needles, visually resembled leafless deciduous trees during the early breeding season. Since deciduous trees in southeastern Latvia do not typically leaf out until the second week of May, and Flycatchers arrive and settle by late April, the birds may have been unable to visually distinguish between affected and unaffected patches. This may explain why nest boxes in defoliated stands were occupied at similar rates as those in healthy forest, supporting the presence of an ecological trap.
These cascading effects have been observed in other systems similar to this one. For instance, the leaf-eating autumnal moth (Epirrita autumnata) is a common outbreaking pest species in birch (Betula pendula)-dominated forests [49]. Brambling (Fringilla montifringilla) responds positively to this, even exhibiting breeding nomadism during outbreaks, because the moth larvae greatly increase the amount of food available to adult birds and their fledglings [50]. However, the larvae greatly deteriorate the overall quality of the birch habitat through their overgrazing of birch leaves, twigs, and seeds. This results in a cascading effect that ultimately harms other arthropods reliant on birch trees, which in turn harms bird species reliant on birch trees and these other arthropods for resources [51]. Moreover, the relationship between the fitness of birds and outbreaking insect pest species is dependent on numerous organismal and ecological factors, making it difficult for conservationists to account for all possible scenarios when combatting outbreaks with biological control methods.
Although the sawflies were in different outbreak phases when comparing [28] (non-larval stage; conducted in 2019) and this study (larval stage; conducted in 2020), the three vegetation measurements we took were also very similar. In the larval stage, sawfly populations persist steadily until early June, when they migrate to the forest floor for pupation and no longer become a reliable food source. As a result, the amount of food available to Flycatchers from sawflies ad libitum decreases sharply in a matter of days following this larval transition. This means that exploiting sawflies as a reliable food source is no longer viable about a week before the Flycatchers actually fledge their nests, leading to a situation where this study and [28] show similar trends for fledgling success. In both studies, the nestlings suffered from starvation, but the specific causes of starvation were different. In [28], the Great Tits did not have the sawflies available as a food source, but the outbreak damaged forest patches to such an extent that overall resource availability was poor since overall foraging substrate was impacted by the cascading effects of the larvae overconsuming tree matter and indirectly decreasing the abundances of other insect species. Interestingly, even with the sawflies available for the Flycatchers, the fledglings from damaged patches still suffered compared to those from undamaged forest patches.
Perceived resource availability is a key factor influencing habitat selection in many forest-dwelling species. For instance, brown bears (Ursus arctos) prefer berry-rich areas, Indigo Buntings (Passerina cyanea) select disturbed forest edges, and Sage Sparrows (Amphispiza belli) nest in dense vegetation [52,53,54]. Yet, in each case, individuals choosing seemingly resource-rich sites experienced reduced fitness, primarily due to increased predation risk, illustrating how misleading environmental cues can result in ecological traps. These examples parallel our findings in Pied Flycatchers, where forest patches degraded by pest outbreaks appeared suitable but ultimately reduced reproductive success. Such cross-taxa patterns underscore how even subtle habitat misjudgments in forest systems can have profound fitness consequences. In our study, the assessment of resource availability differences was not possible, as the forest quality appeared similar when migrating Flycatchers arrived. Interestingly, our findings align with those reported for resident Great Tits in a previous study conducted in the same region [25], where birds nesting in pest-affected forest similarly exhibited reduced fledgling body mass and survival. This similarity may reflect the shared ecological niche of the two species during the breeding season—both are cavity-nesting, insectivorous passerines with overlapping dietary preferences and limited foraging ranges while provisioning their young [41,55]. Such parallels suggest that the observed reduction in fitness is more strongly tied to local habitat degradation than migratory status.
Sawfly outbreaks are unpredictable, tending to last for over a decade and causing damage to trees at various levels of severity, depending on which stage of individual development and outbreak the sawflies are in, ref. [56]. Here, we found that larval biomass increased with canopy cover and volume, but decreased with dead and fully defoliated tree proportion along a gradient of outbreak severity. Larvae existed in the unaffected forest patches—but not at the densities in the affected patches—and did not damage trees to such an extent that fledgling fitness was impacted through overall resource reduction. It is clear that larval fitness is also correlated with vegetation availability as their food source. Larvae grow larger (as measured in terms of their average overall biomass) in undamaged forests with denser vegetation, which leads to the following: at sites with greater estimated larval biomass, fledglings tended to have a higher fitness level compared to those where larval biomass was lower. Undamaged forest patches contributed to larger larvae, which then contributed to fitter fledglings, whereas the opposite trend was evident for affected patches. Although pest larvae may constitute significant parts of avian diets, their overarching negative effects on habitat quality may still result in costs to bird species and even reduce their own numbers through interspecific resource competition and overabundance [57].
From a conservation perspective, our findings suggest that using insectivorous species, such as Pied Flycatchers, for biological control during the peak of a pest outbreak may not be effective. Although the birds actively preyed on larvae, their presence did not prevent further forest degradation, and fledgling success was lower in outbreak-affected patches. Migrating pairs were attracted to nest boxes in degraded habitats, incurring unintended fitness costs—thereby meeting the criteria for an ecological trap. This underscores the importance of assessing not only the potential benefits of avian biological control, but also the associated risks to the control species. In severely defoliated areas, it may be more appropriate to temporarily remove nest boxes or delay their deployment until habitat conditions recover. Nest boxes should be used with caution, especially in outbreak zones, where food availability and forest structure may no longer support successful reproduction. While supplementary feeding could potentially mitigate some of these effects, any such intervention must be carefully evaluated to avoid further unintended consequences.
As climate change and land-use intensification drive rapid ecological change, the risk of creating ecological traps through conservation interventions, such as nest box provision, increases. While nest boxes offer valuable opportunities to study cavity nesters and can support bird populations in degraded habitats, their use must be carefully evaluated, particularly in areas affected by long-term disturbances, like pest outbreaks. Birds attracted to such areas may experience reduced reproductive success, negating the intended benefits of biological control. Therefore, identifying conditions under which nest boxes enhance rather than compromise fitness is critical for minimizing unintended conservation costs and ensuring that efforts to support biodiversity do not inadvertently harm target species.
Author Contributions
Conceptualization, T.K., R.K., I.A.K. and G.B.; Methodology, T.K., R.K., I.A.K. and G.B.; Software, G.T. and D.E.; Validation, T.K., R.K., G.B., L.S., I.D., A.Š. and I.A.K.; Formal Analysis, C.B.A., G.T., T.K., G.B., D.E. and I.A.K.; Investigation, T.K., R.K., G.B., L.S., I.D., A.Š. and I.A.K.; Resources, T.K., R.K., G.B., L.S., I.D., A.Š. and I.A.K.; Data Curation, G.T. and D.E.; Writing—Original Draft Preparation, C.B.A. and I.A.K.; Writing—Review and Editing, T.K., R.K., D.E., G.B., G.T., L.S., I.D. and A.Š.; Visualization, C.B.A., I.A.K., G.T. and D.E.; Supervision, I.A.K. and T.K.; Project Administration, I.A.K. and T.K.; Funding Acquisition, R.K., I.A.K. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Latvian Council of Science (grant lzp-2022/1-0348).
Institutional Review Board Statement
Ethical permission #87 was issued by the Food and Veterinary Service of the Republic of Latvia. Birds were caught and banded under bird banding permit #40 issued to Indrikis A. Krams by the Latvian Ringing Centre.
Data Availability Statement
The data that support the findings of this study are available from the Zenodo repository DOI:10.5281/zenodo.14018198.
Acknowledgments
We thank the Fulbright US Student Program, the Latvian Fulbright Post, and the US Department of State. We used ChatGPT via https://openai.com/ to aid in the generation of our Graphical Abstract. The image is original and unpublished elsewhere.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Schlaepfer, M.A.; Runge, M.C.; Sherman, P.W. Ecological and evolutionary traps. Trends Ecol. Evol. 2002, 17, 474–480. [Google Scholar] [CrossRef]
- Battin, J. When good animals love bad habitats: Ecological traps and the conservation of animal populations. Conserv. Biol. 2004, 18, 1482–1491. [Google Scholar] [CrossRef]
- Hildén, O. Habitat selection in birds: A review. In Annales Zoologici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 1965; Volume 2, pp. 53–75. [Google Scholar]
- Cody, M.L. (Ed.) Habitat Selection in Birds; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Huhta, E.; Jokimäki, J.; Rahko, P. Breeding success of pied flycatchers in artificial forest edges: The effect of a suboptimally shaped foraging area. Auk 1999, 116, 528–535. [Google Scholar] [CrossRef]
- Sherley, R.B.; Ludynia, K.; Dyer, B.M.; Lamont, T.; Makhado, A.B.; Roux, J.P.; Scales, K.L.; Underhill, L.G.; Votier, S.C. Metapopulation tracking juvenile penguins reveals an ecosystem-wide ecological trap. Curr. Biol. 2017, 27, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.K.; Yu, W.S.; Jiang, J.J.; Richards, C.; Siemann, E.; Ma, J.; Li, B.; Ju, R.T. Mismatches between the resources for adult herbivores and their offspring suggest invasive Spartina alterniflora is an ecological trap. J. Ecol. 2020, 108, 719–732. [Google Scholar] [CrossRef]
- Hale, R.; Treml, E.A.; Swearer, S.E. Evaluating the metapopulation consequences of ecological traps. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142930. [Google Scholar] [CrossRef]
- Robertson, B.A.; Hutto, R.L. A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 2006, 87, 1075–1085. [Google Scholar] [CrossRef]
- Hale, R.; Swearer, S.E. Ecological traps: Current evidence and future directions. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152647. [Google Scholar] [CrossRef]
- Hale, R.; Swearer, S.E. When good animals love bad restored habitats: How maladaptive habitat selection can constrain restoration. J. Appl. Ecol. 2017, 54, 1478–1486. [Google Scholar] [CrossRef]
- Richards, D.R.; Belcher, R.N.; Carrasco, L.R.; Edwards, P.J.; Fatichi, S.; Hamel, P.; Masoudi, M.; McDonnell, M.J.; Peleg, N.; Stanley, M.C. Global variation in contributions to human well-being from urban vegetation ecosystem services. One Earth 2022, 5, 522–533. [Google Scholar] [CrossRef]
- Barbosa, P.; Letourneau, D.K.; Agrawal, A.A. (Eds.) Insect Outbreaks Revisited; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Moulinier, J.; Lorenzetti, F.; Bergeron, Y. Effects of a forest tent caterpillar outbreak on the dynamics of mixedwood boreal forests of eastern Canada. Ecoscience 2013, 20, 182–193. [Google Scholar] [CrossRef]
- Vindstad, O.P.L.; Jepsen, J.U.; Ims, R.A. Resistance of a sub-arctic bird community to severe forest damage caused by geometrid moth outbreaks. Eur. J. For. Res. 2015, 134, 725–736. [Google Scholar] [CrossRef][Green Version]
- Daily, G.C.; Matson, P.A. Ecosystem services: From theory to implementation. Proc. Natl. Acad. Sci. USA 2008, 105, 9455–9456. [Google Scholar] [CrossRef] [PubMed]
- Swinton, S.M.; Lupi, F.; Robertson, G.P.; Hamilton, S.K. Ecosystem services and agriculture: Cultivating agricultural ecosystems for diverse benefits. Ecol. Econ. 2007, 64, 245–252. [Google Scholar] [CrossRef]
- Tscharntke, T.; Clough, Y.; Wanger, T.C.; Jackson, L.; Motzke, I.; Perfecto, I.; Vandermeer, J.; Whitbread, A. Global food security, biodiversity conservation and the future of agricultural intensification. Biol. Conserv. 2012, 151, 53–59. [Google Scholar] [CrossRef]
- Langelier, L.A.; Garton, E.O. Management Guidelines for Increasing Populations of Birds That Feed on Western Spruce Budworm (No. 653); US Department of Agriculture, Forest Service, Cooperative State Research Service: Washington, DC, USA, 1986. [Google Scholar]
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Ulyshen, M.D.; Van Driesche, R.G. Population dynamics of an invasive forest insect and associated natural enemies in the aftermath of invasion: Implications for biological control. J. Appl. Ecol. 2015, 52, 1246–1254. [Google Scholar] [CrossRef]
- Mols, C.M.; Visser, M.E. Great tits can reduce caterpillar damage in apple orchards. J. Appl. Ecol. 2002, 39, 888–899. [Google Scholar] [CrossRef]
- Mols, C.M.; Visser, M.E. Great tits (Parus major) reduce caterpillar damage in commercial apple orchards. PLoS ONE 2007, 2, e202. [Google Scholar] [CrossRef]
- Mols, C.M.; Van Noordwijk, A.J.; Visser, M.E. Assessing the reduction of caterpillar numbers by Great Tits Parus major breeding in apple orchards. Ardea 2005, 93, 259. [Google Scholar]
- Wesoowski, T. Reports from nestbox studies: A review of inadequacies. Acta Ornithol. 2011, 46, 13–17. [Google Scholar] [CrossRef]
- Maziarz, M.; Wesołowski, T.; Hebda, G.; Cholewa, M.; Broughton, R.K. Breeding success of the Great Tit Parus major in relation to attributes of natural nest cavities in a primeval forest. J. Ornithol. 2016, 157, 343–354. [Google Scholar] [CrossRef]
- Mänd, R.; Tilgar, V.; Lõhmus, A.; Leivits, A. Providing nest boxes for hole-nesting birds–Does habitat matter? Biodivers. Conserv. 2005, 14, 1823–1840. [Google Scholar] [CrossRef]
- Kilgas, P.; Tilgar, V.; Mägi, M.; Mänd, R. Physiological condition of incubating and brood rearing female Great Tits Parus major in two contrasting habitats. Acta Ornithol. 2007, 42, 129–136. [Google Scholar] [CrossRef]
- Krams, R.; Krama, T.; Brūmelis, G.; Elferts, D.; Strode, L.; Dauškane, I.; Luoto, S.; Šmits, A.; Krams, I.A. Ecological traps: Evidence of a fitness cost in a cavity-nesting bird. Oecologia 2021, 196, 735–745. [Google Scholar] [CrossRef]
- Lundberg, A.; Alatalo, R.V. The Pied Flycatcher; T. & A. D. Poyser: London, UK, 1992. [Google Scholar]
- Merino, S.; Potti, J. Pied flycatchers prefer to nest in clean nest boxes in an area with detrimental nest ectoparasites. Condor 1995, 97, 828–831. [Google Scholar] [CrossRef]
- Atlegrim, O.; Sjöberg, K. Effects of clear-cutting and selective felling in Swedish boreal coniferous forest: Response of invertebrate taxa eaten by birds. Entomol. Fenn. 1995, 6, 79–90. [Google Scholar] [CrossRef]
- Voolma, K.; Hiiesaar, K.; Williams, I.H.; Ploomi, A.; Jõgar, K. Cold hardiness in the pre-imaginal stages of the great web-spinning pine-sawfly A cantholyda posticalis. Agric. For. Entomol. 2016, 18, 432–436. [Google Scholar] [CrossRef]
- Brichta, J.; Vacek, S.; Vacek, Z.; Cukor, J.; Mikeska, M.; Bílek, L.; Šimůnek, V.; Gallo, J.; Brabec, P.; Štefančík, I. Importance and potential of Scots pine (Pinus sylvestris L.) in 21st century. Cent. Eur. For. J. 2023, 69, 3–20. [Google Scholar] [CrossRef]
- Brūmelis, G.; Dauškane, I.; Elferts, D.; Strode, L.; Krama, T.; Krams, I. Estimates of tree canopy closure and basal area as proxies for tree crown volume at a stand scale. Forests 2020, 11, 1180. [Google Scholar] [CrossRef]
- Yazdanian, M.; Kankaanpää, T.; Merckx, T.; Huikkonen, I.M.; Itämies, J.; Jokimäki, J.; Lehikoinen, A.; Leinonen, R.; Pöyry, J.; Sihvonen, P.; et al. Evidence for bottom-up effects of moth abundance on forest birds in the north-boreal zone alone. Ecol. Lett. 2024, 27, e14467. [Google Scholar] [CrossRef]
- Schebeck, M.; Lehmann, P.; Laparie, M.; Bentz, B.J.; Ragland, G.J.; Battisti, A.; Hahn, D.A. Seasonality of forest insects: Why diapause matters. Trends Ecol. Evol. 2024, 39, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Krama, T.; Vrublevska, J.; Freeberg, T.M.; Kullberg, C.; Rantala, M.J.; Krams, I. You mob my owl, I’ll mob yours: Birds play tit-for-tat game. Sci. Rep. 2012, 2, 800. [Google Scholar] [CrossRef]
- Loukola, O.J.; Seppänen, J.T.; Krams, I.; Torvinen, S.S.; Forsman, J.T. Observed fitness may affect niche overlap in competing species via selective social information use. Am. Nat. 2013, 182, 474–483. [Google Scholar] [CrossRef]
- Dhondt, A.A. Interspecific Competition in Birds; Oxford University Press: Oxford, UK, 2012; Volume 2. [Google Scholar]
- Kilgas, P.; Tilgar, V.; Mänd, R. Hematological health state indices predict local survival in a small passerine bird, the great tit (Parus major). Physiol. Biochem. Zool. 2006, 79, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Rytkönen, S.; Krams, I. Does foraging behaviour explain the poor breeding success of great tits Parus major in northern Europe? J. Avian Biol. 2003, 34, 288–297. [Google Scholar] [CrossRef]
- Rytkönen, S.; Orell, M. Great tits, Parus major, lay too many eggs: Experimental evidence in mid-boreal habitats. Oikos 2001, 93, 439–450. [Google Scholar] [CrossRef]
- Tinbergen, J.M.; Dietz, M.W. Parental energy expenditure during brood rearing in the great tit (Parus major) in relation to body mass, temperature, food availability and clutch size. Funct. Ecol. 1994, 8, 563–572. [Google Scholar] [CrossRef]
- Zandt, H.S. A comparison of three sampling techniques to estimate the population size of caterpillars in trees. Oecologia 1994, 97, 399–406. [Google Scholar] [CrossRef]
- Rautiainen, M.; Mõttus, M.; Stenberg, P.; Ervasti, S. Crown envelope shape measurements and models. Silva Fenn. 2008, 42, 19. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Bürkner, P.C. brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Makowski, D.; Ben-Shachar, M.S.; Lüdecke, D. bayestestR: Describing effects and their uncertainty, existence and significance within the Bayesian framework. J. Open Source Softw. 2019, 4, 1541. [Google Scholar] [CrossRef]
- Enemar, A.; Sjöstrand, B.; Andersson, G.; Von Proschwitz, T. The 37-year dynamics of a subalpine passerine bird community, with special emphasis on the influence of environmental temperature and Epirrita autumnata cycles. Ornis Svec. 2004, 14, 63–106. [Google Scholar] [CrossRef]
- Lindström, Å. Breeding nomadism and site tenacity in the brambling Fringilla montifringilla. Ornis Fenn. 1987, 64, 50–56. [Google Scholar]
- Enemar, A.; Nyström, B. Population fluctuations, food and breeding of the Redpoll Carduelis flammea in a mountain birch forest, Swedish Lapland. Vår Fågelvärld 1981, 40, 409–426. [Google Scholar]
- Lamb, C.T.; Mowat, G.; McLellan, B.N.; Nielsen, S.E.; Boutin, S. Forbidden fruit: Human settlement and abundant fruit create an ecological trap for an apex omnivore. J. Anim. Ecol. 2017, 86, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Misenhelter, M.D.; Rotenberry, J.T. Choices and consequences of habitat occupancy and nest site selection in Sage Sparrows. Ecology 2000, 81, 2892–2901. [Google Scholar] [CrossRef]
- Weldon, A.J.; Haddad, N.M. The effects of patch shape on Indigo Buntings: Evidence for an ecological trap. Ecology 2005, 86, 1422–1431. [Google Scholar] [CrossRef]
- Robinson, S.K.; Holmes, R.T. Foraging behavior of forest birds: The relationships among search tactics, diet, and habitat structure. Ecology 1982, 63, 1918–1931. [Google Scholar] [CrossRef]
- Ghimire, R.P.; Markkanen, J.M.; Kivimaenpaa, M.; Lyytikainen-Saarenmaa, P.; Holopainen, J.K. Needle removal by pine sawfly larvae increases branch-level VOC emissions and reduces below-ground emissions of Scots pine. Environ. Sci. Technol. 2013, 47, 4325–4332. [Google Scholar] [CrossRef]
- Mopper, S.; Whitham, T.G.; Price, P.W. Plant phenotype and interspecific competition between insects determine sawfly performance and density. Ecology 1990, 71, 2135–2144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).