The Migration of the Eurasian Woodcock (Scolopax rusticola L.) in the Carpathian Basin at the Turn of the 19–20th Centuries
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
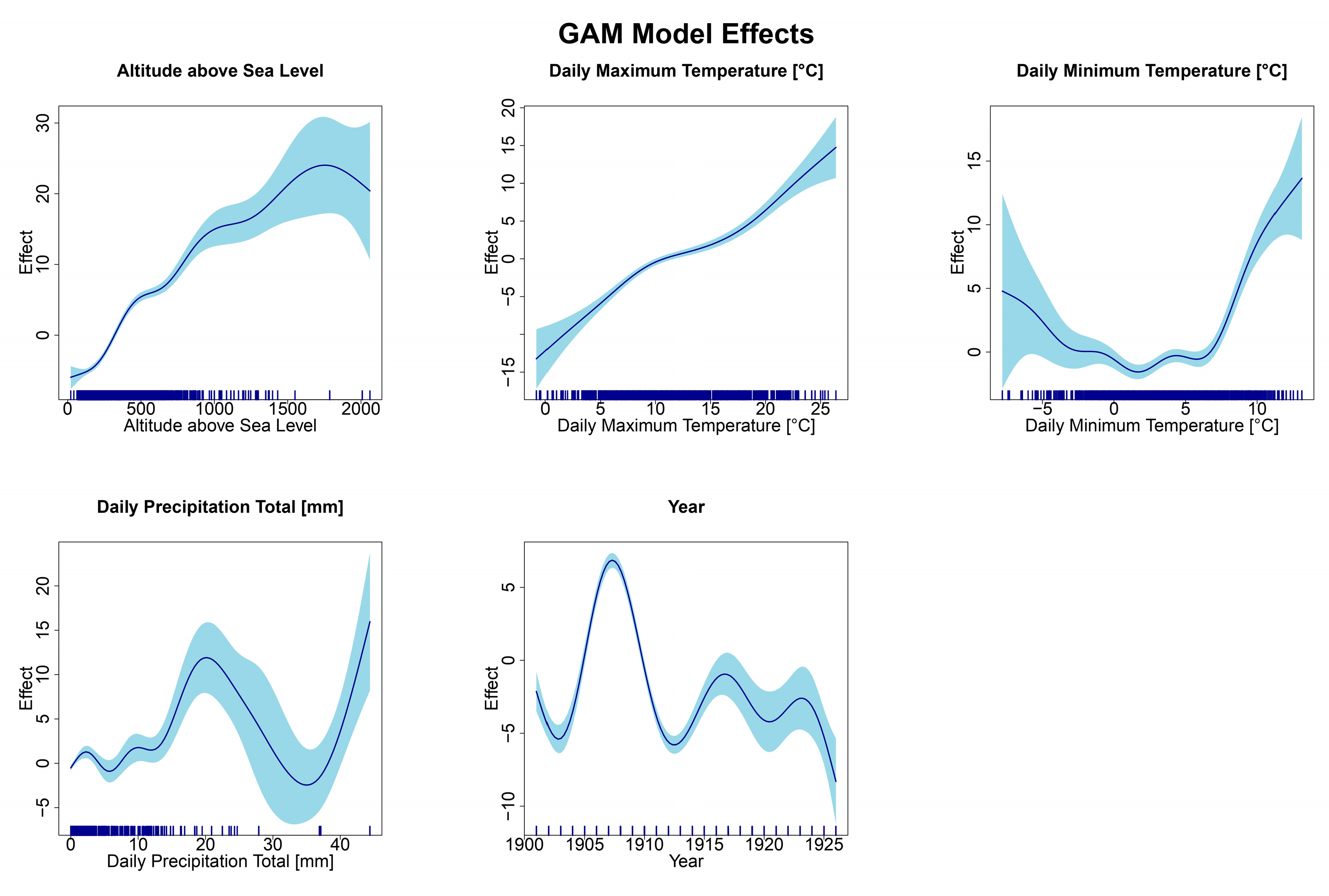
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cramp, S.; Simmons, K.E.L. Handbook of the Birds of Europe, the Middle East and North Africa; Oxford University Press: Oxford, UK, 1983; Volume 3. [Google Scholar]
- Glutz von Blotzheim, U.N. Handbuch der Vögel Mitteleuropas. Band 7. Charadriiformes; AULA-Verlag: Wiesbaden, Germany, 1986; pp. 121–174. [Google Scholar]
- Ericson, P.G.P.; Envall, I.; Irestedt, M.; Norman, J.A. Inter-familial relationships of the shorebirds (Aves: Charadriiformes) based on nuclear DNA sequence data. BMC Evol. Biol. 2003, 3, 16. [Google Scholar] [CrossRef]
- Baker, A.J.; Pereira, S.L.; Paton, T.A. Phylogenetic relationships and divergence times of Charadriiformes genera: Multi-gene evidence for the Cretaceous origin of at least 14 clades of shorebirds. Biol. Lett. 2007, 3, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Fain, M.G.; Houde, P. Multilocus perspectives on the monophyly and phylogeny of the order Charadriiformes. BMC Evol. Biol. 2007, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.; Baker, A. Multiple gene sequences resolve phylogenetic relationships in the shorebird suborder Scolopaci (Aves: Charadriiformes). Mol. Phylogenet. Evol. 2012, 64, 66–72. [Google Scholar] [CrossRef]
- Banks, R.C. Classification and nomenclature of the Sandpipers (Aves: Arenariinae). Zootaxa 2012, 3513, 86–88. [Google Scholar] [CrossRef]
- Barth, J.M.I.; Matschiner, M.; Robertson, B.C. Phylogenetic position and subspecies divergence of the endangered New Zealand Dotterel (Charadrius obscurus). PLoS ONE 2013, 8, e78068. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, J.; Wiersma, P.; Kirwan, G.M. Eurasian Woodcock (Scolopax rusticola), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaka, NY, USA, 2020. [Google Scholar]
- Sørensen, I.H. Eurasian Woodcock. Scolopax rusticola. In European Breeding Bird Atlas 2. Distribution, Abundance and Change; Keller, V., Herrando, S., Voríšek, P., Franch, M., Kipson, M., Milanesi, P., Martí, D., Anton, M., Klvanová, A., et al., Eds.; European Bird Census Council; Lynx Edicions: Barcelona, Spain, 2020; pp. 334–335. [Google Scholar]
- Valkama, J.; Vepsäläinen, V.; Lehikoinen, A. The Third Finnish Breeding Bird Atlas; Finnish Museum of Natural History and Ministry of Environment: Helsinki, Finland, 2011. [Google Scholar]
- Blokhin, Y.Y.; Artemenkov, D.V. Impact of the Hunting Ban on the Population and Hunting Bag of the Eurasian Woodcock Scolopax rusticola (Scolopacidae, Aves) in European Russia. Biol. Bull. 2023, 50, 2615–2622. [Google Scholar] [CrossRef]
- Vönöczky-Schenk, J. Az erdei szalonka fészkelő területei a történelmi Magyarországon—Die Nistareale von Scolopax r. rusticola L. im historischen Ungarn. Aquila 1944, 50, 310–313. [Google Scholar]
- Hadarics, T.; Zalai, T. Magyarország madarainak névjegyzéke. Nomenclator avium Hungariae. In An Annotated List of the Birds of Hungary; Magyar Madártani és Természetvédelmi Egyesület: Budapest, Hungary, 2008; p. 118. [Google Scholar]
- Bende, A.; László, R. Woodcock (Scolopax rusticola L.) nestings in Carpatian Basin from the second half of the 19th century to present days. Ornis Hung. 2020, 28, 92–103. [Google Scholar] [CrossRef]
- Faragó, S. Erdei szalonka. In Magyar Madárvonulási Atlasz; Csörgő, T., Karcza, Z., Halmos, G., Magyar, G., Gyurácz, J., Szép, T., Bankovics, A., Schmidt, A., Schmidt, E., Eds.; Kossuth Kiadó Zrt.: Budapest, Hungary, 2009; pp. 304–305. [Google Scholar]
- Boland, J.M. Leapfrog migration in North American shorebirds: Intra- and interspecific examples. Condor 1990, 92, 284–290. [Google Scholar] [CrossRef]
- Alerstam, T.; Hendenström, A. The development of bird migration theory. J. Avian Biol. 1998, 29, 343–369. [Google Scholar] [CrossRef]
- Swarth, H.S. Revision of the Avian Genus Passerella with Special Reference to the Distribution and Migration of the Races in California; University of California Press: Berkeley, CA, USA, 1920; Volume 21, pp. 75–224. [Google Scholar]
- Pienkowski, M.W. Differences in habitat requirements and distribution patterns of plovers and sandpipers as investigated by studies of feeding behaviour. Verhandlungen Ornithol. Ges. Bayern 1979, 23, 105–124. [Google Scholar]
- Guzmán, J.L.; Ferrand, Y.; Arroyo, B. Origin and migration of Woodcock Scolopax rusticola wintering in Spain. European. J. Wildl. Res. 2011, 57, 647–655. [Google Scholar] [CrossRef]
- Faragó, S. A vadászható vízivadfajok magyarországi vonulása jelölt madarak megkerülése alapján. Magy. Vízivad Közlemények 2000, 6, 337–375. [Google Scholar]
- Spina, F.; Baillie, S.R.; Bairlein, F.; Fiedler, W.; Thorup, K. The Eurasian African Bird Migration Atlas. EURING/CMS, 2022. Available online: https://migrationatlas.org/node/1702 (accessed on 31 August 2024).
- Schally, G. Woodcock ringing in Hungary between 1913 and 2014. WI/IUCN-WSSG Newsl. 2015, 41, 33–36. [Google Scholar]
- Schally, G.; Csányi, S.; Palatitz, P. Spring migration phenology of Eurasian Woodcocks tagged with GPS-Argos transmitters in Central Europe. Ornis Fenn. 2022, 99, 104–116. [Google Scholar] [CrossRef]
- Schally, G. Erdei szalonka gyűrűzés Magyarországon 1913 és 2015 között. Vadbiológia 2017, 19, 77–86. [Google Scholar]
- Tryjanowski, P.; Kuźniak, S.; Sparks, T. Earlier arrival of some farmland migrants in western Poland. Ibis 2002, 144, 62–68. [Google Scholar] [CrossRef]
- Gordo, O. Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Climate Res. 2007, 35, 37–58. [Google Scholar] [CrossRef]
- Kullberg, C.; Fransson, T.; Hedlund, J.; Jonzén, N.; Langvall, O.; Nilsson, J.; Bolmgren, K. Change in spring arrival of migratory birds under an era of climate change, Swedish data from the last 140 years. Ambio 2015, 44, 69–77. [Google Scholar] [CrossRef]
- Mason, C.F. Long-term trends in the arrival dates of spring migrants. Bird Study 1995, 42, 182–189. [Google Scholar] [CrossRef]
- Sparks, T.H. Phenology and the changing pattern of bird migration in Britain. Int. J. Biometeorol. 1999, 42, 134–138. [Google Scholar] [CrossRef]
- Marra, P.P.; Francis, C.M.; Mulvihill, R.S.; Moore, F.R. The influence of climate on the timing and rate of spring bird migration. Oecologia 2005, 142, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Fiedler, W.; Berthold, P. Effects of Climate Change on Birds; OUP: Oxford, UK, 2010. [Google Scholar]
- Gill, J.A.; Alves, J.A.; Sutherland, W.J.; Appleton, G.F.; Potts, P.M.; Gunnarsson, T.G. Why is timing of bird migration advancing when individuals are not? Proc. R. Soc. Lond. B. Biol. Sci. 2014, 281, 20132161. [Google Scholar] [CrossRef] [PubMed]
- Lehikoinen, E.; Sparks, T.H. Changes in migration. Eff. Clim. Change Birds 2010, 1, 89–112. [Google Scholar]
- Kolářová, E.; Matiu, M.; Menzel, A.; Nekovář, J.; Lumpe, P.; Adamík, P. Changes in spring arrival dates and temperature sensitivity of migratory birds over two centuries. Int. J. Biometeorol. 2017, 61, 1279–1289. [Google Scholar] [CrossRef]
- Vitale, J.; Schlesinger, W.H. Historical analysis of the spring arrival of migratory birds to Dutchess County, New York: A 123-year record. Northeast. Nat. 2011, 18, 335–346. [Google Scholar] [CrossRef]
- Travers, S.E.; Marquardt, B.; Zerr, N.J.; Finch, J.B.; Boche, M.J.; Wilk, R.; Burdick, S.C. Climate change and shifting arrival date of migratory birds over a century in the northern Great Plains. Wilson J. Ornithol. 2015, 127, 43–51. [Google Scholar] [CrossRef]
- Marja, R.; Elts, J. The Eurasian Woodcock (Scolopax rusticola) is arriving in Estonia earlier than hundred years ago. Hirundo 2022, 35, 17–27. [Google Scholar]
- Bairlein, F.; Dierschke, J.; Dierschke, V.; Salewski, V.; Geiter, O.; Hüppop, K.; Köppen, U.; Fiedler, W. Atlas des Vogelzugs. Ringfunde deutscher Brut- und Gastvögel; Aula Verlag: Wiebelsheim, Germany, 2014; pp. 221–222. [Google Scholar]
- Haraszthy, L. A Magyar Madártani és Természetvédelmi Egyesület első 50 éve; Magyar Madártani és Természetvédelmi Egyesület: Budapest, Hungary, 2014. [Google Scholar]
- Hungarian Ornithological Centre A madárvonulás Magyarországon az 1894. év tavaszán. Aquila 1895, 2, 3–84.
- Gaal, G. A madárvonulás Magyarországon az 1895. év tavaszán. Aquila 1896, 3, 7–116. [Google Scholar]
- Gaal, G. A madárvonulás Magyarországon az 1896. év tavaszán. Aquila 1897, 4, 44–104. [Google Scholar]
- Gaal, G. A madárvonulás Magyarországon az 1897. év tavaszán. Aquila 1898, 5, 226–279. [Google Scholar]
- Schenk, J. A madárvonulás Magyarországon az 1898. év tavaszán. Aquila 1899, 6, 168–251. [Google Scholar]
- Schenk, J. A madárvonulás Magyarországon az 1899. év tavaszán. Aquila 1901, 8, 50–122. [Google Scholar]
- Schenk, J. A madárvonulás Magyarországon az 1903. év tavaszán. Aquila 1905, 12, 83–202. [Google Scholar]
- Schenk, J. A madárvonulás Magyarországon az 1904. év tavaszán. Aquila 1906, 13, 9–66. [Google Scholar]
- Schenk, J. A madárvonulás Magyarországon az 1906. év tavaszán. Aquila 1907, 14, 1–119. [Google Scholar]
- Schenk, J. A madárvonulás Magyarországon az 1907. év tavaszán. Aquila 1908, 15, 1–152. [Google Scholar]
- Schenk, J. A madárvonulás Magyarországon az 1908. év tavaszán. Aquila 1909, 16, 1–128. [Google Scholar]
- Schenk, J. A madárvonulás Magyarországon az 1913. év tavaszán. Aquila 1914, 21, 137–187. [Google Scholar]
- Schenk, J. A madárvonulás Magyarországon az 1914. év tavaszán. Aquila 1915, 22, 5–56. [Google Scholar]
- Schenk, J. A madárvonulás Magyarországon az 1915 és 1916 tavaszán. Aquila 1916, 23, 13–49. [Google Scholar]
- Schenk, J. Madárvonulási adatok Magyarországból. Aquila 1919, 26, 46–75. [Google Scholar]
- Schenk, J. Madárvonulási adatok Magyarországból. Aquila 1920, 27, 39–55. [Google Scholar]
- Schenk, J. Madárvonulási adatok Magyarországból. Aquila 1921, 28, 97–126. [Google Scholar]
- Vezényi, Á. A madárvonulás Magyarországon az 1900. év tavaszán. Aquila 1902, 9, 81–155. [Google Scholar]
- Vezényi, Á. A madárvonulás Magyarországon az 1901. év tavaszán. Aquila 1903, 10, 104–187. [Google Scholar]
- Vezényi, Á. A madárvonulás Magyarországon az 1902. év tavaszán. Aquila 1905, 12, 1–77. [Google Scholar]
- Greschik, J. A madárvonulás Magyarországon az 1909. év tavaszán. Aquila 1910, 17, 1–129. [Google Scholar]
- Lambrecht, K. A madárvonulás Magyarországon az 1910. év tavaszán. Aquila 1911, 18, 9–134. [Google Scholar]
- Lambrecht, K. A madárvonulás Magyarországon az 1911. év tavaszán. Aquila 1912, 19, 43–165. [Google Scholar]
- Lambrecht, K. A madárvonulás Magyarországon az 1912. év tavaszán. Aquila 1913, 20, 16–145. [Google Scholar]
- Hegyfoky, K. Az 1899–1916. évi tavaszi madárvonulás vidékenkint. Aquila 1917, 24, 107–111. [Google Scholar]
- Warga, K. Madárvonulási adatok Magyarországból. Aquila 1922, 29, 91–131. [Google Scholar]
- Warga, K. Madárvonulási adatok Magyarországból. Aquila 1924, 30–31, 179–237. [Google Scholar]
- Warga, K. Madárvonulási adatok Magyarországból. Aquila 1926, 32–33, 66–127. [Google Scholar]
- Warga, K. Madárvonulási adatok Magyarországból. Aquila 1928, 34–35, 257–305. [Google Scholar]
- Faragó, S. A tavaszi erdei szalonka vadászat kialakulásának története és fenntartásának indokai Magyarországon. Magy. Vízivad Közlemények 2013, 23, 311–332. [Google Scholar]
- Bende, A. Az Erdei Szalonka (Scolopax rusticola L.) Tavaszi Vonulásdinamikája, Kor-, Ivarviszonyai és Költésbiológiája Magyarországon. Ph.D. Thesis, Soproni Egyetem, Sopron, Hungary, 2021. [Google Scholar]
- Url. 1.: HungaroMet Nonprofit Zrt. Meteorological Databank. Available online: https://www.met.hu/rolunk/tevekenysegek/adattar/ (accessed on 15 April 2024).
- Schenk, J. Az erdei szalonka tavaszi vonulásának prognózisa Magyarországon—Die Prognose des Frühjahrszuges der Waldschnepfe in Ungarn. Aquila 1930, 36–37, 33–44. [Google Scholar]
- Clausager, I. Migration of Scandinavian Woodcock (Scolopax rusticola) with special reference to Denmark. Dan. Rev. Game Biol. 1974, 8, 38. [Google Scholar]
- Bettmann, H. Die Waldschnepfe, 2. überarbeitete Auflage; BLV Verlagsgesellschaft: München, Germany, 1975; p. 110. [Google Scholar]
- Moritz, D.; Nemetschek, G. Der Zug der Waldschnepfe auf Helgoland. Corax 1976, 5, 176–191. [Google Scholar]
- Spina, F.; Volponi, S. Atlante Della Migrazione Degli Uccelli in Italia; I. non-passeriformi; ISPRA: Roma, Italy, 2008; pp. 514–521.
- Haraszthy, L. Erdei szalonka Scolopax rusticola. In Magyarország Fészkelő Madarainak Költésbiológiája; 1. kötet. Fácánféléktől a sólyomfélékig (Non-Passeriformes); Haraszthy, L., Ed.; Pro Vértes Nonprofit Zrt.: Csákvár, Hungary, 2019; pp. 508–512. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 31 August 2024).
- RStudio Tea RStudio: Integrated Development Environment for R. RStudio, PBC. Available online: https://www.rstudio.com/ (accessed on 31 August 2024).
- Wood, S.N. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Statist. Ass. 2004, 99, 673–686. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. B 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Wood, S.N. A simple test for random effects in regression models. Biometrika 2013, 100, 1005–1010. [Google Scholar] [CrossRef]
- Wood, S.N. On p-values for smooth components of an extended generalized additive model. Biometrika 2013, 100, 221–228. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: New York, NY, USA, 2017. [Google Scholar]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; Chapman and Hall/CRC: Boca Raton, FL, USA, 1990. [Google Scholar]
- Bende, A.; Faragó, S.; László, R. Variations in the spring migration of Eurasian Woodcock (Scolopax rusticola L.) in Hungary. Ornis Hung. 2022, 31, 133–146. [Google Scholar] [CrossRef]
- Kralj, J.; Barišić, S.; Ćiković, D.; Tutiš, V. Atlas Selibde Ptica Hrvatske; Croatian Academy of Sciences and Arts: Zagreb, Croatia, 2013; pp. 97–98. [Google Scholar]
- Stadie, R. Groß-Wetterlage und Frühjahrsschnepfenzug 1934 im Reich. Ver. Schlesischer Ornithol. 1938, 23, 1–6. [Google Scholar]
- Duchein, P. Migration de la Bécasse en Suisse 1998–2018, 20 ans d’observations et de suivis Etude réalisée par l’Association Suisse des Bécassiers; Verlag nicht ermittelbar: Chester, UK, 2019; p. 21. [Google Scholar]
- Clausager, I. Skovsneppen som Ynglefugl i Danmark. Dan. Viltunders. 1972, 19, 1–39. [Google Scholar]
- Clausager, I. Migration of Scandinavian Woodcock (Scolopax rusticola) with Special Reference to Denmark; Vildtbiologisk Station: Kalo, Denmark, 1974. [Google Scholar]
- Bettmann, H. Die Waldschnepfe: Scolopax rusticola; F. C.: München-Solln: Mayer, MN, USA, 1961; p. 230. [Google Scholar]
- Bønløkke, J.; Madsen, J.J.; Thorup, K.; Pedersen, K.T.; Bjerrum, M.; Rahbek, C. Dansk Trækfugleatlas; Rhodos: Humlebæk, Denmark, 2006; pp. 364–368. [Google Scholar]
- Saurola, P.; Valkama, J.; Velmala, W. Suomen Rengastusatlas, Osa I; Luonnontieteellinen keskusmuseo: Helsinki, Finland, 2013. [Google Scholar]
- Berthold, P. Control of Bird Migration; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Berthold, P. Bird Migration: A General Survey; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Coppack, T.; Both, C. Predicting life-cycle adaptation of migratory birds to global climate change. Ardea 2002, 55, 369–378. [Google Scholar] [CrossRef]
- Miller-Rushing, A.J.; Lloyd-Evans, T.L.; Primack, R.B.; Satzinger, P. Bird migration times, climate change, and changing population sizes. Glob. Change Biol. 2008, 14, 1959–1972. [Google Scholar] [CrossRef]
- Calvert, A.M.; Mackenzie, S.A.; Flemming, J.M.; Taylor, P.D.; Walde, S.J. Variation in songbird migratory behavior offers clues about adaptability to environmental change. Oecologia 2012, 168, 849–861. [Google Scholar] [CrossRef]
- Lehikoinen, E.S.A.; Sparks, T.H.; Zalakevicius, M. Arrival and departure dates. Adv. Ecol. Res. 2004, 35, 1–31. [Google Scholar]
- Gienapp, P.; Leimu, R.; Merilä, J. Responses to climate change in avian migration time—Microevolution versus phenotypic plasticity. Climate Res. 2007, 35, 25–35. [Google Scholar] [CrossRef]
- Clarke, W.E. Studies in Bird Migration; Gurney and Jackson; Oliver and Boyd: London, UK, 1912; p. 323. [Google Scholar]
- Le Rest, K.; Hoodless, A.; Heward, C.; Cazenave, J.L.; Ferrand, Y. Effect of weather conditions on the spring migration of Eurasian Woodcock and consequences for breeding. Ibis 2019, 161, 559–572. [Google Scholar] [CrossRef]
- Pátkai, I. Az erdei szalonka vonulása 1947. és 1948. évek tavaszán. Aquila 1951, 55–58, 109–113. [Google Scholar]
- Alerstam, T. Bird Migration in Relation to Wind and Topography. Ph.D. Thesis, University of Lund, Lund, Sweden, 1976. [Google Scholar]
- Schenk, J. Az erdei szalonka vonulása Európában—Der Zug der Waldschnepfe in Europa. Aquila 1924, 30–31, 26–74. [Google Scholar]
- Stadie, R. Wetterlage und Frühjahrs-Schnepfenzug 1933. Ber. Des Ver. Schlesischer Ornithol. 1934, 19, 17–22. [Google Scholar]
- Richardson, W.J. Timing and amount of bird migration in relation to weather: A review. Oikos 1978, 30, 224–272. [Google Scholar] [CrossRef]
- Bulte, M.; McLaren, J.D.; Bairlein, F.; Bouten, W.; Schmaljohann, H.; Shamoun-Baranes, J.; Alerstam, T. Can wheatears weather the Atlantic? Modeling nonstop trans-Atlantic flights of a small migratory songbird. Auk 2014, 131, 363–370. [Google Scholar] [CrossRef]
- Kranstauber, B.; Weinzierl, R.; Wikelski, M.; Safi, K. Global aerial flyways allow efficient travelling. Ecol. Lett. 2015, 18, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Bende, A.; László, R. Spectrum of animal and plant in the diet of Woodcock (Scolopax rusticola L.) based on literature data. Ornis Hung. 2022, 30, 188–194. [Google Scholar] [CrossRef]
- Hadarics, T. Erdei szalonka Scolopax rusticola. In Magyarország Madáratlasza; Szép, T., Csörgő, T., Halmos, G., Lovászi, P., Nagy, K., Schmidt, A., Eds.; Agrárminisztérium, Magyar Madártani és Természetvédelmi Egyesület: Budapest, Hungary, 2021; pp. 247–248. [Google Scholar]
- Mătieș, M.; Munteanu, D. La dinamique saisonnière de la bécasse des bois (Scolopax rusticola) en Roumanie. Trav. Mus. D’histoire Nat. Grigore Antipa 1979, 20, 455–478. [Google Scholar]
- Bende, A.; Csanády, V.; Szász, B.; László, R. Az erdei szalonka (Scolopax rusticola L.) tavaszi vonulásának vizsgálata Erdélyben. Magy. Vízivad Közlemények 2023, 37, 59–70. [Google Scholar] [CrossRef]
- Szabolcs, J. Az Erdei Szalonka; Mezőgazdasági Kiadó: Budapest, Hungary, 1971. [Google Scholar]
- Faragó, S. Trends of Woodcock hunting bags in Hungary during the last 15 years. IWRB-WSRG Newsl. 1985, 11, 33–39. [Google Scholar]
- Knefély, M. Szalonkavarázs V. Nimród 1987, 107, 7–9. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2000-ben Magyarországon. Magy. Vízivad Közlemények 2002, 9, 323–340. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2001-ben Magyarországon. Magy. Vízivad Közlemények 2003, 11, 343–359. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2002-ben Magyarországon. Magy. Vízivad Közlemények 2005, 12, 247–261. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2003-ban Magyarországon. Magy. Vízivad Közlemények 2006, 13, 235–249. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2004-ben Magyarországon. Magy. Vízivad Közlemények 2007, 14, 211–225. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2005-ben Magyarországon. Magy. Vízivad Közlemények 2007, 15, 221–235. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2006-ban Magyarországon. Magy. Vízivad Közlemények 2008, 17, 215–229. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2007-ben Magyarországon. Magy. Vízivad Közlemények 2010, 18–19, 205–220. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2008-ban Magyarországon. Magy. Vízivad Közlemények 2010, 18–19, 421–435. [Google Scholar]
- Faragó, S.; László, R. Long-term monitoring of the Hungarian Woodcock bag during 1995–2008. In Seventh European Woodcock and Snipe Workshop. Proceedings of the International Symposium of the IUCN/WI Woodcock & Snipe Specialist Group, Office National de la Chasse et de la Faune Sauvage, Saint-Petersburg, Russia, 16–18 May 2011; Ferrand, Y., Ed.; Office National de la Chasse et de la faune sauvage: Paris, France, 2013; pp. 41–44. [Google Scholar]
- Faragó, S.; László, R.; Bende, A. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2011-ben Magyarországon. Magyar Vízivad Közlemények 2012, 22, 297–310. [Google Scholar]
- Faragó, S.; László, R.; Bende, A. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2012-ben Magyarországon–Results of the Hungarian Woodcock (Scolopax rusticola) Bag Monitoring in 2012. Magy. Vízivad Közlemények 2014, 24, 283–295. [Google Scholar]
- Faragó, S.; László, R.; Bende, A. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2013-ban Magyarországon-Results of the Hungarian Woodcock (Scolopax rusticola) Bag Monitoring in 2013. Magy. Vízivad Közlemények 2015, 25, 289–302. [Google Scholar] [CrossRef]
- Faragó, S.; László, R.; Bende, A. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2014-ben Magyarországon-Results of the Hungarian Woodcock (Scolopax rusticola) bag monitoring in 2014. Magy. Vízivad Közlemények 2016, 27, 284–296. [Google Scholar] [CrossRef]
- Faragó, S.; László, R.; Fluck, D.; Bende, A. Erdei szalonka monitoring mintavételi programjának eredményei 2010-ben. In Kari Tudományos Konferencia. Sopron. Konferenciakötet; Lakatos, F., Szabó, Z., Eds.; Nyugat-magyarországi Egyetem Soproni Egyetem Kiadó: Sopron, Hungary, 2011; pp. 308–311. [Google Scholar]
- Faragó, S.; László, R.; Sándor, G. Az erdei szalonka (Scolopax rusticola) testméretei, ivari és korviszonyai 1990–1999 között Magyarországon. Magy. Vízivad Közlemények 2000, 6, 409–461. [Google Scholar]
- Bende, A.; Faragó, S.; László, R. Regional differences the development of the spatial and temporal pattern of Woodcock (Scolopax rusticola L.) migration in Hungary. Magy. Vízivad Közlemények 2023, 37, 23–30. [Google Scholar] [CrossRef]
- Haest, B.; Hüppop, O.; van de Pol, M.; Bairlein, F. Autumn bird migration phenology: A potpourri of wind, precipitation and temperature effects. Glob. Change Biol. 2019, 25, 4064–4080. [Google Scholar] [CrossRef] [PubMed]
- Schally, G. Assessment of the breeding and wintering sites of Eurasian Woodcock (Scolopax rusticola) occurring in Hungary based on ringing recovery data. Ornis Hung. 2019, 27, 110–116. [Google Scholar] [CrossRef]
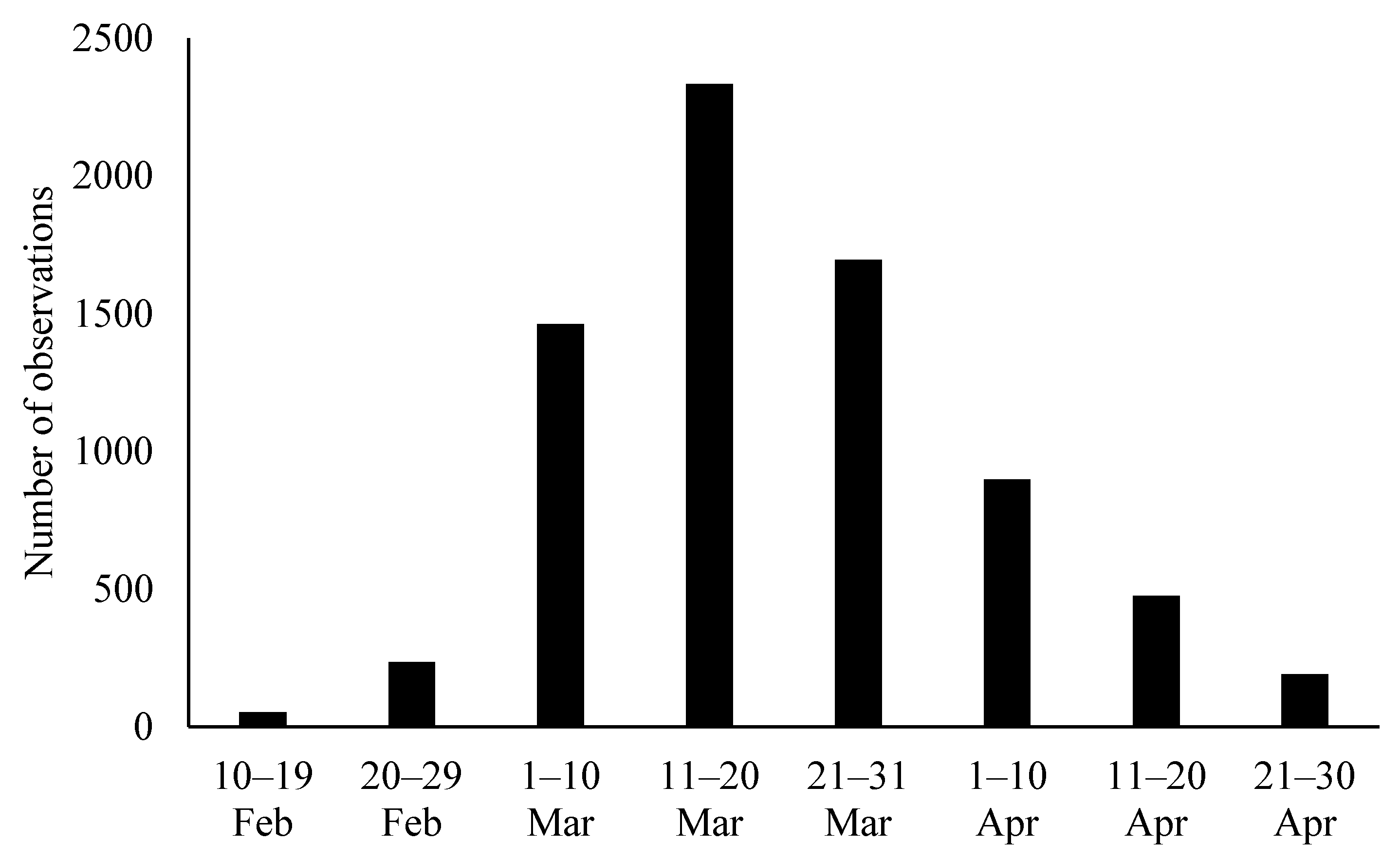
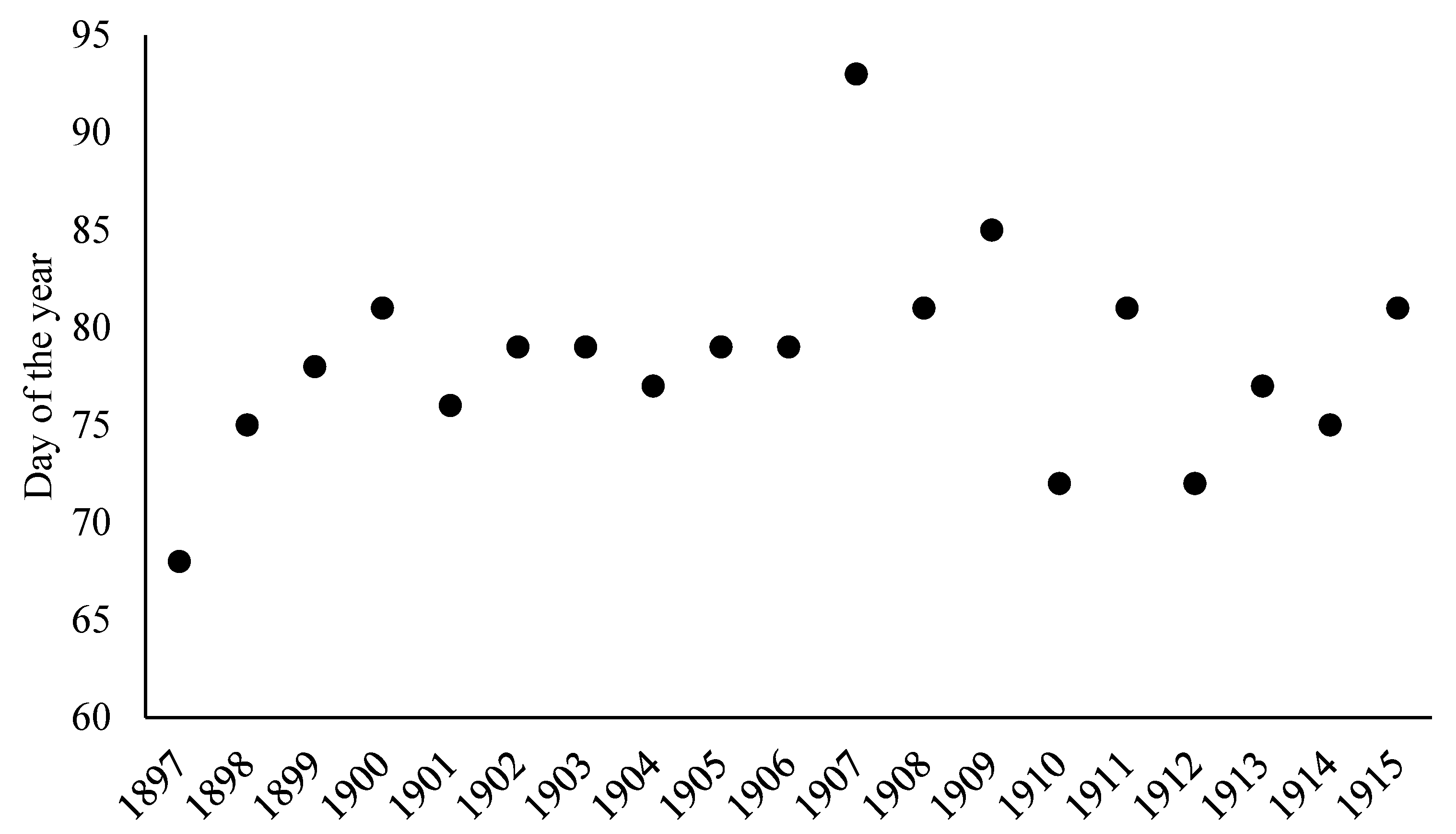
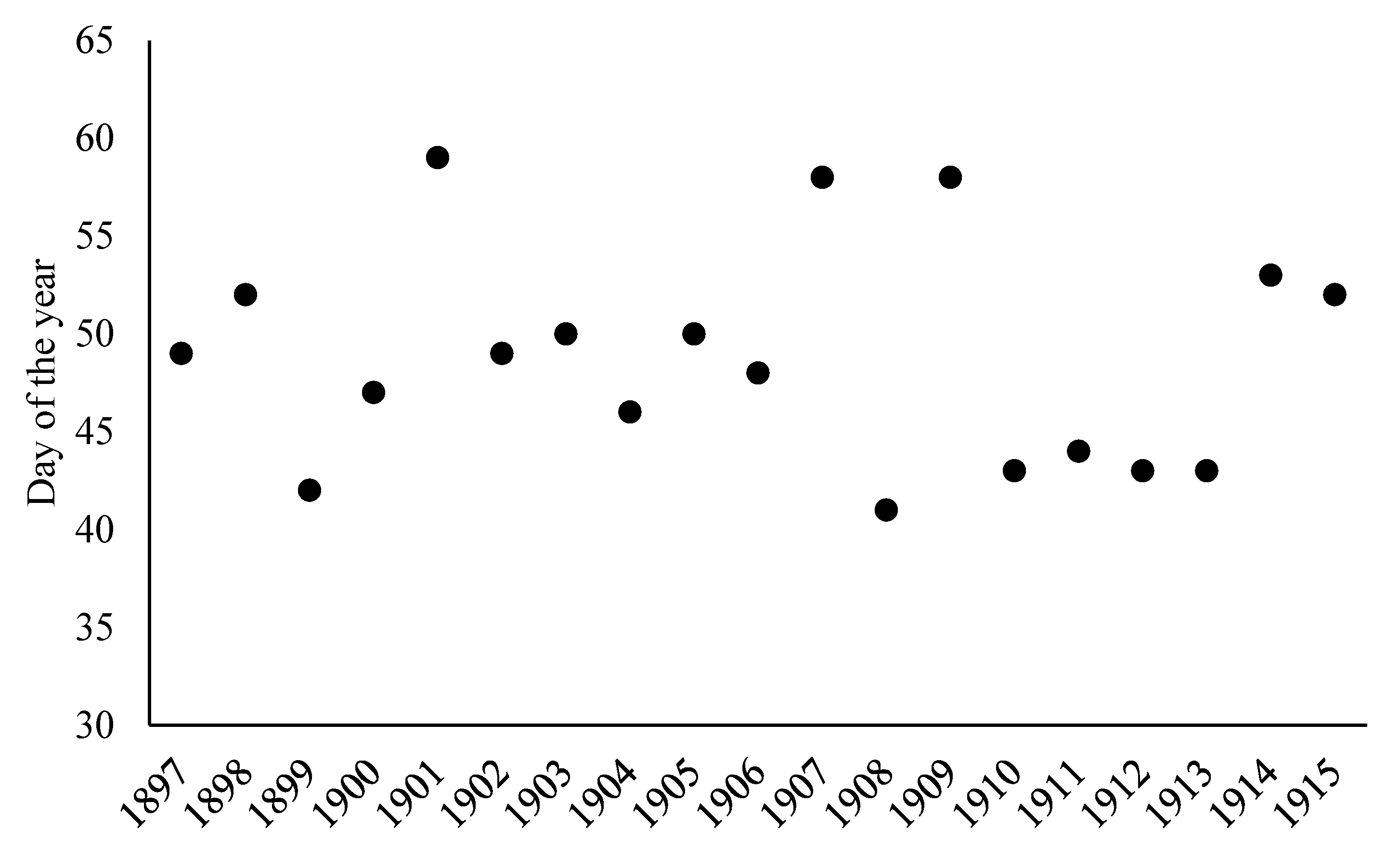
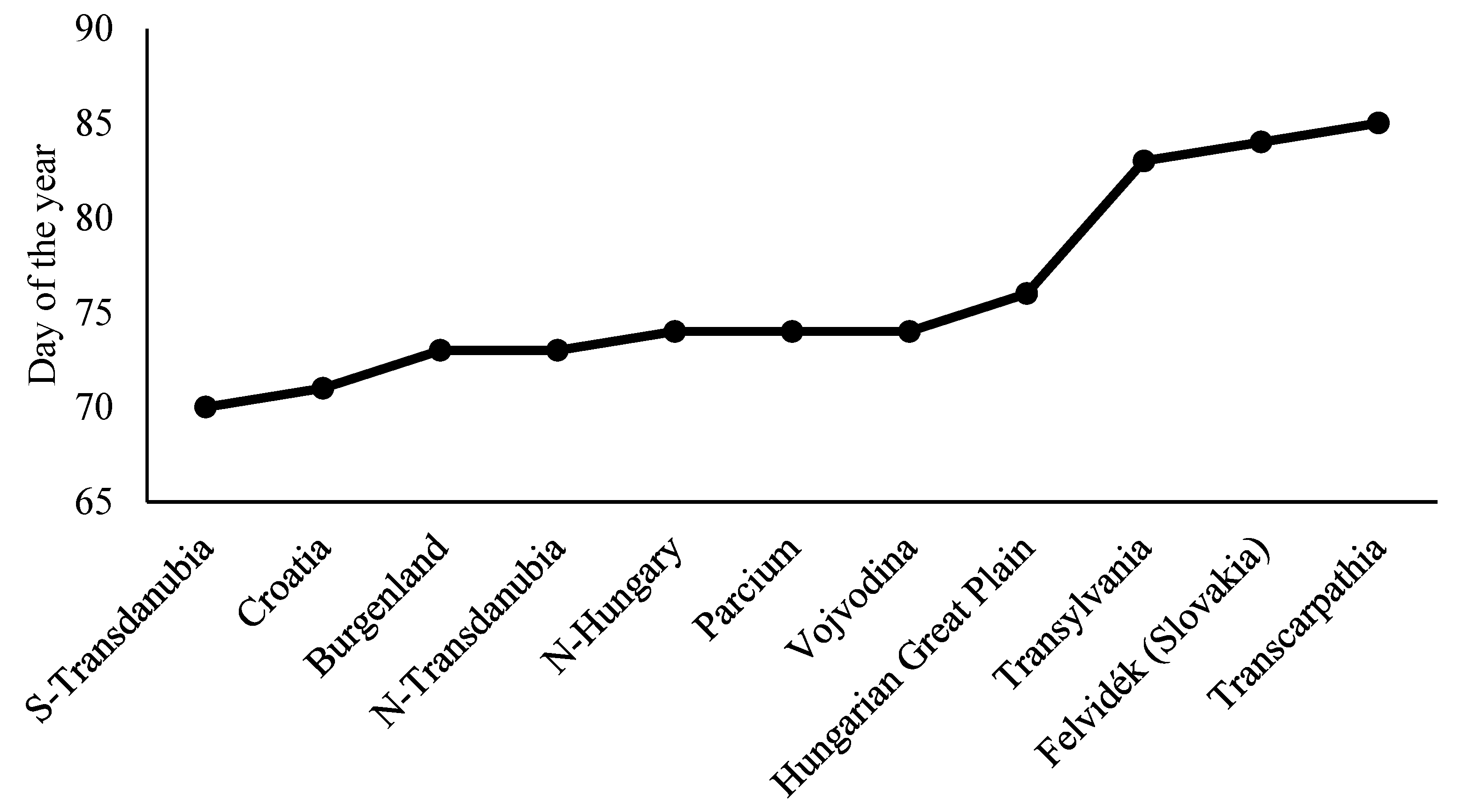
| Large Region | Number of Data Points | Number of Municipalities within Large Region |
|---|---|---|
| Burgenland | 188 | 55 |
| S-Transdanubia | 438 | 151 |
| Transylvania | 2040 | 534 |
| N-Transdanubia | 871 | 223 |
| N-Hungary | 257 | 77 |
| Felvidék (Slovakia) | 1811 | 431 |
| Croatia | 156 | 69 |
| Transcarpathia | 544 | 130 |
| Hungarian Great Plain | 172 | 60 |
| Parcium | 638 | 188 |
| Vojvodina | 229 | 53 |
| Variable | Description |
|---|---|
| Month | Categorical variable capturing seasonal effects. This variable was excluded from the analyses since the variables “days” and “years” adequately capture migratory patterns. |
| Number of days from beginning of migration compared to January with leap years from January to the end of the month | Numeric variable that captures intra-monthly timing effects. |
| Beginning of migration in days compared to January with leap years compared to January | Numeric variable that is related to timing within the year. This is our most important variable of interest (beside year). This variable shows in days how many days have passed since 1st January of each year considering leap years. |
| Geographic latitude and geography length | These variables were excluded from the analyses since the variables “days” and “years” adequately capture migratory patterns. |
| Altitude above sea level | Numeric variable that could influence the dependent variables due to geographical effects. |
| Region, county, geographical unit | Categorical variables that might capture regional differences affecting the dependent variables. |
| Daily mean temperature, daily maximum temperature, daily minimum temperature, daily precipitation total, type of daily precipitation total (the last variable is categorical) | These climate-related numeric variables may impact the dependent variables. |
| Parametric Coefficients | ||||
|---|---|---|---|---|
| Estimate | SE | t-Value | p-Value | |
| Intercept | 80.4108 | 0.1265 | 635.5 | p < 0.001 |
| Approximate significance of smooth terms | ||||
| Smooth term | Edf | Ref. df. | F-value | p-value |
| s(Altitude_above_sea_level) | 7.660 | 8.490 | 179.16 | p < 0.001 |
| s(Daily_maximum_temperature) | 4.576 | 5.674 | 56.08 | p < 0.001 |
| s(Daily_minimum_temperature) | 7.435 | 8.326 | 23.33 | p < 0.001 |
| s(Daily_precipitation_total) | 8.145 | 8.769 | 10.47 | p < 0.001 |
| s(Year) | 8.759 | 8.977 | 124.51 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozó, L.; Fekete, I.; Bende, A. The Migration of the Eurasian Woodcock (Scolopax rusticola L.) in the Carpathian Basin at the Turn of the 19–20th Centuries. Birds 2024, 5, 571-585. https://doi.org/10.3390/birds5030038
Bozó L, Fekete I, Bende A. The Migration of the Eurasian Woodcock (Scolopax rusticola L.) in the Carpathian Basin at the Turn of the 19–20th Centuries. Birds. 2024; 5(3):571-585. https://doi.org/10.3390/birds5030038
Chicago/Turabian StyleBozó, László, István Fekete, and Attila Bende. 2024. "The Migration of the Eurasian Woodcock (Scolopax rusticola L.) in the Carpathian Basin at the Turn of the 19–20th Centuries" Birds 5, no. 3: 571-585. https://doi.org/10.3390/birds5030038
APA StyleBozó, L., Fekete, I., & Bende, A. (2024). The Migration of the Eurasian Woodcock (Scolopax rusticola L.) in the Carpathian Basin at the Turn of the 19–20th Centuries. Birds, 5(3), 571-585. https://doi.org/10.3390/birds5030038






