Avifaunal Diversity and Abundance in the Proposed Sarasalai Mangrove Reserve, Jaffna, Sri Lanka
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Analyses
3. Results
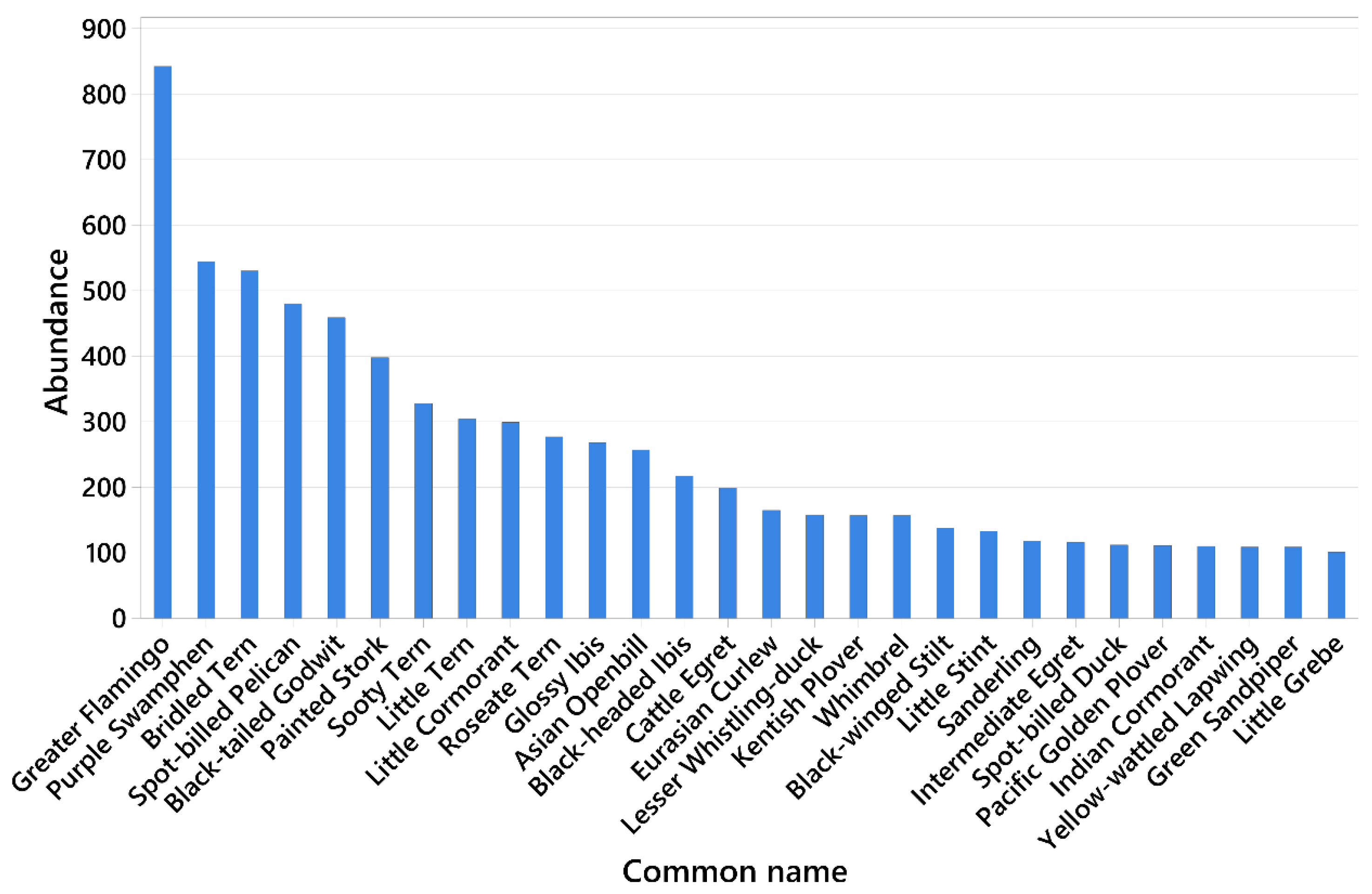
3.1. Waterbird Diversity
3.2. Threatened and Northern Restricted Species Recorded during This Study
4. Discussion
4.1. Livelihood Activities of People Depend
4.2. Threats to the Proposed Sarasalai Mangrove
4.3. Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Scientific Name | Common Name | Family | M/R | NCS | |
|---|---|---|---|---|---|
| 1 | Francolinus pondicerianus | Grey Francolin | Phasianidae | R | EN |
| 2 | Dendrocygna javanica | Lesser Whistling Duck | Anatidae | R | LC |
| 3 | Tadorna ferruginea | Ruddy Shelduck | Anatidae | M | LC |
| 4 | Anas poecilorhyncha | Spot-Billed Duck | Anatidae | R | CR |
| 5 | Mareca penelope | Eurasian Wigeon | Anatidae | M | LC |
| 6 | Spatula clypeata | Northern Shoveler | Anatidae | M | LC |
| 7 | Anas acuta | Northern Pintail | Anatidae | M | LC |
| 8 | Spatula querquedula | Garganey | Anatidae | M | LC |
| 9 | Phoenicopterus roseus | Greater Flamingo | Phoenicopteridae | M | LC |
| 10 | Mycteria leucocephala | Painted Stork | Ciconiidae | R | LC |
| 11 | Anastomus oscitans | Asian Openbill | Ciconiidae | R | LC |
| 12 | Threskiornis melanocephalus | Black-Headed Ibis | Threskiornithidae | R | LC |
| 13 | Plegadis falcinellus | Glossy Ibis | Threskiornithidae | M | DD |
| 14 | Nycticorax nycticorax | Black-crowned Night Heron | Ardeidae | R | LC |
| 15 | Butorides striata | Striated Heron | Ardeidae | R | LC |
| 16 | Ardeola grayii | Indian Pond Heron | Ardeidae | R | LC |
| 17 | Ardea cinerea | Grey Heron | Ardeidae | R | LC |
| 18 | Ardea purpurea | Purple Heron | Ardeidae | R | LC |
| 19 | Bubulcus ibis | Cattle Egret | Ardeidae | R | LC |
| 20 | Ardea alba | Great Egret | Ardeidae | R | LC |
| 21 | Ardea intermedia | Intermediate Egret | Ardeidae | R | LC |
| 22 | Egretta garzetta | Little Egret | Ardeidae | R | LC |
| 23 | Pelecanus philippensis | Spot-Billed Pelican | Pelecanidae | R | LC |
| 24 | Tachybaptus ruficollis | Little Grebe | Podicipedidae | R | LC |
| 25 | Microcarbo niger | Little Cormorant | Phalacrocoracidae | R | LC |
| 26 | Phalacrocorax fuscicollis | Indian Cormorant | Phalacrocoracidae | R | LC |
| 27 | Anhinga melanogaster | Oriental Darter | Anhingidae | R | LC |
| 28 | Milvus migrans | Black Kite | Accipitridae | R | LC |
| 29 | Haliastur indus | Brahminy Kite | Accipitridae | R | LC |
| 30 | Accipiter badius | Shikra | Accipitridae | R | LC |
| 31 | Amaurornis phoenicurus | White-breasted Waterhen | Rallidae | R | LC |
| 32 | Porphyrio porphyrio | Purple Swamphen | Rallidae | R | LC |
| 33 | Burhinus oedicnemus | Eurasian Thick-knee | Burhinidae | R | LC |
| 34 | Haematopus ostralegus | Eurasian Oystercatcher | Haematopodidae | M | LC |
| 35 | Himantopus himantopus | Black-Winged Stilt | Recurvirostridae | R | LC |
| 36 | Recurvirostra avosetta | Pied Avocet | Recurvirostridae | M | LC |
| 37 | Vanellus malabaricus | Yellow-Wattled Lapwing | Charadriidae | R | LC |
| 38 | Vanellus indicus | Red-wattled Lapwing | Charadriidae | R | LC |
| 39 | Pluvialis fulva | Pacific Golden Plover | Charadriidae | M | LC |
| 40 | Charadrius asiaticus | Caspian Plover | Charadriidae | M | LC |
| 41 | Charadrius alexandrinus | Kentish Plover | Charadriidae | R | EN |
| 42 | Lymnocryptes minimus | Jack Snipe | Scolopacidae | M | LC |
| 43 | Limosa limosa | Black-tailed Godwit | Scolopacidae | M | LC |
| 44 | Numenius phaeopus | Whimbrel | Scolopacidae | M | LC |
| 45 | Numenius arquata | Eurasian Curlew | Scolopacidae | M | LC |
| 46 | Tringa totanus | Common Redshank | Scolopacidae | M | LC |
| 47 | Tringa stagnatilis | Marsh Sandpiper | Scolopacidae | M | LC |
| 48 | Tringa nebularia | Common Greenshank | Scolopacidae | M | LC |
| 49 | Tringa ochropus | Green Sandpiper | Scolopacidae | M | LC |
| 50 | Tringa glareola | Wood Sandpiper | Scolopacidae | M | LC |
| 51 | Actitis hypoleucos | Common Sandpiper | Scolopacidae | M | LC |
| 52 | Arenaria interpres | Ruddy Turnstone | Scolopacidae | M | LC |
| 53 | Calidris tenuirostris | Great Knot | Scolopacidae | M | LC |
| 54 | Calidris alba | Sanderling | Scolopacidae | M | LC |
| 55 | Calidris minuta | Little Stint | Scolopacidae | M | LC |
| 56 | Calidris temminckii | Temminck’s Stint | Scolopacidae | M | LC |
| 57 | Calidris ferruginea | Curlew Sandpiper | Scolopacidae | M | LC |
| 58 | Sterna dougallii | Roseate Tern | Laridae | R | CR |
| 59 | Sternula albifrons | Little Tern | Laridae | R | VU |
| 60 | Onychoprion anaethetus | Bridled Tern | Laridae | M, R | CR |
| 61 | Onychoprion fuscatus | Sooty Tern | Laridae | M, R | CR |
| 62 | Columba livia | Common Pigeon | Columbidae | R | LC |
| 63 | Streptopelia decaocto | Eurasian Collard Dove | Columbidae | R | NT |
| 64 | Spilopelia chinensis | Spotted Dove | Columbidae | R | LC |
| 65 | Psittacula krameri | Rose-Ringed Parakeet | Psittaculidae | R | LC |
| 66 | Clamator jacobinus | Jacobin Cuckoo | Cuculidae | R | LC |
| 67 | Eudynamys scolopaceus | Asian Koel | Cuculidae | R | LC |
| 68 | Centropus sinensis | Greater Coucal | Cuculidae | R | LC |
| 69 | Aerodramus unicolor | Indian Swiftlet | Apodidae | R | LC |
| 70 | Coracias benghalensis | Indian Roller | Coraciidae | R | LC |
| 71 | Merops orientalis | Green Bee-Eater | Meropidae | R | LC |
| 72 | Merops philippinus | Blue-tailed Bee-eater | Meropidae | M | CR |
| 73 | Pelargopsis capensis | Stork-Billed Kingfisher | Alcedinidae | R | LC |
| 74 | Halcyon smyrnensis | White-throated Kingfisher | Alcedinidae | R | LC |
| 75 | Alcedo atthis | Common Kingfisher | Alcedinidae | R | LC |
| 76 | Ceryle rudis | Pied Kingfisher | Alcedinidae | R | LC |
| 77 | Megalaima zeylanica | Brown-headed Barbet | Megalaimidae | R | LC |
| 78 | Dinopium benghalense | Lesser Golden-Backed Woodpecker | Picidae | R | LC |
| 79 | Pitta brachyura | Indian Pitta | Pittidae | M | LC |
| 80 | Oriolus xanthornus | Black Hooded Oriole | Oriolidae | R | LC |
| 81 | Corvus splendens | House Crow | Corvidae | R | LC |
| 82 | Corvus macrorhynchos | Large-billed Crow | Corvidae | R | LC |
| 83 | Hirundo rustica | Barn Swallow | Hirundinidae | M | LC |
| 84 | Artamus fuscus | Ashy Woodswallow | Artamidae | R | LC |
| 85 | Dicrurus macrocercus | Black Drongo | Dicruridae | R | LC |
| 86 | Dicrurus leucophaeus | Ashy Drongo | Dicruridae | M | LC |
| 87 | Anthus richardi | Richard’s Pipit | Motacillidae | M | LC |
| 88 | Anthus rufulus | Paddyfield Pipit | Motacillidae | R | LC |
| 89 | Anthus godlewskii | Blyth’s Pipit | Motacillidae | M | LC |
| 90 | Alauda gulgula | Oriental Skylark | Alaudidae | R | LC |
| 91 | Eremopterix griseus | Ashy-crowned Sparrow Lark | Alaudidae | R | LC |
| 92 | Pycnonotus cafer | Red-vented Bulbul | Pycnonotidae | R | LC |
| 93 | Pycnonotus luteolus | White-browed Bulbul | Pycnonotidae | R | LC |
| 94 | Prinia hodgsonii | Grey-breasted Prinia | Cisticolidae | R | LC |
| 95 | Prinia inornata | Plain Prinia | Cisticolidae | R | LC |
| 96 | Orthotomus sutorius | Common Tailorbird | Cisticolidae | R | LC |
| 97 | Phylloscopus trochiloides | Greenish Warbler | Parulidae | M | LC |
| 98 | Turdoides affinis | Yellow-billed Babbler | Leiothrichidae | R | LC |
| 99 | Acridotheres tristis | Common Myna | Sturnidae | R | LC |
| 100 | Copsychus saularis | Oriental Magpie-robin | Muscicapidae | R | LC |
| 101 | Saxicoloides fulicatus | Indian Robin | Muscicapidae | R | LC |
| 102 | Dicaeum erythrorhynchos | Pale-billed Flowerpecker | Dicaeidae | R | LC |
| 103 | Cinnyris asiaticus | Purple Sunbird | Nectariniidae | R | LC |
| 104 | Cinnyris lotenius | Loten’s Sunbird | Nectariniidae | R | LC |
| 105 | Ploceus philippinus | Baya Weaver | Ploceidae | R | LC |
| 106 | Passer domesticus | House Sparrow | Passeridae | R | LC |
| 107 | Euodice malabarica | Indian Silverbill | Passeridae | R | LC |
Appendix B

References
- Blankespoor, B.; Dasgupta, S.; Lange, G.M. Mangroves as a protection from storm surges in a changing climate. Ambio 2017, 46, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Ranawana, K.B. Mangroves of Sri Lanka. Publ. Seacology-Sudeesa Mangrove Mus. 2017, 1, 25–28. [Google Scholar]
- Howard, J.; Andradi-Brown, D.A.; Hagger, V.; Sasmito, S.D.; Bosire, J. Editorial: Drivers of mangrove forest change and its effects on biodiversity and ecosystem services. Front. For. Glob. Change 2022, 5, 989665. [Google Scholar] [CrossRef]
- Getzner, M.; Islam, M.S. Ecosystem services of mangrove forests: Results of a meta-analysis of economic values. Int. J. Environ. Res. Public Health 2020, 17, 5830. [Google Scholar] [CrossRef]
- Mukherjee, N.; Sutherland, W.J.; Dicks, L.; Huge´, J.; Koedam, N.; Dahdouh-Guebas, F. Ecosystem Service Valuations of Mangrove Ecosystems to Inform Decision Making and Future Valuation Exercises. PLoS ONE 2014, 9, e107706. [Google Scholar] [CrossRef]
- Friess, D.A.; Yando, E.S.; Alemu, J.B.; Wong, L.W.; Soto, S.D.; Bhatia, N. Ecosystem services and disservices of mangrove forests and salt marshes. Oceanogr. Mar. Biol. Annu. Rev. 2020, 58, 107–142. [Google Scholar]
- Goldberg, L.; Lagomasino, D.; Thomas, N.; Fatoyinbo, T. Global declines in human-driven mangrove loss. Glob. Change Biol. 2020, 26, 5844–5855. [Google Scholar] [CrossRef]
- Harris, N.L.; Gibbs, D.A.; Baccini, A.; Birdsey, R.A.; De Bruin, S.; Farina, M.; Fatoyinbo, L.; Hansen, M.C.; Herold, M.; Houghton, R.A.; et al. Global maps of twenty-first century forest carbon fluxes. Nat. Clim. Change 2021, 11, 234–240. [Google Scholar] [CrossRef]
- Kotagama, S.W.; Bambaradeniya, C.N.B. An Overview of the Wetlands of Sri Lanka and Their Conservation Significance. Colombo: The Central Environmental Authority (CEA); The World Conservation Union (IUCN) and the International Water Management Institute (IWMI): Colombo, Sri Lanka, 2006. [Google Scholar]
- Thanusanth, S.; Ahamed, A.M.R.; Harris, J.H.; Sumuthini, S.; Priyanka, K. Avian species diversity in Sathurukondan wetland mangrove habitat, Batticaloa district, Sri Lanka. In Proceedings of the 9th International Symposium (Full Paper), South Eastern University of Sri Lanka, Oluvi, Sri Lanka, 27–28 November 2019; pp. 389–396. [Google Scholar]
- Wetlands International. Guidance on Waterbird Monitoring Methodology: Field Protocol for Waterbird Counting; Wetlands International: Wagenigen, The Netherlands, 2010; p. 15. [Google Scholar]
- Hattori, A.; Mae, S. Habitat use and diversity of waterbirds in a coastal lagoon Lake Biwa, Japan. Ecol. Res. 2001, 16, 543–553. [Google Scholar] [CrossRef]
- Wei, D.L.Z.; Bloem, A.; Delany, S.; Martakis, G.; Quintero, J.O. Status of Water Birds in Asia: Results of the Asian Water Bird Census: 1987–2007; Wetlands International: Kuala Lumpur, Malaysia, 2009. [Google Scholar]
- Warakagoda, D.; Sirivardana, U. The Avifauna of Sri Lanka: An Overview of the Current Status. Taprobanica 2011, 1, 28–35. [Google Scholar] [CrossRef]
- Kotagama, S.; Ratnavira, G. Birds of Sri Lanka: An Illustrated Guide; University of Colombo, Field Ornithology Group of Sri Lanka: Colombo, Sri Lanka, 2017; p. 540. [Google Scholar]
- Lepage, D. Checklist of the birds of Sri Lanka. Colombo, Sri Lanka: Avibase, The World Bird Database, 2021, 2022. Available online: https://avibase.bsc-eoc.org/checklist.jsp?region=LK (accessed on 12 September 2022).
- Kandasamy, G.; Weerakoon, D.K.; Sivaruban, A.; Jayasiri, H.B. Diversity and Abundance of Waterbird Communities in the Jaffna and Kilinochchi Districts: Where do we have to go from here? Vingnanam J. Sci. 2019, 14, 15–21. [Google Scholar] [CrossRef]
- Wijesundara, C.S.; Warakagoda, D.; Sirivardana, U.; Chathuranga, D.; Hettiarachchi, T.; Perera, N.; Rajkumar, P.; Wanniarachchi, S.; Weerakoon, G. Diversity and conservation of waterbirds in the northern avifaunal region of Sri Lanka. Ceylon J. Sci. 2017, 46, 143. [Google Scholar] [CrossRef]
- Kannan, V.; Pandiyan, J. Shorebirds (Charadriidae) of Pulicat Lake, India with special reference to conservation. J. Zool. 2012, 7, 178–191. [Google Scholar]
- Aarif, K.M.; Sara, K.; Aymen, N.; Sama, A. Over-summering abundance, species composition, and habitat use patterns at a globally important site for migratory shorebirds. J. Ornithol. 2020, 132, 165–172. [Google Scholar] [CrossRef]
- Warakagoda, D.; Sirivardana, U. Status of waterfowl in Sri Lanka. In The Fauna of Sri Lanka, 1st ed.; Bambaradeniya, C., Ed.; The World Conservation Union (IUCN): Colombo, Sri Lanka, 2006; pp. 204–215. [Google Scholar]
- Kotagama, S.; Ratnavira, G. An Illustrated Guide to the Birds of Sri Lanka; Field Ornithology Group of Sri Lanka: Colombo, Sri Lanka, 2010; p. 382. [Google Scholar]
- Giri, B.; Chalise, M.K. Seasonal Diversity and Population Status of Waterbirds in Phewa Lake, Pokhara, Nepal. J. Wetl. Ecol. 2008, 1, 3–7. [Google Scholar] [CrossRef]
- Sellathurai, T.; Sivananthawerl, T.; Sivakumar, S.S.; Mikunthan, T. Trend analysis of rainfall and temperature in Jaffna districts of Sri Lanka Trend analysis. In Proceedings of the WaSo 2020 Final Conference, Online, 15–16 December 2020; pp. 57–64. [Google Scholar]
- Weerakoon, D.; Goonatilake, S.D.A.; Wijewickrama, T.; Rajasuriya, A.; Perera, N.; Kumara, T.P.; De Silva, G.; Miththapala, S.; Mallawatantri, A. Conservation and Sustainable use of Biodiversity in the Islands and Lagoons of Northern Sri Lanka; IUCN, International Union for Conservation of Nature: Grand, Switzerland, 2020; p. 327. [Google Scholar]
- Sathiyaseelan, V. Medicinal potential of mangrove and mangrove associated plants at Sarasalai North in Jaffna. In Proceedings of the Wayamba University International Conference, Kuliyapitiya, Sri Lanka, 29–30 August 2014 2014; p. 87. [Google Scholar]
- Sellathurai, T.; Mikunthan, T.; Sivakumar, S.S.; Karunainathan, T. Seasonal variation of rainfall in Vadamaradchi area in Jaffna District Sri Lanka. In Proceedings of the 6th International Conference on Dry Zone Agriculture (ICDA 2020), Jaffna, Sri Lanka, 3–4 December 2020; p. 30. [Google Scholar]
- Sutherland, W.J. Ecological Census Techniques, a Handbook, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; p. 450. [Google Scholar]
- Wijesundara, C.S. Significance of a Forest Fragment as a Bird Habitat and its Importance in Biodiversity Conservation. Int. J. Earth Sci. Eng. 2015, 08, 320–325. [Google Scholar]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling: Estimating Abundance of Biological Populations; Oxford University Press: Oxford, UK, 2001; p. 448. [Google Scholar]
- Harrison, J.A. A Field Guide to the Birds of Sri Lanka; Oxford University Press: Oxford, UK, 2011; p. 208. [Google Scholar]
- Warakagoda, D.; Inskipp, C.; Inskipp, T.; Grimmett, R. Birds of Sri Lanka: Helm Field Guides; Bloomsbury Publishing: London, UK, 2012; p. 400. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; p. 256. [Google Scholar]
- Youn, T.M. Biodiversity Calculator. 2022. Available online: https://www.alyoung.com/labs/biodiversity_calculator.html (accessed on 22 December 2022).
- Henry, G.M. A guide to the Birds of Ceylon, 1st ed.; Oxford University Press: Oxford, UK, 1955; pp. 272–273. [Google Scholar]
- Rodrigo, M. In lockdown’s Calm, Glossy Ibis Finds Prime Nesting Conditions in Sri Lanka. 2020. Available online: https://news.mongabay.com/2020/06/in-lockdowns-calm-glossy-ibis-finds-prime-nesting-conditions-in-sri-lanka/ (accessed on 31 January 2023).
- MOE. The National Red List 2021; Conservation Status of the Birds of Sri Lanka (2021); Biodiversity Secretariat, Ministry of Environment: Colombo, Sri Lanka, 2021; pp. 52–72.
- Kishoran, S.; Harris, J.M.; Vinobaba, M.; Vinobaba, P. Bird diversity and threaten to their habitat in sathurukondan birding site in Batticaloa, Sri Lanka. J. Agric. Sci. Technol. 2017, 7, 123–128. [Google Scholar] [CrossRef]
- Sivasubramanian, C. Birds Diversity of Muthupet Mangroves Southeast Coast of India. In National Symposium on Conservation and Valuation of Marine Biodiversity; Zoological Survey of India Address: Kolkata, India, 2007; pp. 365–377. [Google Scholar]
- Wijesundara, C.S.; Wanniarachchi, S.; Hettiarachchi, T.; Galappaththi, S.; Weerawardhana, A.; Rajkumar, P. Population size and movements of the Greater Flamingo (Phoenicopterus roseus) in the Jaffna peninsula, Sri Lanka: Results from a long-term study. Ceylon J. Sci. 2018, 47, 373–378. [Google Scholar] [CrossRef]
- De Silva, R.I. Waterbird study in Malala Lewaya (Sri Lanka). Loris 1999, 22, 45–51. [Google Scholar]
- Balachandra, S. Avian Diversity in Coastal Wetlands of India and their Conservation Needs. Int. Day Biol. Divers. Mar. Biodivers. 2012, 155–162. [Google Scholar]
- Kandasamy, G.; Weerakoon, D.K.; Sivaruban, A. Spatial variation of waterbirds in Pallai and Thadduvankoddy in the Northern Province of Sri Lanka. In Proceedings of the Third International Conference on Science, Engineering and Environment, SEEUSQ, Brisbane, Australia, 13–15 November 2017; pp. 124–129. [Google Scholar]
- Cunningham, J.A.; Kesler, D.C.; Lanctot, R.B. Habitat and social factors influence nest site selection in Arctic Breeding shorebirds. Auk 2016, 133, 364–377. [Google Scholar] [CrossRef]
- Aarif, K.M.; Nefla, A.; Nasser, M.; Prasadan, P.K.; Athira, T.R.; Muzaffar, S.B. Multiple environmental factors and prey depletion determine declines in abundance and timing of departure in migratory shorebirds in the west coast of India. Glob. Ecol. Conserv. 2021, 26, e01518. [Google Scholar] [CrossRef]
- Panagoda, P.A.B.G.; Mundkur, T.; Balachandran, S.; Kotagama, S.W.; Meng, F.; Zhang, B.; Lei, C.; Seneviratne, S.S. Overflying the Himalayas; The Northward Migration of Sri Lankan-Wintering Brown-Headed Gulls. General Sir John Kotelawala Defence University—15th International Research Conference 2022; p. 19. Available online: http://ir.kdu.ac.lk/handle/345/6176 (accessed on 24 December 2022).
- Rashiba, A.P.; Jishnu, K.; Byju, H.; Shifa, C.T.; Anand, J.; Vichithra, K.; Xu, Y.; Nefla, A.; Muzaffar, S.B.; Aarif, K.M.; et al. The Paradox of Shorebird Diversity and Abundance in the West Coast and East Coast of India: A Comparative Analysis. Diversity 2022, 14, 885. [Google Scholar] [CrossRef]
- Bart, J.A.; Manning, A.; Thomas, S.; Wightman, C. Preparation of Regional Shorebird Monitoring Plans; General Technical Report PSW-GTR-191; U.S. Department of Agriculture: Albany, CA, USA, 2005.
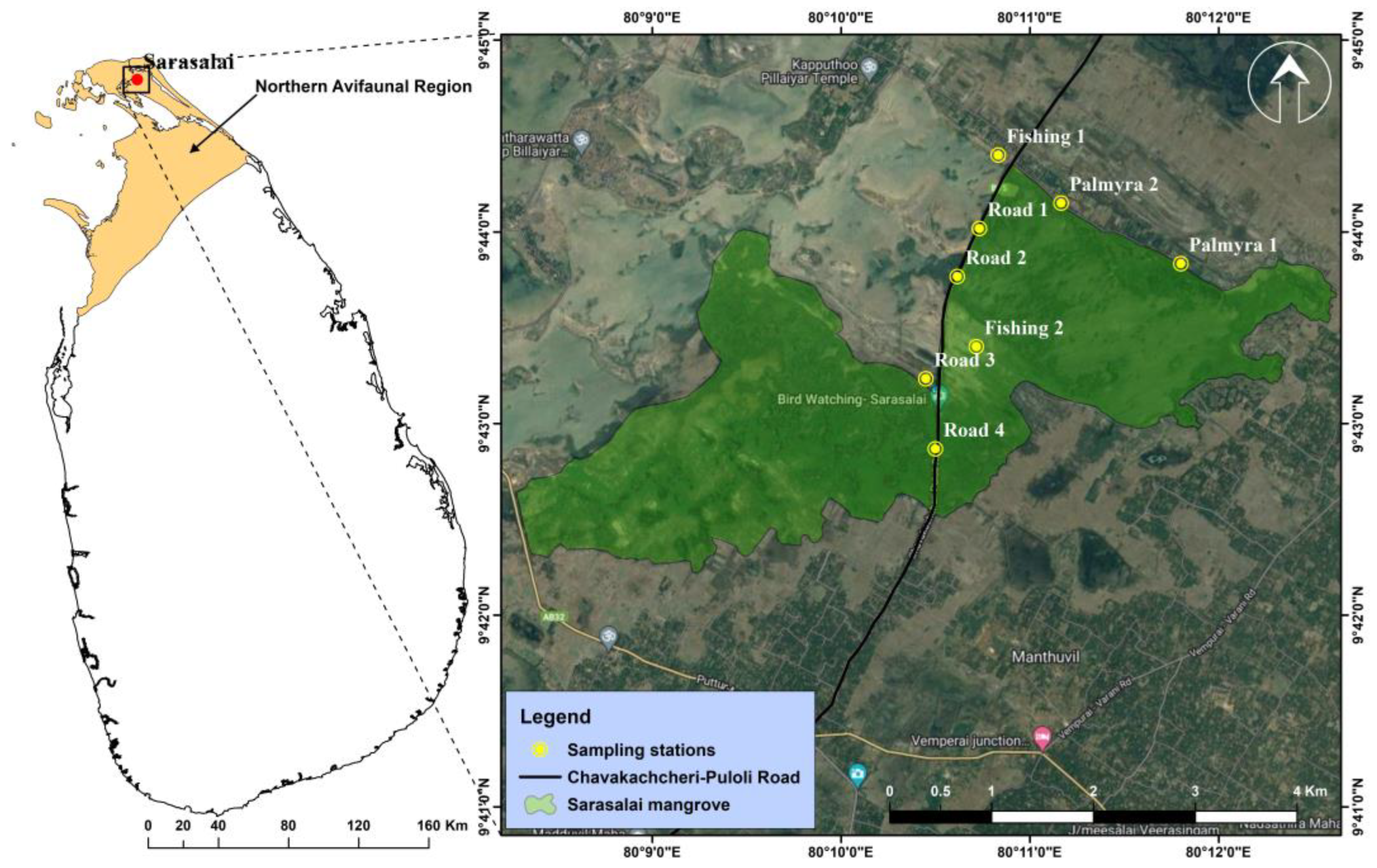


| Site | Description | Vegetation | Water Level | Human Disturbance |
|---|---|---|---|---|
| Palmyra 1 | Located on a narrow footpath to the main road | Dense mangrove/paddy field/palmyra | Very low in dry season | Medium |
| Palmyra 2 | Located on the same footpath close to the main road | Low mangrove/palmyra/home garden | Low in dry season and high in wet season | High |
| Fishing 1 | Located outside of the main road | Low mangrove/open grasses/palmyra/home garden | Low in dry season and high in wet season | High |
| Fishing 2 | Located outside of the road network | Low mangrove/open areas | Zero during dry season | High |
| Road 1 | Located on the main road | Low mangrove/scattered trees/open areas with tall grasses | Medium | High |
| Road 2 | Located on the main road | Dense mangrove/grasses/pond | High | High |
| Road 3 | Located a little away from the main road | Low mangrove/open grasses | Zero during dry season | High |
| Road 4 | Located on the main road | Dense mangrove | Medium | Low |
| Location | Palmyra 1 | Palmyra 2 | Fishing 1 | Road 1 | Road 2 | Fishing 2 | Road 3 | Road 4 |
|---|---|---|---|---|---|---|---|---|
| Abundance (no. of records) | 1512 | 1460 | 1620 | 3103 | 1770 | 1120 | 1047 | 1219 |
| Richness | 87 | 88 | 86 | 91 | 88 | 83 | 89 | 89 |
| Shannon Diversity Index (H′) | 3.47 | 3.83 | 3.72 | 3.59 | 3.59 | 3.83 | 4.06 | 4.06 |
| Simpson Index | 0.0949 | 0.0325 | 0.0387 | 0.0534 | 0.0575 | 0.0304 | 0.0231 | 0.0229 |
| Berger-Parker Dominance Index | 0.2877 | 0.0767 | 0.1130 | 0.1650 | 0.1864 | 0.0634 | 0.0573 | 0.0673 |
| Margalef Richness Index | 11.7467 | 11.9410 | 11.5017 | 11.1939 | 11.633 | 11.6791 | 12.6552 | 12.3843 |
| Reciprocal Simpson Index | 10.4595 | 30.1329 | 25.4440 | 18.6045 | 17.2234 | 31.9218 | 41.6572 | 42.1609 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloysius, N.; Madhushanka, S.; Chandrika, C. Avifaunal Diversity and Abundance in the Proposed Sarasalai Mangrove Reserve, Jaffna, Sri Lanka. Birds 2023, 4, 103-116. https://doi.org/10.3390/birds4010009
Aloysius N, Madhushanka S, Chandrika C. Avifaunal Diversity and Abundance in the Proposed Sarasalai Mangrove Reserve, Jaffna, Sri Lanka. Birds. 2023; 4(1):103-116. https://doi.org/10.3390/birds4010009
Chicago/Turabian StyleAloysius, Nitharsan, Shashi Madhushanka, and Chathuri Chandrika. 2023. "Avifaunal Diversity and Abundance in the Proposed Sarasalai Mangrove Reserve, Jaffna, Sri Lanka" Birds 4, no. 1: 103-116. https://doi.org/10.3390/birds4010009
APA StyleAloysius, N., Madhushanka, S., & Chandrika, C. (2023). Avifaunal Diversity and Abundance in the Proposed Sarasalai Mangrove Reserve, Jaffna, Sri Lanka. Birds, 4(1), 103-116. https://doi.org/10.3390/birds4010009





