Discriminant Criteria for Field Sexing in the Eurasian Tree Sparrow by Combining Body Size and Plumage Features
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
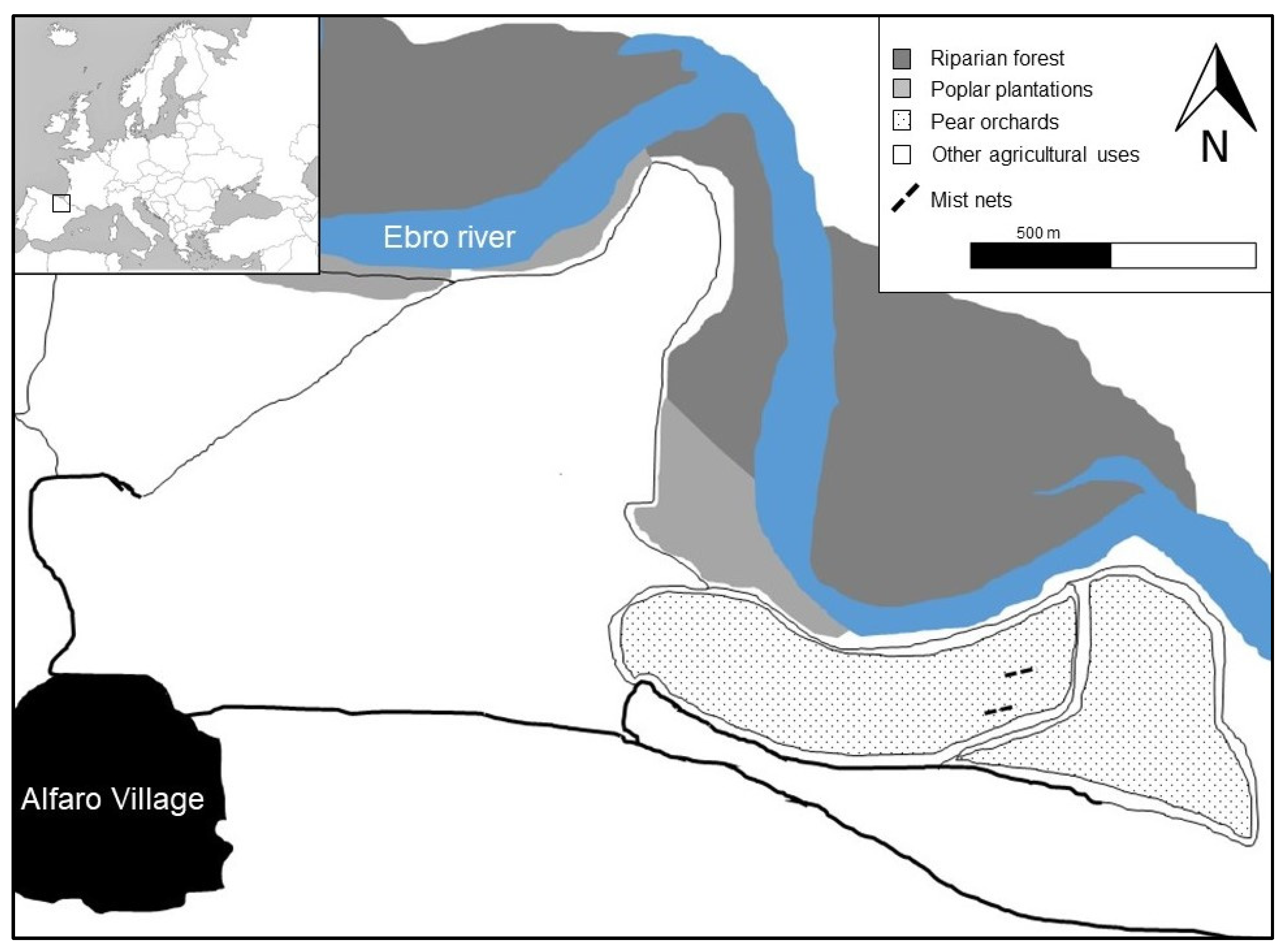
2.1. Study Area
2.2. Field Data Collection
2.3. Molecular Analyses
2.4. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newton, I. Population Limitation in Birds; Academic Press: London, UK, 1998; 597p. [Google Scholar]
- Yaremych, S.A.; Levengood, J.A.; Novak, R.J.; Mankin, P.C.; Warner, R.E. Gender Determination and Lack of Sex-Specific West Nile Virus Mortality in American Crows. Wildl. Soc. Bull. 2004, 32, 893–899. [Google Scholar] [CrossRef]
- O’Dwyer, T.W. Breeding Biology of Gould’s Petrels Pterpodroma leucoptera: Predicting Breeding Outcomes from a Physiological and Morphological Appraisal of Adults. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2004. [Google Scholar]
- Tomalty, K.M. Differential Migration and Phenology of Adult Red-Tailed Hawks in California. J. Raptor Res. 2016, 50, 45–53. [Google Scholar] [CrossRef]
- Redlisiak, M.; Mazur, A.; Remisiewicz, M. Size dimorphism and sex determination in the song thrush (Turdus philomelos) migrating through the southern Baltic coast. Ann. Zool. Fenn. 2019, 57, 31–40. [Google Scholar] [CrossRef]
- Blanco, G.; Tella, J.L. Temporal, spatial and social segregation of red-billed choughs between two types of communal roost: A role for mating and territory acquisition. Anim. Behav. 1999, 57, 1219–1227. [Google Scholar] [CrossRef]
- McCloskey, J.T.; Thompson, J.E. Sex-Related differences in migration chronology and winter habitat use of Common Snipe. Wilson J. Ornithol. 2000, 112, 143–148. [Google Scholar] [CrossRef]
- Owens, I.P.F.; Hartley, I.R. Sexual dimorphism in birds: Why are there so many different forms of dimorphism? Proc. R. Soc. B Biol. Sci. 1998, 265, 397–407. [Google Scholar] [CrossRef]
- Svensson, L. Identification Guide to European Passerines; British Trust for Ornithology: Thetford, UK, 1992; 368p. [Google Scholar]
- Morinha, F.; Cabral, J.A.; Bastos, E. Molecular sexing of birds: A comparative review of polymerase chain reaction (PCR)-based methods. Theriogenology 2012, 78, 703–714. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Newton, I.; Green, R. Bird Ecology and Conservation: A Handbook of Techniques (Techniques in Ecology & Conservation); Oxford University Press: Oxford, UK, 1982; 408p. [Google Scholar]
- Bertolero, A.; Rivaes, S.; Mougeot, F.; Sanchez-Barbudo, I.S.; Andree, K.B.; Ibañez, C. Sexisng and ageing the Purple swamphen Porphyrio porphyrio porphyrio by plumage and biometry. Ardeola 2016, 63, 261–277. [Google Scholar] [CrossRef]
- Nam, H.Y.; Lee, S.Y.; Cho, S.Y.; Choi, C.Y.; Park, S.Y.; Bing, G.C.; Park, C.U.; Seo, S.G.; Kim, Y.M. Effectiveness of Morphological Sex Determination in the East Asian Barn Swallow (Hirundo rustica gutturalis) on Spring Migration. Zool. Stud. 2018, 57, e43. [Google Scholar]
- Costa, J.S.; Hahn, S.; Rocha, A.D.; Araújo, P.A.; Olano-Marín, J.; Emmenegger, T.; Alves, J.A. The discriminant power of biometrics for sex determination in European Bee-eaters Merops apiaster. Bird Study 2020, 67, 19–28. [Google Scholar] [CrossRef]
- Monus, F.; Szabo, K.; Lozsa, A.; Penzes, Z.; Barta, Z. Intersexual size and plumage differences in tree sparrows (Passer montanus)—A morphological study based on molecular sex determination. Acta Zool. Acad. Sci. Hung. 2011, 57, 269–276. [Google Scholar]
- Lee, J.H.; Nam, W.H.; Lee, D.Y.; Sung, H.C. Intersexual differences in the monomorphic Eurasian Tree Sparrow (Passer montanus saturatus). Wilson J. Ornithol. 2022, 134, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Del Hoyo, J.; Elliott, A.; Sargatal, J.; Christie, D.A.; De Juana, E. Eurasian Tree Sparrow (Passer montanus). In Handbook of the Birds of the World Alive; Lynx Edicions: Barcelona, Spain, 2013. [Google Scholar]
- Summers-Smith, J.D. Studies of West Palearctic birds. 197. The Tree Sparrow. Br. Birds 1998, 91, 124–138. [Google Scholar]
- Clement, P. Finches & Sparrows; Princeton University Press: Princeton, NJ, USA, 1999; p. 500. [Google Scholar]
- IUCN. Eurasian Tree Sparrow Passer montanus. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/species/22718270/119216586 (accessed on 20 October 2022).
- McHugh, N.M.; Prior, M.; Leather, S.R.; Holland, J.M. The diet of Eurasian Tree Sparrow Passer montanus nestlings in relation to agri environment scheme habitats. Bird Study 2016, 63, 279–283. [Google Scholar] [CrossRef]
- BirdLife International. European Birds of Conservation Concern: Populations, Trends and National Responsibilities; Birdlife International: Cambridge, UK, 2017; p. 172. [Google Scholar]
- Jokimäki, J.; Suhonen, J.; Kaisanlahti-Jokimäki, M.L. Differential Long-Term Population Responses of Two Closely Related Human-Associated Sparrow Species with Respect to Urbanization. Birds 2021, 2, 230–249. [Google Scholar] [CrossRef]
- Piha, M.; Lehikoinen, E. Body mass and wing length of birds based on ringing database—Part 1. Non-corvid passerines. Linnut-Vuosikirja 2015, 2016, 142–151, (In Finnish with English Summary). [Google Scholar]
- Pinowska, B.; Pinowski, J.; Ham, K. Tree Sparrow (Passer montanus saturatus, Stejneger 1886) can be sexed on the basis of plumage characteristics. Int. Stud. Sparrows 1998, 25, 51–55. [Google Scholar]
- Cordero, P.J. Can the sex of tree sparrow (Passer montanus) be recognized from external features? Int. Stud. Sparrows 1992, 19, 27–29. [Google Scholar]
- Matsui, S.; Kasahara, S.; Kato, T.; Izumi, H.; Morimoto, G.; Ueda, K.; Mikami, O.K. Badge size of male Eurasian tree sparrows Passer montanus correlates with hematocrit during the breeding season. Ornithol. Sci. 2017, 16, 87–91. [Google Scholar] [CrossRef]
- Busse, P.; Meissner, W. Bird Ringing Station Manual; De Gruyter Open Ltd.: Warsaw, Poland; Berlin, German, 2015; 211p. [Google Scholar]
- Senar, J.C. La Medición de la Repetibilidad y el Error de Medida. Etologia 1999, 17, 53–64. (In Spanish) [Google Scholar]
- Kaiser, A. A new multicategory classification of subcutaneous fat deposits of songbirds. J. Field Ornithol. 1993, 64, 246–255. [Google Scholar]
- Griffiths, R.; Double, M.C.; Orr, K.; Dawson, R.J. A DNA test to sex most birds. Mol. Ecol. 1998, 7, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Andrew, S.C.; Awasthy, M.; Griffith, A.D.; Nakagawaa, S.; Griffith, S.C. Clinal variation in avian body size is better explained by summer maximum temperatures during development than by cold winter temperatures. Auk 2018, 135, 206–217. [Google Scholar] [CrossRef]
- Ellrich, H.; Salewski, V.; Fiedler, W. Morphological sexing of passerines: Not valid over larger geographical scales. J. Ornithol. 2010, 151, 449–458. [Google Scholar] [CrossRef]
- McCollin, D.; Hodgson, J.; Crockett, R. Do British birds conform to Bergmann’s and Allen’s rules? An analysis of body size variation with latitude for four species. Bird Study 2015, 62, 404–410. [Google Scholar] [CrossRef]
- Hernández, J.A. Gorrión molinero Passer montanus. In III Atlas de las Aves en Época de Reproducción en España; Molina, B., Nebreda, A., Muñoz, A.R., Seoane, J., Real, R., Bustamante, J., del Moral, J.C., Eds.; SEO/BirdLife: Madrid, Spain, 2022; Available online: https://atlasaves.seo.org/ave/gorrion-molinero/ (accessed on 24 November 2022).
- Zuberogoitia, I.; Alonso, R.; Palomares, L.E.; Martínez, J.A. Sex determination in Eurasian Sparrowhawks (Accipiter nisus). J. Raptor Res. 2011, 45, 48–55. [Google Scholar] [CrossRef]
- Cresswell, W. Diurnal and seasonal mass variation in blackbirds Turdus merula: Consequences for mass-dependent predation risk. J. Anim. Ecol. 1998, 67, 78–90. [Google Scholar] [CrossRef]
- Liker, A.; Papp, Z.; Bókony, V.; Lendvai, A.Z. Lean birds in the city: Body size and condition of house sparrows along the urbanization gradient. J. Anim. Ecol. 2008, 77, 789–795. [Google Scholar] [CrossRef]
- Guallar, S.; Quesada, J.; Gargallo, G.; Herrando, S.; Romero, J.M. Use of discriminant analysis in the sex determination of passerines breeding in the western Mediterranean. Rev. Catalana d’Ornitologia 2010, 26, 38–50. [Google Scholar]
- Evans, M.R.; Goldsmith, A.R.; Norris, S.R.A. The effects of testosterone on antibody production and plumage coloration in male House Sparrows (Passer domesticus). Behav. Ecol. Sociobiol. 2000, 47, 156–163. [Google Scholar] [CrossRef]
- Torda, G.; Liker, A.; Barta, Z. Dominance hierarchy and status signalling in captive tree sparrow (Passer montanus) flocks. Acta Zool. Acad. Sci. Hung. 2004, 50, 35–44. [Google Scholar]
- Monus, F.; Liker, A.; Penzes, Z.; Barta, Z. Status badge-signalling in male but not in female Eurasian Tree Sparrows Passer montanus. Ibis 2017, 159, 180–192. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, L.; Jovani, R.; Stevens, M. Shape matters: Animal colour patterns as signals of individual quality. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162446. [Google Scholar] [CrossRef] [PubMed]

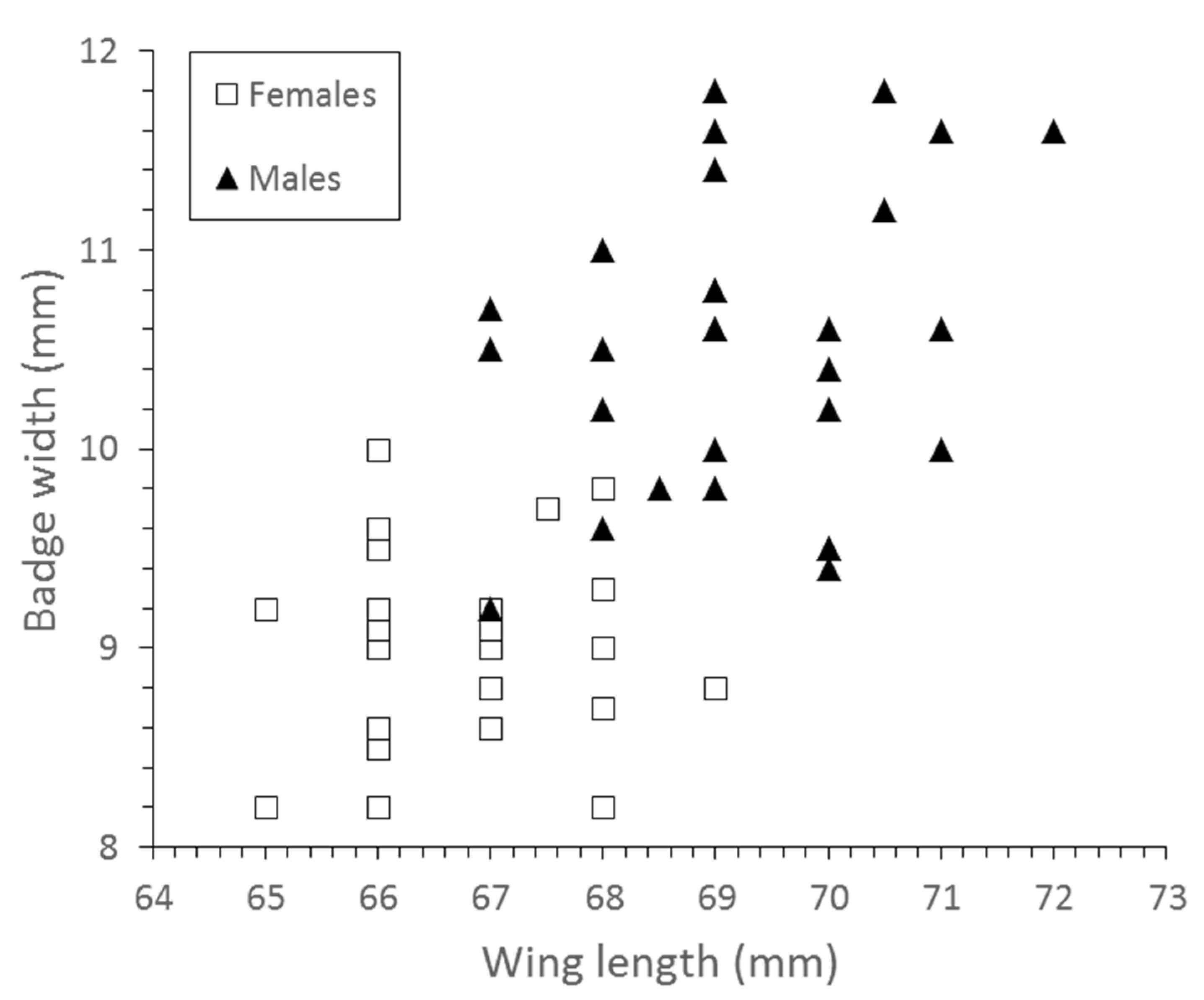
| Traits | Males (n = 32) | Females (n = 34) | Statistics |
|---|---|---|---|
| Wing length (mm) | 69.19 (±1.52) | 66.41 (±1.49) | F = 67.6, df = 1, p < 0.001 |
| 3rd primary (mm) | 53.22 (±1.22) | 51.26 (±1.54) | F = 32.24, df = 1, p < 0.001 |
| Tarsus length (mm) | 17.35 (±0.69) | 17.18 (±0.81) | F = 0.64, df = 1, p = 0.41 |
| Badge width (mm) | 10.53 (±0.76) | 8.96 (±0.49) | F = 85.91, df = 1, p < 0.05 |
| Badge height (mm) | 12.82 (±1.93) | 11.04 (±1.83) | F = 13.44, df = 1, p < 0.001 |
| Weight (g) | 21.05 (±1.26) | 19.96 (±0.99) | F = 13.51, df = 1, p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, S.; Morinha, F.; Villanúa, D.; Goñi, L.; Blanco, G. Discriminant Criteria for Field Sexing in the Eurasian Tree Sparrow by Combining Body Size and Plumage Features. Birds 2022, 3, 402-409. https://doi.org/10.3390/birds3040027
González S, Morinha F, Villanúa D, Goñi L, Blanco G. Discriminant Criteria for Field Sexing in the Eurasian Tree Sparrow by Combining Body Size and Plumage Features. Birds. 2022; 3(4):402-409. https://doi.org/10.3390/birds3040027
Chicago/Turabian StyleGonzález, Sergio, Francisco Morinha, Diego Villanúa, Lander Goñi, and Guillermo Blanco. 2022. "Discriminant Criteria for Field Sexing in the Eurasian Tree Sparrow by Combining Body Size and Plumage Features" Birds 3, no. 4: 402-409. https://doi.org/10.3390/birds3040027
APA StyleGonzález, S., Morinha, F., Villanúa, D., Goñi, L., & Blanco, G. (2022). Discriminant Criteria for Field Sexing in the Eurasian Tree Sparrow by Combining Body Size and Plumage Features. Birds, 3(4), 402-409. https://doi.org/10.3390/birds3040027






