When to Return to Normal? Temporal Dynamics of Vigilance in Four Situations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Data Analysis
2.3. Ethical Note
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paus, T.; Zatorre, R.J.; Hofle, N.; Caramanos, Z.; Gotman, J.; Petrides, M.; Evans, A.C. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J. Cogn. Neurosci. 1997, 9, 392–408. [Google Scholar] [CrossRef] [PubMed]
- Lea, A.J.; Blumstein, D.T. Age and sex influence marmot antipredator behavior during periods of heightened risk. Behav. Ecol. Sociobiol. 2011, 65, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Pecorella, I.; Fattorini, N.; Macchi, E.; Ferretti, F. Sex/age differences in foraging, vigilance and alertness in a social herbivore. Acta Ethol. 2019, 22, 1–8. [Google Scholar] [CrossRef]
- Han, L.; Blank, D.; Wang, M.; Yanget, W. Vigilance behaviour in Siberian ibex (Capra sibirica): Effect of group size, group type, sex and age. Behav Proc. 2020, 170, 104021. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Stillman, R.A.; Smart, S.L.; Bullock, J.M.; Norris, K.J. Are the costs of routine vigilance avoided by granivorous foragers? Func. Ecol. 2011, 25, 617–627. [Google Scholar] [CrossRef]
- Saltz, D.; Berger-Tal, O.; Motro, U.; Shkedy, Y.; Raanan, N. Conservation implications of habituation in Nubian ibex in response to ecotourism. Anim. Cons 2019, 22, 220–227. [Google Scholar] [CrossRef]
- Scheijen, C.P.J.; van der Merwe, S.; Ganswindt, A.; Deacon, F. Anthropogenic influences on distance travelled and vigilance behavior and stress-related endocrine correlates in free-roaming giraffes. Animals 2021, 11, 1239. [Google Scholar] [CrossRef]
- Mettke-Hofmann, C. Morph Composition Matters in the Gouldian Finch (Chloebia gouldiae): Involvement of Red-Headed Birds Increases Vigilance. Birds 2021, 2, 404–414. [Google Scholar] [CrossRef]
- Monclus, R.; Roedel, H.G.; von Holst, D. Fox odour increases vigilance in European rabbits: A study under semi-natural conditions. Ethology 2006, 112, 1186–1193. [Google Scholar] [CrossRef]
- Sauter, C.; Danker-Hopfe, H.; Loretz, E.; Zeitlhofer, J.; Geisler, P.; Popp, R. The assessment of vigilance: Normative data on the Siesta sustained attention test. Sleep Med. 2013, 14, 542–548. [Google Scholar] [CrossRef]
- Hume, G.; Brunton, E.; Burnett, S. Eastern grey kangaroo (Macropus giganteus) vigilance behaviour varies between human-modified and natural environments. Animals 2019, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Flamand, A.; Rebout, N.; Bordes, C.; Guinnefollau, L.; Berges, M.; Ajak, F.; Siutz, C.; Millesi, E.; Weber, C.; Petit, O. Hamsters in the city: A study on the behaviour of a population of common hamsters (Cricetus cricetus) in urban environment. PLoS ONE 2019, 14, e0225347. [Google Scholar] [CrossRef] [PubMed]
- Sirot, E.; Pays, O. On the dynamics of predation risk perception for a vigilant forager. J. Theor. Biol. 2011, 276, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Juricic, E. Sensory basis of vigilance behavior in birds: Synthesis and future prospects. Behav. Proc. 2012, 89, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Welp, T.; Rushen, J.; Kramer, D.L.; Festa-Bianchet, M.; de Passille, A.M.B. Vigilance as a measure of fear in dairy cattle. Appl. Anim. Behav. Sci 2004, 87, 1–13. [Google Scholar] [CrossRef]
- Feyten, L.E.A.; Brown, G.E. Ecological uncertainty influences vigilance as a marker of fear. Anim. Sent. 2018, 15, 7. [Google Scholar] [CrossRef]
- Goldenberg, S.U.; Borcherding, J.; Heynen, M. Balancing the response to predation—The effects of shoal size, predation risk and habituation on behaviour of juvenile perch. Behav. Ecol. Sociobiol. 2014, 68, 989–998. [Google Scholar] [CrossRef]
- Poudel, B.S.; Spooner, P.G.; Matthews, A. Behavioural changes in marmots in relation to livestock grazing disturbance: An experimental test. Eur. J. Wildl. Res. 2016, 62, 491–495. [Google Scholar] [CrossRef]
- Montero-Quintana, A.N.; Vazquez-Haikin, J.A.; Merkling, T.; Blanchard, P.B.; Osorio-Beristain, M. Ecotourism impacts on the behaviour of whale sharks: An experimental approach. Oryx 2020, 54, 270–275. [Google Scholar] [CrossRef]
- Kittendorf, A.; Dantzer, B. Urban fox squirrels exhibit tolerance to humans but respond to stimuli from natural predators. Ethology 2021, 127, 697–709. [Google Scholar] [CrossRef]
- Uchida, K.; Blumstein, D.T. Habituation or sensitization? Long-term responses of yellow-bellied marmots to human disturbance. Behav. Ecol. 2021, 32, 668–678. [Google Scholar] [CrossRef]
- Carbone, C.; Thompson, W.A.; Zadorina, L.; Rowcliffe, J.M. Competition, predation risk and patterns of flock expansion in barnacle geese (Branta leucopsis). J. Zool. 2003, 259, 301–308. [Google Scholar] [CrossRef]
- Beale, C.M.; Monaghan, P. Behavioural responses to human disturbance: A matter of choice? Anim. Behav. 2004, 68, 1065–1069. [Google Scholar] [CrossRef]
- Barros, M.; Alencar, C.; de Souza Silva, M.A.; Tomaz, C. Changes in experimental conditions alter anti-predator vigilance and sequence predictability in captive marmosets. Behav. Proc. 2008, 77, 351–356. [Google Scholar] [CrossRef]
- Dupuch, A.; Morris, D.W.; Halliday, W.D. Patch use and vigilance by sympatric lemmings in predator and competitor-driven landscapes of fear. Behav. Ecol. Sociobiol. 2014, 68, 299–308. [Google Scholar] [CrossRef]
- Kautz, T.M.; Beyer, D.E., Jr.; Farley, Z.; Fowler, N.L.; Kellner III, K.F.; Lutto, A.L.; Petroelje, T.R.; Belant, J.L. American martens use vigilance and short-term avoidance to navigate a landscape of fear from fishers at artificial scavenging sites. Sci. Rep. 2021, 11, 12146. [Google Scholar] [CrossRef]
- Vallino, C.; Caprio, E.; Genco, F.; Chamberlain, D.; Palestrini, C.; Roggero, A.; Bocca, M.; Rolando, A. Behavioural responses to human disturbance in an alpine bird. J. Orn. 2019, 160, 763–772. [Google Scholar] [CrossRef]
- Hammer, T.L.; Bize, P.; Saraux, C.; Gineste, B.; Robin, J.-P.; Groscolas, R.; Viblanc, V.A. Repeatability of alert and flight initiation distances in king penguins: Effects of colony, approach speed, and weather. Ethology 2022, 128, 303–316. [Google Scholar] [CrossRef]
- Meeker, T.J.; Emerson, N.M.; Chien, J.-H.; Saffer, M.I.; Bienvenu, O.J.; Korzeniewska, A.; Greenspan, J.D.; Lenz, F.A. During vigilance to painful stimuli: Slower response rate is related to high trait anxiety, whereas faster response rate is related to high state anxiety. J. Neurophysiol. 2021, 125, 305–319. [Google Scholar] [CrossRef]
- Jun, J.; Remington, R.W.; Koutstaal, W.; Jiang, Y.V. Characteristics of sustaining attention in a gradual-onset continuous performance task. J. Exp. Psychol. Human Perc. Perf. 2019, 45, 386–401. [Google Scholar] [CrossRef]
- Siddle, D.A.T. Vigilance decrement and speed of habituation of the GSR component of the orienting response. Br. J. Psychol. 1972, 63, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, B.; Helton, W.S.; Bedoya, C.; Dolev, Y.; Nelson, X.J. Psychophysical investigation of vigilance decrement in jumping spiders: Overstimulation or understimulation? Anim. Cogn. 2018, 21, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.T.; Whittaker, A.H.; Johnston, S.J. Pupillometry and the vigilance decrement: Task-evoked but not baseline pupil measures reflect declining performance in visual vigilance tasks. Eur. J. Neurosci. 2022, 55, 778–799. [Google Scholar] [CrossRef] [PubMed]
- Melrose, A.; Nelson, X.J.; Dolev, Y.; Helton, W.S. Vigilance all the way down: Vigilance decrement in jumping spiders resembles that of humans. Q. J. Exp. Psychol. 2019, 72, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Terhune, J.M.; Brilliant, S.W. Harbour seal vigilance decreases over time since haul out. Anim. Behav. 1996, 51, 757–763. [Google Scholar] [CrossRef]
- Beauchamp, G.; Ruxton, G.D. Vigilance Decreases with Time at Loafing Sites in Gulls (Larus spp.). Ethology 2012, 118, 733–739. [Google Scholar] [CrossRef]
- Dostine, P.L.; Johnson, G.C.; Franklin, D.C.; Zhang, Y.; Hempel, C. Seasonal use of savanna landscapes by the Gouldian finch, Erythrura gouldiae, in the Yinberrie Hills area, Northern Territory. Wildl. Res. 2001, 28, 445–458. [Google Scholar] [CrossRef]
- Brush, A.H.; Seifried, H. Pigmentation and feather structure in genetic variants of the Gouldian finch, Poephila gouldiae. Auk Ornithol. Adv. 1968, 85, 416–430. [Google Scholar] [CrossRef]
- Fernandez-Juricic, E.; Gall, M.D.; Dolan, T.; O’Rourke, C.; Thomas, S.; Lynch, J. Visual systems and vigilance behaviour of two ground-foraging avian prey species: White-crowned sparrows and California towhees. Anim. Behav. 2011, 81, 705–713. [Google Scholar] [CrossRef]
- Jones, K.A.; Krebs, J.R.; Whittingham, M.J. Vigilance in the third dimension: Head movement not scan duration varies in response to different predator models. Anim. Behav. 2007, 74, 1181–1187. [Google Scholar] [CrossRef]
- Fernandez-Juricic, E.; Beauchamp, G.; Treminio, R.; Hoover, M. Making heads turn: Association between head movements during vigilance and perceived predation risk in brown-headed cowbird flocks. Anim. Behav. 2011, 82, 573–577. [Google Scholar] [CrossRef]
- Krebs, H.; Weyers, P.; Macht, M.; Weijers, H.-G.; Janke, W. Scanning behavior of rats during eating under stressful noise. Physiol. Behav. 1997, 62, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Mettke-Hofmann, C.; Winkler, H.; Leisler, B. The significance of ecological factors for exploration and neophobia in parrots. Ethology 2002, 108, 249–272. [Google Scholar] [CrossRef]
- ASAB. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2020, 159, i–xi. [Google Scholar] [CrossRef]
- Dacier, A.; Maia, R.; Agustinho, D.P.; Barros, M. Rapid habituation of scan behavior in captive marmosets following brief predator encounters. Behav. Proc. 2006, 71, 66–69. [Google Scholar] [CrossRef]
- Pascal, J.; Senar, J.C. Antipredator behavioural compensation of proactive personality trait in male Eurasian siskins. Anim. Behav. 2014, 90, 297–303. [Google Scholar] [CrossRef]
- Risko, E.F.; Anderson, N.C.; Lanthier, S.; Kingstone, A. Curious eyes: Individual differences in personality predict eye movement behavior in scene-viewing. Cogn. 2012, 122, 86–90. [Google Scholar] [CrossRef]
- Sol, D.; Griffin, A.S.; Bartomeus, I.; Boyce, H. Exploring or Avoiding Novel Food Resources? The Novelty Conflict in an Invasive Bird. PLoS ONE 2011, 6, e19535. [Google Scholar] [CrossRef]
- Barros, M.; de Souza Silva, M.A.; Huston, J.P.; Tomaz, C. Multibehavioral analysis of fear and anxiety before, during, and after experimentally induced predatory stress in Callithrix penicillate. Pharm. Biochem. Behav. 2004, 78, 357–367. [Google Scholar] [CrossRef]
- Atkins, A.; Little, R.B.; Redpath, S.M.; Amar, A. Impact of increased predation risk on vigilance behaviour in a gregarious waterfowl, the Egyptian goose Alopochen aegyptiana. J. Avian Biol. 2019, 50, e02121. [Google Scholar] [CrossRef]
- Biedenweg, T.A.; Parsons, M.H.; Fleming, P.A.; Blumstein, D.T. Sounds scary? Lack of habituation following the presentation of novel sounds. PLoS ONE 2011, 6, e14549. [Google Scholar] [CrossRef] [PubMed]
- Rinck, M.; Becker, E.S. Spider fearful individuals attend to threat, then quickly avoid it: Evidence from eye movements. J. Abnorm. Psychol. 2006, 115, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Suzuki, K.K.; Shimamoto, T.; Yanagawa, H.; Koizumi, I. Decreased vigilance or habituation to humans? Mechanisms on increased boldness in urban animals. Behav. Ecol. 2019, 30, 1583–1590. [Google Scholar] [CrossRef]
- Reimers, E.; Loe, L.E.; Eftestol, S.; Colman, J.E.; Dahle, B. Effects of hunting on response behaviors of wild reindeer. J. Wildl. Manag. 2009, 73, 844–851. [Google Scholar] [CrossRef]
- Samia, D.S.M.; Blumstein, D.T.; Stankowich, T.; Cooper, W.E., Jr. Fifty years of chasing lizards: New insights advance optimal escape theory. Biol. Rev. 2016, 91, 349–366. [Google Scholar] [CrossRef]
- Samia, D.S.M.; Blumstein, D.T.; Díaz, M.; Grim, T.; Ibanez-Alamo, J.D.; Jokimäki, J.; Tätte, K.; Marko, G.; Tryjanowski, P.; Moeller, A.P. Rural-urban differences in escape behavior of European birds across a latitudinal gradient. Front. Ecol. Evol. 2017, 5, 66. [Google Scholar] [CrossRef]
- Bergvall, U.A.; Schäpers, A.; Kjellander, P.; Weiss, A. Personality and foraging decisions in fallow deer, Dama dama. Anim. Behav. 2011, 81, 101–112. [Google Scholar] [CrossRef]
- Couchoux, C.; Cresswell, W. Personality constraints versus flexible antipredation behaviors: How important is boldness in risk management of redshanks (Tringa totanus) foraging in a natural system? Behav. Ecol. 2011, 23, 290–301. [Google Scholar] [CrossRef]
- Mazza, V.; Jacob, J.; Dammhahn, M.; Zaccaroni, M.; Eccard, J.A. Individual variation in cognitive style reflects foraging and antipredator strategies in a small mammal. Sci. R. 2019, 9, 10157. [Google Scholar] [CrossRef]
- Perez-Duenas, C.; Acosta, A.; Lupianez, J. Reduced habituation to angry faces: Increased attentional capture as to override inhibition of return. Psychol. Res. 2014, 78, 196–208. [Google Scholar] [CrossRef]
- Childress, M.J.; Lung, M.A. Predation risk, gender and the group size effect: Does elk vigilance depend upon the behaviour of conspecifics? Anim. Behav. 2003, 66, 389–398. [Google Scholar] [CrossRef]
- Giambra, L.M.; Quilter, R.E. Sex differences in sustained attention across the adult life span. J. Appl. Psychol. 1989, 74, 91–95. [Google Scholar] [CrossRef] [PubMed]

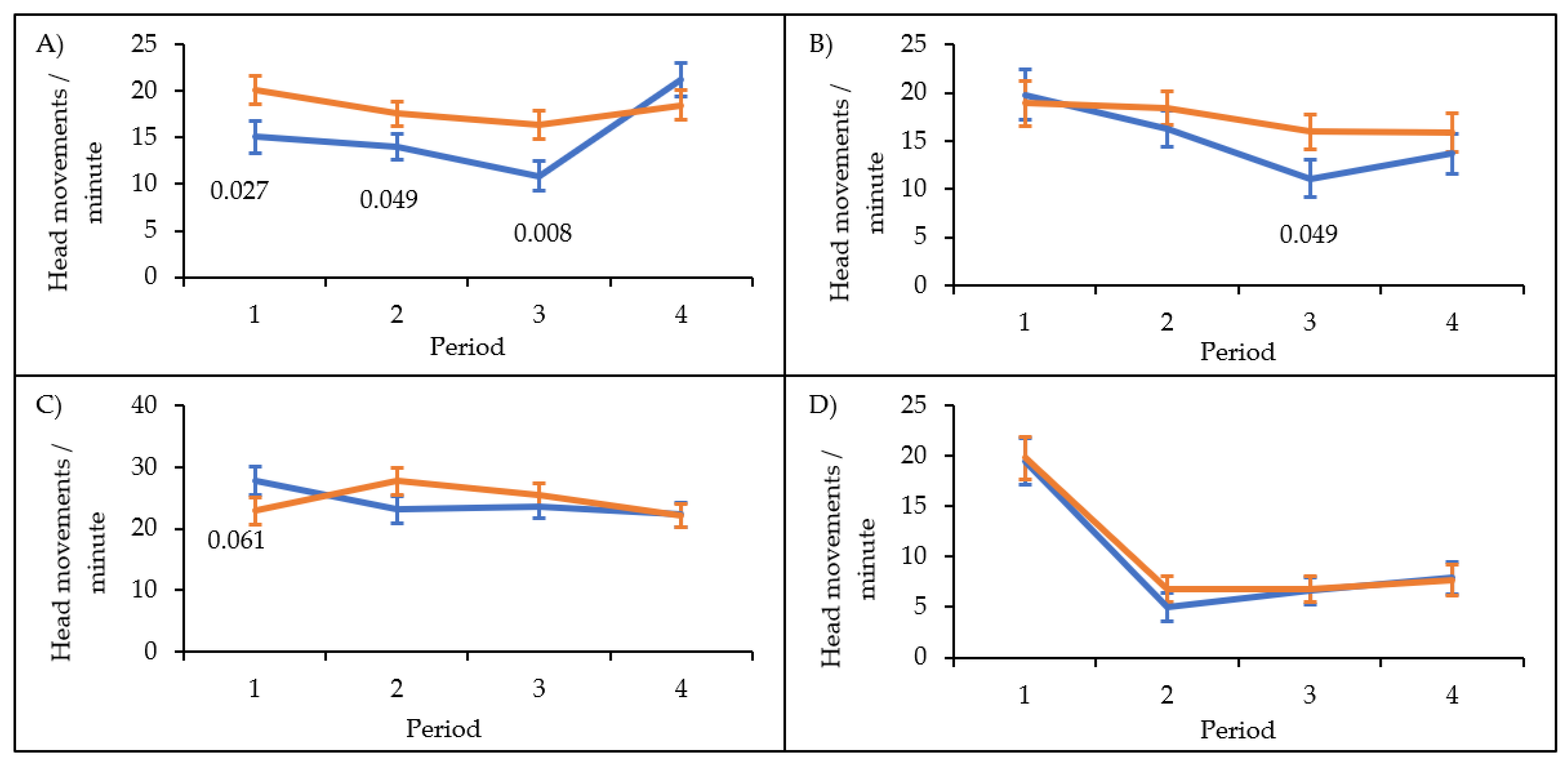
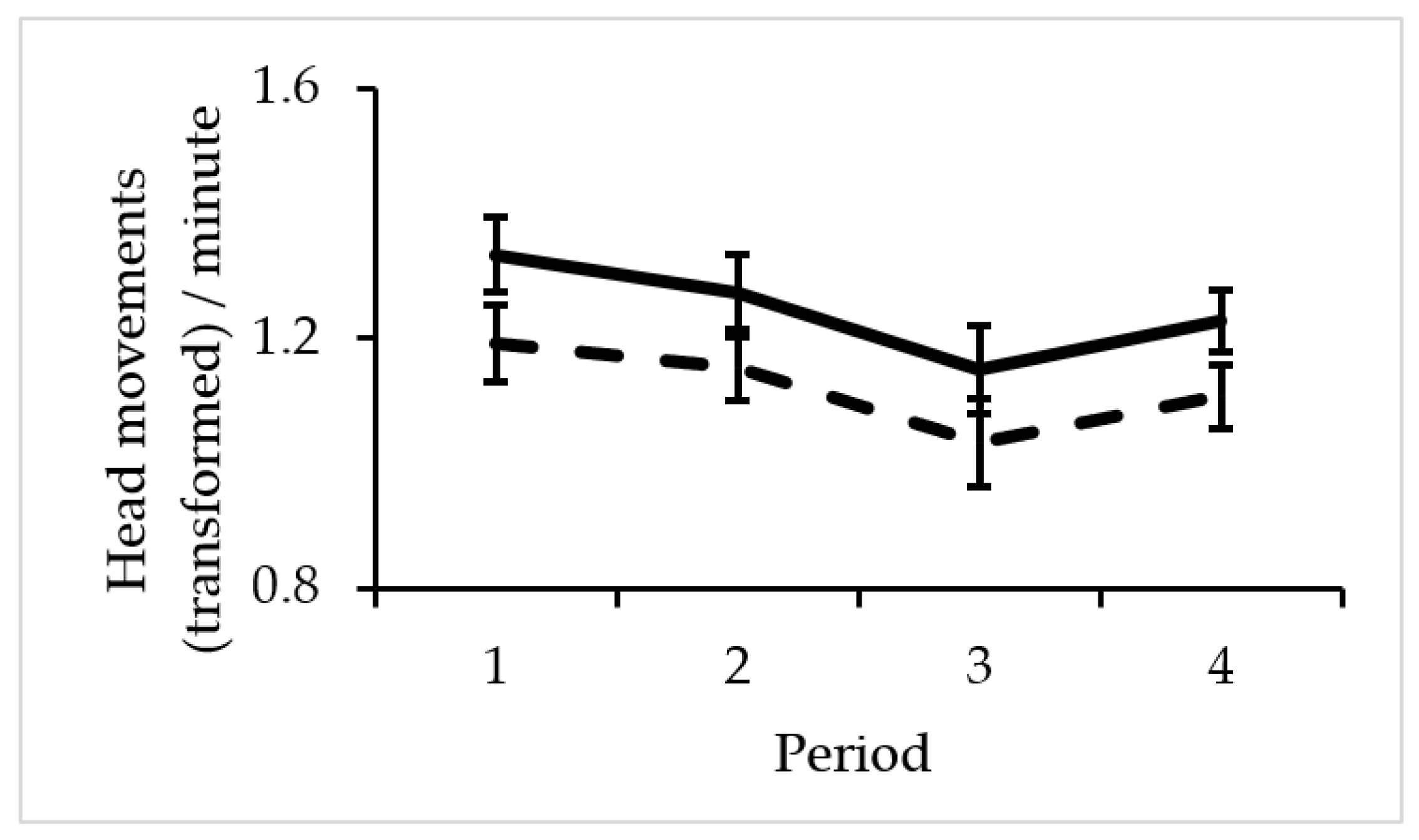

| Variables | F | Df1 | Df2 | p |
|---|---|---|---|---|
| Corrected model | 10.182 | 95 | 256 | <0.001 |
| Period | 14.066 | 3 | 256 | <0.001 |
| Period × situation | 41.122 | 12 | 256 | <0.001 |
| Age class × period × situation | 1.364 | 32 | 256 | 0.100 |
| Sex × period × situation | 2.090 | 16 | 256 | 0.009 |
| Colour morph × period × situation | 1.128 | 16 | 256 | 0.329 |
| Partner colour morph × period × situation | 0.790 | 16 | 256 | 0.696 |
| Variables | F | Df1 | Df2 | p |
|---|---|---|---|---|
| Corrected model | 1.897 | 15 | 70 | 0.038 |
| Period | 5.847 | 3 | 70 | 0.001 |
| Approach | 3.311 | 1 | 70 | 0.073 |
| Period × approach | 0.044 | 3 | 70 | 0.988 |
| Age class × period | 1.397 | 8 | 70 | 0.213 |
| Best Model | ||||
|---|---|---|---|---|
| Variables | F | Df1 | Df2 | p |
| Corrected model | 1.161 | 6 | 81 | 0.336 |
| Period | 1.325 | 3 | 81 | 0.272 |
| Approach | 0.072 | 1 | 81 | 0.789 |
| Period × approach | 0.873 | 2 | 81 | 0.422 |
| Second best model | ||||
| Corrected model | 4.211 | 10 | 77 | <0.001 |
| Period | 1.642 | 3 | 77 | 0.187 |
| Approach | 0.494 | 1 | 77 | 0.484 |
| Period × approach | 4.087 | 2 | 77 | 0.021 |
| Sex × period | 9.213 | 4 | 77 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mettke-Hofmann, C. When to Return to Normal? Temporal Dynamics of Vigilance in Four Situations. Birds 2023, 4, 1-14. https://doi.org/10.3390/birds4010001
Mettke-Hofmann C. When to Return to Normal? Temporal Dynamics of Vigilance in Four Situations. Birds. 2023; 4(1):1-14. https://doi.org/10.3390/birds4010001
Chicago/Turabian StyleMettke-Hofmann, Claudia. 2023. "When to Return to Normal? Temporal Dynamics of Vigilance in Four Situations" Birds 4, no. 1: 1-14. https://doi.org/10.3390/birds4010001
APA StyleMettke-Hofmann, C. (2023). When to Return to Normal? Temporal Dynamics of Vigilance in Four Situations. Birds, 4(1), 1-14. https://doi.org/10.3390/birds4010001






