High Blood Parasite Infection Rate and Low Fitness Suggest That Forest Water Bodies Comprise Ecological Traps for Pied Flycatchers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Birds
2.2. Blood Parasites
2.3. Estimation of Vector Numbers
2.4. Statistics
3. Results
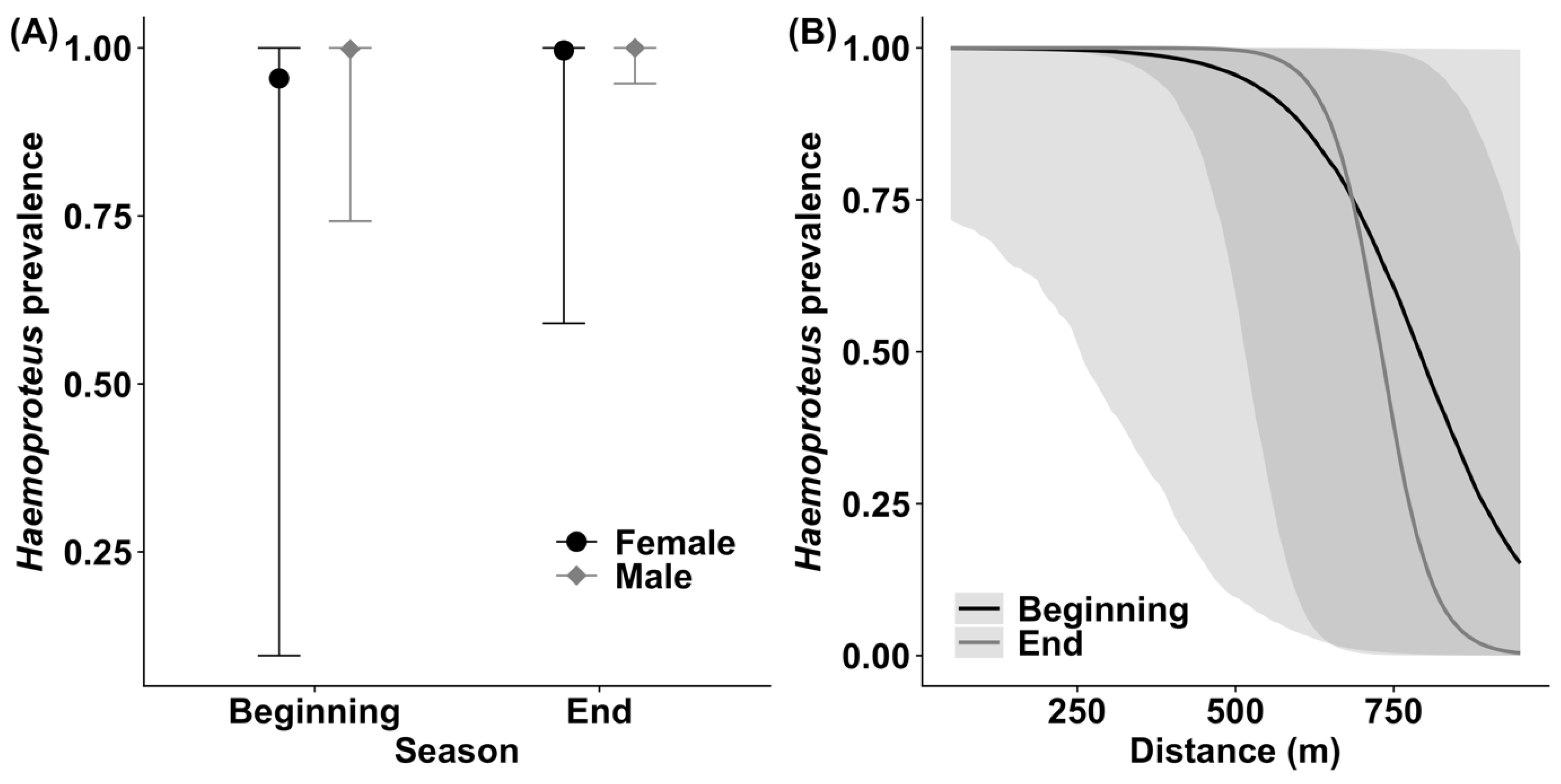
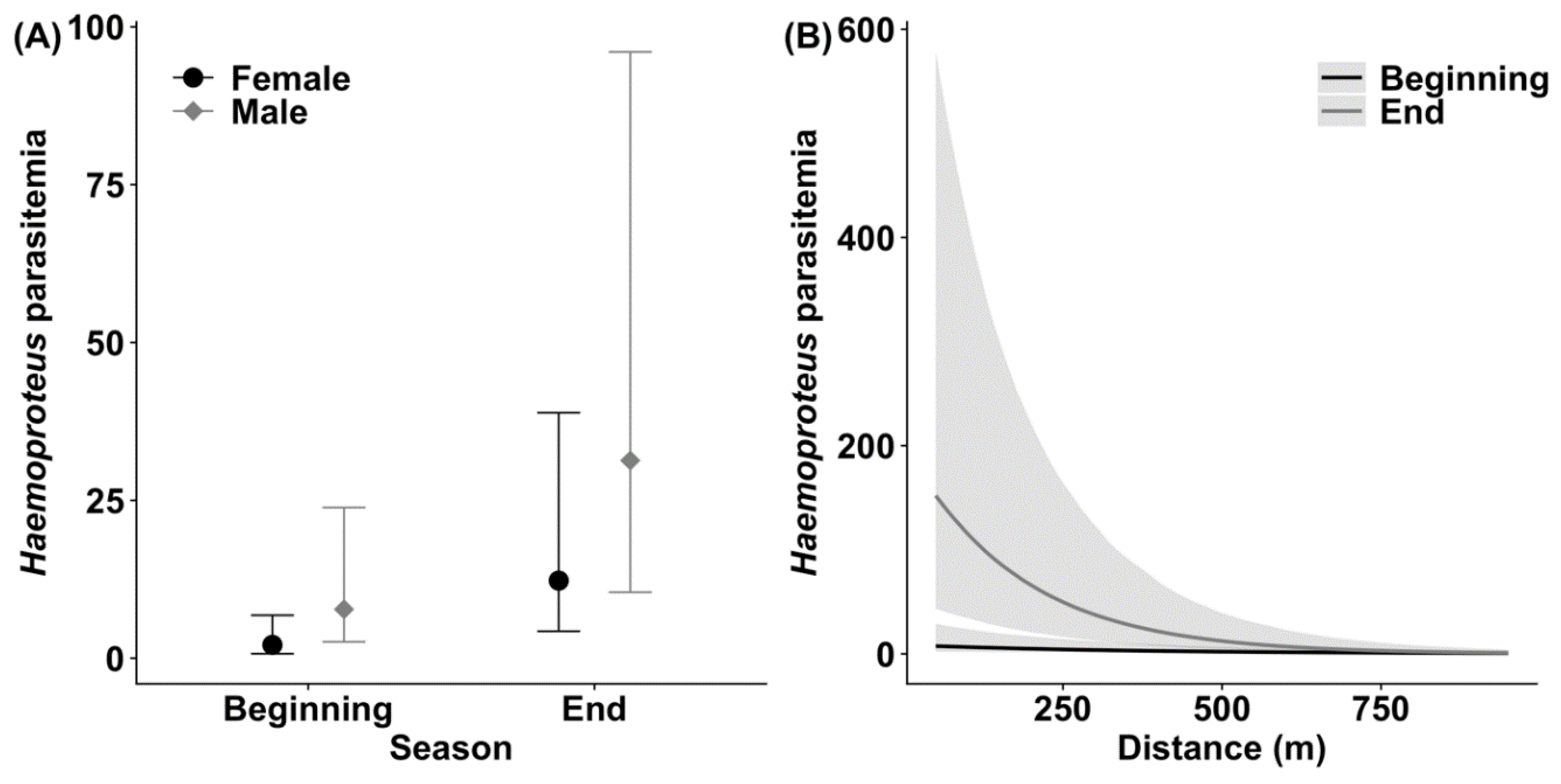
3.1. Haemoproteus
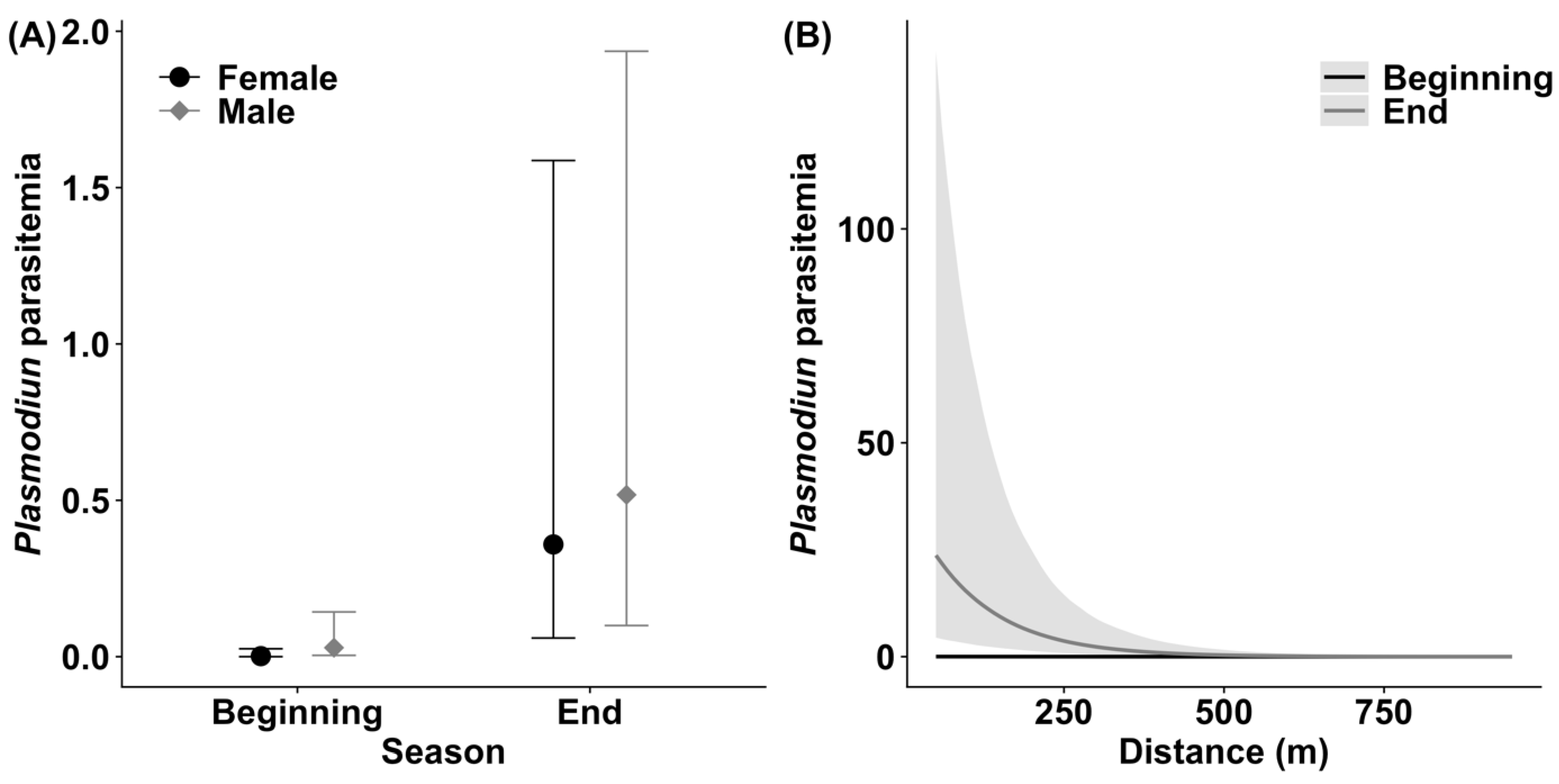
3.2. Plasmodium
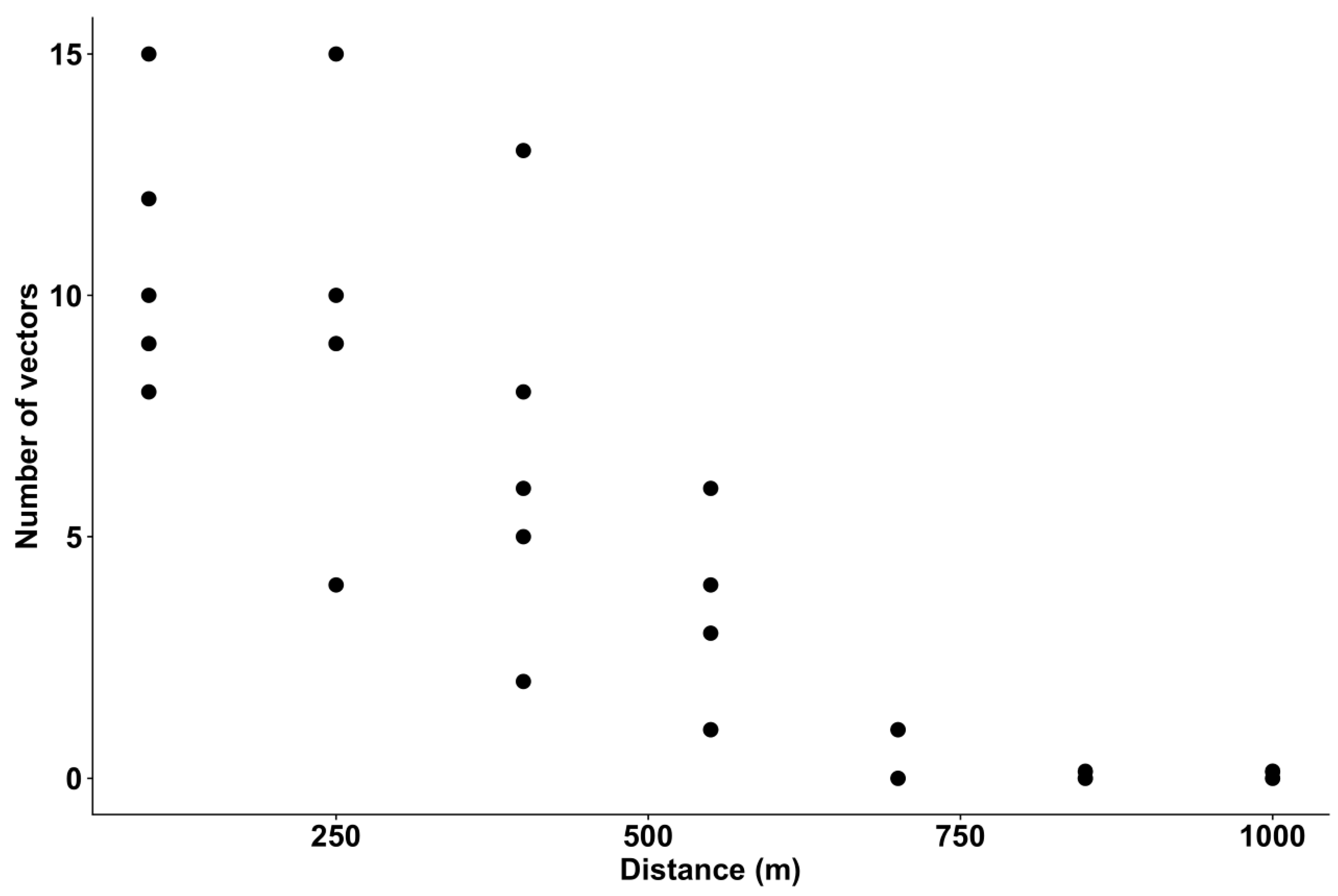
3.3. Vector Abundance
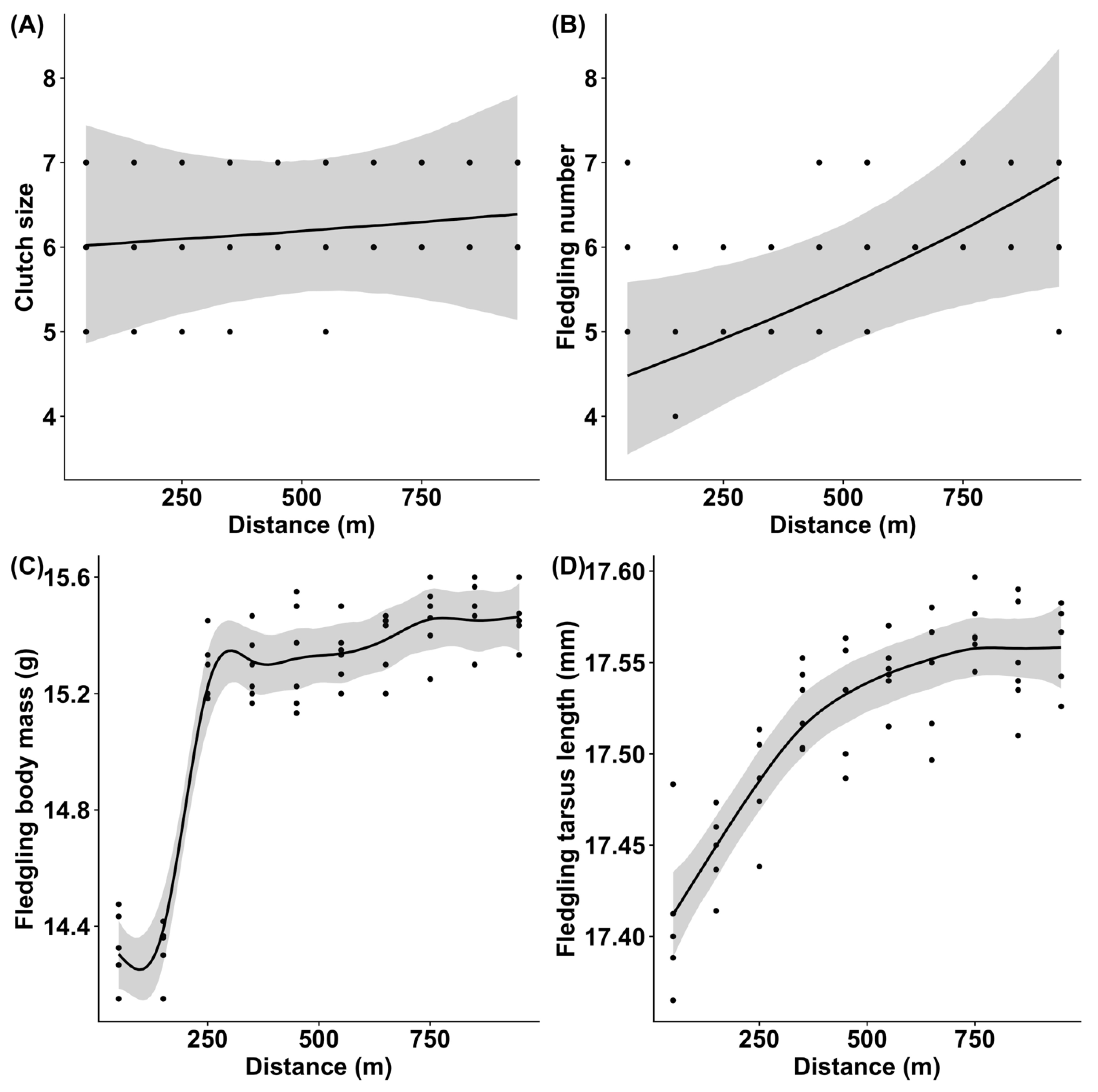
3.4. Fitness Parameters of Pied Flycatchers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmid-Hempel, P. Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Svensson-Coelho, M.; Ricklefs, R.E. Host Phylogeography and Beta Diversity in Avian Haemosporidian (Plasmodiidae) Assemblages of the Lesser Antilles: Haemospordian Beta Diversity and Host Phylogeography. J. Anim. Ecol. 2011, 80, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Krams, I.A.; Suraka, V.; Rantala, M.J.; Sepp, T.; Mierauskas, P.; Vrublevska, J.; Krama, T. Acute Infection of Avian Malaria Impairs Concentration of Haemoglobin and Survival in Juvenile Altricial Birds: Costs of Malaria Infection. J. Zool. 2013, 291, 34–41. [Google Scholar] [CrossRef]
- García-Longoria, L.; Garamszegi, L.Z.; Møller, A.P. Host Escape Behavior and Blood Parasite Infections in Birds. Behav. Ecol. 2014, 25, 890–900. [Google Scholar] [CrossRef]
- Heil, M. Host Manipulation by Parasites: Cases, Patterns, and Remaining Doubts. Front. Ecol. Evol. 2016, 4, 80. [Google Scholar] [CrossRef]
- Asghar, M.; Hasselquist, D.; Hansson, B.; Zehtindjiev, P.; Westerdahl, H.; Bensch, S. Hidden Costs of Infection: Chronic Malaria Accelerates Telomere Degradation and Senescence in Wild Birds. Science 2015, 347, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Krama, T.; Krams, R.; Cīrule, D.; Moore, F.R.; Rantala, M.J.; Krams, I.A. Intensity of Haemosporidian Infection of Parids Positively Correlates with Proximity to Water Bodies, but Negatively with Host Survival. J. Ornithol. 2015, 156, 1075–1084. [Google Scholar] [CrossRef]
- Rätti, O.; Dufva, R.; Alatalo, R.V. Blood Parasites and Male Fitness in the Pied Flycatcher. Oecologia 1993, 96, 410–414. [Google Scholar] [CrossRef]
- Krams, I.; Suraka, V.; Rattiste, K.; Āboliņš-Ābols, M.; Krama, T.; Rantala, M.J.; Mierauskas, P.; Cīrule, D.; Saks, L. Comparative Analysis Reveals a Possible Immunity-Related Absence of Blood Parasites in Common Gulls (Larus canus) and Black-Headed Gulls (Chroicocephalus ridibundus). J. Ornithol. 2012, 153, 1245–1252. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Martínez, J.; Rivero-De-Aguilar, J.; Del Cerro, S.; Merino, S. Vector Abundance Determines Trypanosoma Prevalence in Nestling Blue Tits. Parasitology 2013, 140, 1009–1015. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; Bensch, S.; Roiz, D.; Ruiz, S.; Viana, D.S.; Soriguer, R.C.; Figuerola, J. Ecological Determinants of Avian Malaria Infections: An Integrative Analysis at Landscape, Mosquito and Vertebrate Community Levels. J. Anim. Ecol. 2018, 87, 727–740. [Google Scholar] [CrossRef]
- Gonzalez-Quevedo, C.; Davies, R.G.; Richardson, D.S. Predictors of Malaria Infection in a Wild Bird Population: Landscape-Level Analyses Reveal Climatic and Anthropogenic Factors. J. Anim. Ecol. 2014, 83, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Piersma, T. Do Global Patterns of Habitat Use and Migration Strategies Co-Evolve with Relative Investments in Immunocompetence Due to Spatial Variation in Parasite Pressure? Oikos 1997, 80, 623. [Google Scholar] [CrossRef]
- Warner, R.E. The Role of Introduced Diseases in the Extinction of the Endemic Hawaiian Avifauna. Condor 1968, 70, 101–120. [Google Scholar] [CrossRef]
- van Riper, C.; van Riper, S.G.; Goff, M.L.; Laird, M. The Epizootiology and Ecological Significance of Malaria in Hawaiian Land Birds. Ecol. Monogr. 1986, 56, 327–344. [Google Scholar] [CrossRef]
- Little, R.M.; Earlé, R.A. Sandgrouse (Pterocleidae) and Sociable Weavers Philetarius Socius Lack Avian Haematozoa in Semi-Arid Regions of South Africa. J. Arid Environ. 1995, 30, 367–370. [Google Scholar] [CrossRef]
- Figuerola, J. Effects of Salinity on Rates of Infestation of Waterbirds by Haematozoa. Ecography 1999, 22, 681–685. [Google Scholar] [CrossRef]
- Sol, D.; Jovani, R.; Torres, J. Geographical Variation in Blood Parasites in Feral Pigeons: The Role of Vectors. Ecography 2000, 23, 307–314. [Google Scholar] [CrossRef]
- Hellgren, O.; Bensch, S.; Malmqvist, B. Bird Hosts, Blood Parasites and Their Vectors—Associations Uncovered by Molecular Analyses of Blackfly Blood Meals. Mol. Ecol. 2008, 17, 1605–1613. [Google Scholar] [CrossRef]
- Arriero, E.; Moreno, J.; Merino, S.; Martínez, J. Habitat Effects on Physiological Stress Response in Nestling Blue Tits Are Mediated through Parasitism. Physiol. Biochem. Zool. 2008, 81, 195–203. [Google Scholar] [CrossRef]
- Arriero, E. Rearing Environment Effects on Immune Defence in Blue Tit Cyanistes caeruleus Nestlings. Oecologia 2009, 159, 697–704. [Google Scholar] [CrossRef]
- Sehgal, R.N.M. Deforestation and Avian Infectious Diseases. J. Exp. Biol. 2010, 213, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Kettle, D. Medical and Veterinary Entomology; CAB International: Wallingford, UK, 1995. [Google Scholar]
- Wood, M.J.; Cosgrove, C.L.; Wilkin, T.A.; Knowles, S.C.L.; Day, K.P.; Sheldon, B.C. Within-Population Variation in Prevalence and Lineage Distribution of Avian Malaria in Blue Tits, Cyanistes caeruleus: Within-population variation in blue tit malaria. Mol. Ecol. 2007, 16, 3263–3273. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, F.; Guillaumot, L.; Clegg, S.M.; Phillimore, A.B.; Black, R.A.; Owens, I.P.F.; Mundy, N.I.; Sheldon, B.C. Avian Haematozoan Parasites and Their Associations with Mosquitoes across Southwest Pacific Islands. Mol. Ecol. 2008, 17, 4545–4555. [Google Scholar] [CrossRef] [PubMed]
- Njabo, K.Y.; Cornel, A.J.; Sehgal, R.N.; Loiseau, C.; Buermann, W.; Harrigan, R.J.; Pollinger, J.; Valkiūnas, G.; Smith, T.B. Coquillettidia (Culicidae, Diptera) Mosquitoes Are Natural Vectors of Avian Malaria in Africa. Malar. J. 2009, 8, 193. [Google Scholar] [CrossRef]
- Krams, I.; Cīrule, D.; Krama, T.; Hukkanen, M.; Rytkönen, S.; Orell, M.; Iezhova, T.; Rantala, M.J.; Tummeleht, L. Effects of Forest Management on Haematological Parameters, Blood Parasites, and Reproductive Success of the Siberian Tit (Poecile cinctus) in Northern Finland. Ann. Zool. Fenn. 2010, 47, 335–346. [Google Scholar] [CrossRef]
- Santiago-Alarcon, D.; Palinauskas, V.; Schaefer, H.M. Diptera Vectors of Avian Haemosporidian Parasites: Untangling Parasite Life Cycles and Their Taxonomy. Biol. Rev. 2012, 87, 928–964. [Google Scholar] [CrossRef]
- Minakawa, N.; Dida, G.O.; Sonye, G.O.; Futami, K.; Njenga, S.M. Malaria Vectors in Lake Victoria and Adjacent Habitats in Western Kenya. PLoS ONE 2012, 7, e32725. [Google Scholar] [CrossRef]
- Kibret, S.; Wilson, G.G.; Ryder, D.; Tekie, H.; Petros, B. The Influence of Dams on Malaria Transmission in Sub-Saharan Africa. EcoHealth 2017, 14, 408–419. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Kim, K.S.; Tsuda, Y.; Yamada, A. Bloodmeal Identification and Detection of Avian Malaria Parasite From Mosquitoes (Diptera: Culicidae) Inhabiting Coastal Areas of Tokyo Bay, Japan. J. Med. Entomol. 2009, 46, 1230–1234. [Google Scholar] [CrossRef][Green Version]
- Ferraguti, M.; Martínez-de la Puente, J.; Muñoz, J.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Avian Plasmodium in Culex and Ochlerotatus Mosquitoes from Southern Spain: Effects of Season and Host-Feeding Source on Parasite Dynamics. PLoS ONE 2013, 8, e66237. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; Ruiz, S.; Soriguer, R.; Figuerola, J. On the Study of the Transmission Networks of Blood Parasites from SW Spain: Diversity of Avian Haemosporidians in the Biting Midge Culicoides Circumscriptus and Wild Birds. Parasites Vectors 2013, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Hendry, G. Midges in Scotland; Aberdeen University Press: Aberdeen, UK, 1989. [Google Scholar]
- Both, C.; Bijlsma, R.G.; Visser, M.E. Climatic Effects on Timing of Spring Migration and Breeding in a Long-Distance Migrant, the Pied Flycatcher Ficedula hypoleuca. J. Avian Biol. 2005, 36, 368–373. [Google Scholar] [CrossRef]
- Goffin, B.; Felgueiras, M.; Hof, A.R. Increased Stopover Duration and Low Body Condition of the Pied Flycatcher (Ficedula hypoleuca) at an Autumn Stopover Site. Animals 2020, 10, 2208. [Google Scholar] [CrossRef] [PubMed]
- Rytkönen, S.; Krams, I. Does Foraging Behaviour Explain the Poor Breeding Success of Great Tits Parus major in Northern Europe? J. Avian Biol. 2003, 34, 288–297. [Google Scholar] [CrossRef]
- Brūmelis, G.; Dauškane, I.; Elferts, D.; Strode, L.; Krama, T.; Krams, I. Estimates of Tree Canopy Closure and Basal Area as Proxies for Tree Crown Volume at a Stand Scale. Forests 2020, 11, 1180. [Google Scholar] [CrossRef]
- Ruuskanen, S.; Siitari, H.; Eeva, T.; Belskii, E.; Järvinen, A.; Kerimov, A.; Krams, I.; Moreno, J.; Morosinotto, C.; Mänd, R.; et al. Geographical Variation in Egg Mass and Egg Content in a Passerine Bird. PLoS ONE 2011, 6, e25360. [Google Scholar] [CrossRef]
- Samplonius, J.M.; Bartošová, L.; Burgess, M.D.; Bushuev, A.V.; Eeva, T.; Ivankina, E.V.; Kerimov, A.B.; Krams, I.; Laaksonen, T.; Mägi, M.; et al. Phenological Sensitivity to Climate Change Is Higher in Resident than in Migrant Bird Populations among European Cavity Breeders. Glob. Chang. Biol. 2018, 24, 3780–3790. [Google Scholar] [CrossRef]
- Lundberg, A.; Alatalo, R.V. The Pied Flycatcher; T & AD Poyser: London, UK, 1992. [Google Scholar]
- Ojanen, M. A Method for Age Determination of Pied Flycatchers Ficedula hypoleuca in Spring. Acta Regiae Soc. Sci. Litt. Gothobg. Zool. 1987, 14, 95–101. [Google Scholar]
- Forsman, J.T.; Seppänen, J.-T.; Mönkkönen, M.; Thomson, R.L.; Kivelä, S.M.; Krams, I.; Loukola, O.J. Is It Interspecific Information Use or Aggression between Putative Competitors That Steers the Selection of Nest-Site Characteristics? A Reply to Slagsvold and Wiebe. J. Avian Biol. 2018, 49, jav-01558. [Google Scholar] [CrossRef]
- Loukola, O.J.; Seppänen, J.-T.; Krams, I.; Torvinen, S.S.; Forsman, J.T. Observed Fitness May Affect Niche Overlap in Competing Species via Selective Social Information Use. Am. Nat. 2013, 182, 474–483. [Google Scholar] [CrossRef]
- Bennett, G.F. Simple Techniques for Making Avian Blood Smears. Can. J. Zool. 1970, 48, 585–586. [Google Scholar] [CrossRef]
- Bennett, G.F.; Siikamäki, P.; Jokimäki, J.; Hovi, M.; Huhta, E. Leucocytozoon Muscicapa n. Sp. (Leucocytozoidae: Apicomplexa) from the Pied Flycatcher Ficedula hypoleuca (Pallas) (Passeriformes: Muscicapinae). Syst. Parasitol. 1995, 31, 33–36. [Google Scholar] [CrossRef]
- Rintamäki, P.T.; Huhta, E.; Jokimäki, J.; Squires-Parsons, D. Leucocytozoonosis and Trypanosomiasis in Redstarts in Finland. J. Wildl. Dis. 1999, 35, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Hostedde, A.I.; Zinner, B.; Millar, J.S.; Hickling, G.J. Restitution of Mass-Size Residuals: Validating Body Condition Indices. Ecology 2005, 86, 155–163. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 3 April 2022).
- Bürkner, P.-C. Brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Hale, R.; Swearer, S.E. Ecological Traps: Current Evidence and Future Directions. Proc. R. Soc. B. 2016, 283, 20152647. [Google Scholar] [CrossRef] [PubMed]
- Hildén, O. Habitat Selection in Birds: A Review. Ann. Zool. Fenn. 1965, 2, 53–75. [Google Scholar]
- Mägi, M.; Mänd, R.; Tamm, H.; Sisask, E.; Kilgas, P.; Tilgar, V. Low Reproductive Success of Great Tits in the Preferred Habitat: A Role of Food Availability. Écoscience 2009, 16, 145–157. [Google Scholar] [CrossRef]
- Jokimäki, J.; Huhta, E.; Itämies, J.; Rahko, P. Distribution of Arthropods in Relation to Forest Patch Size, Edge, and Stand Characteristics. Can. J. For. Res. 1998, 28, 1068–1072. [Google Scholar] [CrossRef]
- Muriel, J.; Marzal, A.; Magallanes, S.; García-Longoria, L.; Suarez-Rubio, M.; Bates, P.J.J.; Lin, H.H.; Soe, A.N.; Oo, K.S.; Aye, A.A.; et al. Prevalence and Diversity of Avian Haemosporidians May Vary with Anthropogenic Disturbance in Tropical Habitats in Myanmar. Diversity 2021, 13, 111. [Google Scholar] [CrossRef]
- Illera, J.C.; López, G.; García-Padilla, L.; Moreno, Á. Factors Governing the Prevalence and Richness of Avian Haemosporidian Communities within and between Temperate Mountains. PLoS ONE 2017, 12, e0184587. [Google Scholar] [CrossRef] [PubMed]
- Dale, S.; Kruszewicz, A.; Slagsvold, T. Effects of Blood Parasites on Sexual and Natural Selection in the Pied Flycatcher. J. Zool. 1996, 238, 373–393. [Google Scholar] [CrossRef]
- Magrath, R.D. Nestling Weight and Juvenile Survival in the Blackbird, Turdus merula. J. Anim. Ecol. 1991, 60, 335. [Google Scholar] [CrossRef]
- Naef-Daenzer, B.; Keller, L.F. The Foraging Performance of Great and Blue Tits (Parus major and P. caeruleus) in Relation to Caterpillar Development, and Its Consequences for Nestling Growth and Fledging Weight. J. Anim. Ecol. 1999, 68, 708–718. [Google Scholar] [CrossRef]
- Grüebler, M.U.; Naef-Daenzer, B. Survival Benefits of Post-Fledging Care: Experimental Approach to a Critical Part of Avian Reproductive Strategies. J. Anim. Ecol. 2010, 79, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Grüebler, M.U.; Naef-Daenzer, B. Fitness Consequences of Timing of Breeding in Birds: Date Effects in the Course of a Reproductive Episode. J. Avian Biol. 2010, 41, 282–291. [Google Scholar] [CrossRef]
- Naef-Daenzer, B.; Grüebler, M.U. Post-fledging Survival of Altricial Birds: Ecological Determinants and Adaptation. J. Field Ornithol. 2016, 87, 227–250. [Google Scholar] [CrossRef]
- Sheldon, B.C.; Verhulst, S. Ecological Immunology: Costly Parasite Defences and Trade-Offs in Evolutionary Ecology. Trends Ecol. Evol. 1996, 11, 317–321. [Google Scholar] [CrossRef]
- Marzal, A.; de Lope, F.; Navarro, C.; Møller, A.P. Malarial Parasites Decrease Reproductive Success: An Experimental Study in a Passerine Bird. Oecologia 2005, 142, 541–545. [Google Scholar] [CrossRef]
- Gutiérrez-López, R.; Gangoso, L.; Martínez-de la Puente, J.; Fric, J.; López-López, P.; Mailleux, M.; Muñoz, J.; Touati, L.; Samraoui, B.; Figuerola, J. Low Prevalence of Blood Parasites in a Long-Distance Migratory Raptor: The Importance of Host Habitat. Parasites Vectors 2015, 8, 189. [Google Scholar] [CrossRef]
- Piperaki, E.T.; Daikos, G.L. Malaria in Europe: Emerging Threat or Minor Nuisance? Clin. Microbiol. Infect. 2016, 22, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.W.; Koenraadt, C.; Meiswinkel, R. Mosquitoes and Culicoides Biting Midges: Vector Range and the Influence of Climate Change: -EN- -FR- Les Moustiques et Les Moucherons Piqueurs Culicoides: Diversité Des Vecteurs et Influence Du Changement Climatique -ES- Mosquitos y Jejenes Culicoides: Distribución de Los Vectores e Influencia Del Cambio Climático. Rev. Sci. Tech. OIE 2015, 34, 123–137. [Google Scholar] [CrossRef]
- Brand, S.P.C.; Keeling, M.J. The Impact of Temperature Changes on Vector-Borne Disease Transmission: Culicoides Midges and Bluetongue Virus. J. R. Soc. Interface 2017, 14, 20160481. [Google Scholar] [CrossRef] [PubMed]
- Kluiters, G.; Swales, H.; Baylis, M. Local Dispersal of Palaearctic Culicoides Biting Midges Estimated by Mark-Release-Recapture. Parasit Vectors 2015, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Wiersch, S.C.; Lubjuhn, T.; Maier, W.A.; Kampen, H. Haemosporidian Infection in Passerine Birds from Lower Saxony. J. Ornithol. 2007, 148, 17–24. [Google Scholar] [CrossRef]
- Dubiec, A.; Podmokła, E.; Harnist, I.; Mazgajski, T.D. Haemoparasites of the Pied Flycatcher: Inter-Population Variation in the Prevalence and Community Composition. Parasitology 2018, 145, 912–919. [Google Scholar] [CrossRef]
- Jones, W.; Kulma, K.; Bensch, S.; Cichoń, M.; Kerimov, A.; Krist, M.; Laaksonen, T.; Moreno, J.; Munclinger, P.; Slater, F.M.; et al. Interspecific Transfer of Parasites Following a Range-Shift in Ficedula Flycatchers. Ecol. Evol. 2018, 8, 12183–12192. [Google Scholar] [CrossRef]
- Zuk, M.; McKean, K.A. Sex Differences in Parasite Infections: Patterns and Processes. Int. J. Parasitol. 1996, 26, 1009–1024. [Google Scholar] [CrossRef]
- Calero-Riestra, M.; García, J.T. Sex-Dependent Differences in Avian Malaria Prevalence and Consequences of Infections on Nestling Growth and Adult Condition in the Tawny Pipit, Anthus campestris. Malar. J. 2016, 15, 178. [Google Scholar] [CrossRef]
- Grossman, C. Possible Underlying Mechanisms of Sexual Dimorphism in the Immune Response, Fact and Hypothesis. J. Steroid Biochem. 1989, 34, 241–251. [Google Scholar] [CrossRef]
- Folstad, I.; Karter, A.J. Parasites, Bright Males, and the Immunocompetence Handicap. Am. Nat. 1992, 139, 603–622. [Google Scholar] [CrossRef]
- Ots, I.; Hõrak, P. Great Tits Parus major Trade Health for Reproduction. Proc. R. Soc. B Biol. Sci. 1996, 263, 1443–1447. [Google Scholar]
- Grossman, C. Interactions between the Gonadal Steroids and the Immune System. Science 1985, 227, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Alatalo, R.V.; Lundberg, A. Polyterritorial Polygyny in the Pied Flycatcher. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 1990; Volume 19, pp. 1–27. ISBN 978-0-12-004519-8. [Google Scholar]
- Krams, I.; Krams, T.; Cernihovics, J. Selection of Foraging Sites in Mixed Willow and Crested Tit Flocks: Rank-Dependent Survival Strategies. Ornis Fenn. 2001, 78, 1–11. [Google Scholar]
- Mänd, R.; Tilgar, V.; Leivits, A. Calcium, Snails, and Birds: A Case Study. Web Ecol. 2000, 1, 63–69. [Google Scholar] [CrossRef]
- Dhondt, A.A.; Hochachka, W.M. Variations in Calcium Use by Birds During the Breeding Season. Condor 2001, 103, 592–598. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krams, R.; Krama, T.; Elferts, D.; Daukšte, J.; Raibarte, P.; Brūmelis, G.; Dauškane, I.; Strode, L.; Krams, I.A. High Blood Parasite Infection Rate and Low Fitness Suggest That Forest Water Bodies Comprise Ecological Traps for Pied Flycatchers. Birds 2022, 3, 221-233. https://doi.org/10.3390/birds3020014
Krams R, Krama T, Elferts D, Daukšte J, Raibarte P, Brūmelis G, Dauškane I, Strode L, Krams IA. High Blood Parasite Infection Rate and Low Fitness Suggest That Forest Water Bodies Comprise Ecological Traps for Pied Flycatchers. Birds. 2022; 3(2):221-233. https://doi.org/10.3390/birds3020014
Chicago/Turabian StyleKrams, Ronalds, Tatjana Krama, Didzis Elferts, Janīna Daukšte, Patrīcija Raibarte, Guntis Brūmelis, Iluta Dauškane, Linda Strode, and Indrikis A. Krams. 2022. "High Blood Parasite Infection Rate and Low Fitness Suggest That Forest Water Bodies Comprise Ecological Traps for Pied Flycatchers" Birds 3, no. 2: 221-233. https://doi.org/10.3390/birds3020014
APA StyleKrams, R., Krama, T., Elferts, D., Daukšte, J., Raibarte, P., Brūmelis, G., Dauškane, I., Strode, L., & Krams, I. A. (2022). High Blood Parasite Infection Rate and Low Fitness Suggest That Forest Water Bodies Comprise Ecological Traps for Pied Flycatchers. Birds, 3(2), 221-233. https://doi.org/10.3390/birds3020014








