Simple Summary
Many shorebirds are active throughout a 24 h time period, yet few comparisons of nighttime and daytime activity exist. Better understanding of nighttime activities could aid in conservation measures of endangered shorebirds. The Great Lakes population of piping plovers contains fewer than 80 breeding pairs. Within this population, a few pairs breed along Lake Superior in Michigan’s Upper Peninsula. To better understand what leads to success or failure of these nests, we observed behaviors of plovers during both daytime and nighttime. We found they feed more at night than during the day, when their primary predators are active. Our findings give insight into the nighttime activity of these critically endangered birds and help to identify the need for management strategies that limit disturbance at night.
Abstract
Shorebirds commonly exhibit cathemeral activity and commonly forage throughout a 24 h period. Conservation of endangered shorebirds should then extend to protection at night, yet little data exists on overall time budgets of such species at night. The Great Lakes population of piping plovers (Charadrius melodus) is the smallest and most endangered, making each breeding pair an essential part of recovery. Intense monitoring of breeding individuals occurs during the daytime, yet we have little understanding of the time budgets of plovers at night. To gain better insight into the cathemeral behavior of plovers we recorded behaviors of 12 plovers from along Michigan’s Lake Superior shoreline during both day and night in 2018 with the use of a night-vision-capable camera, and compared time budgets of plovers between daytime and nighttime. Overall, piping plovers spent more time and a greater proportion of their time foraging at night and more time devoted to being alert during the day. These differences were especially evident during the chick rearing phase. Limited observations suggest that copulatory activity may also be more common at night. Likely, the threat of avian predation on this population drives the increase in nighttime foraging, despite decreased efficiency. Recognizing the importance of decreasing potential for disturbance during the night should be considered in future management strategies regarding the recovery of this endangered species.
1. Introduction
Cathemerality refers to animal activity throughout both light and dark phases of the 24 h cycle [1,2], and is commonly reported in primates, e.g., [3,4], and other mammals, e.g., [5,6]. It has not been widely used to describe bird behavior, despite numerous studies of both nocturnal and diurnal foraging of bird species, e.g., [7,8,9]. Shorebirds commonly exhibit cathemeral behavior [9,10,11,12], and may use different foraging techniques between daytime and nighttime (i.e., visual or tactile) [10,11,13], or forage in different areas between daytime and night time due to food availability, predation risk, and human activities [11]. Within the shorebirds, many plovers (Charadridae), including the piping plover (Charadrius melodus), rely on visual foraging during daytime and nighttime, and have eye anatomy adapted to allow good vision in both conditions [10,12,14].
The Great Lakes population of piping plover is the smallest, and most endangered, of the three North American subpopulations (Atlantic, Great Lakes, and Great Plains) federally listed under the Endangered Species Act in 1986 [15]. With as few as 12 breeding pairs in 1984 [15] the Great Lakes population has increased to 74 breeding pairs in 2021 [16] through conservation efforts including intense nest monitoring, use of nest exclosures to reduce predation, captive rearing of abandoned eggs, and management aimed at reducing disturbance in breeding areas [15]. Additionally, adult individuals have been marked with unique leg band combinations to allow for resighting and tracking of individuals since 1993 [17], and individuals from each brood are marked with the same unique combination; since 2017 each chick has a unique number to allow individual tracking. Brood band combinations are then changed to individual band combinations if birds return to nest upon maturity.
Continued success of this population on the breeding grounds requires management strategies that increase survival and recruitment in all breeding stages (courtship, incubation, and chick rearing). Several studies have outlined the importance of food availability, body condition, and decreased predation on piping plover nesting success. Pre-nesting forage is essential for females as they transition from migrating to breeding condition [18]. The body condition of chicks is directly related to probability of returning to nest in subsequent years [17], and decreased predation of chicks and adults will be essential for long-term viability of the Great Lakes population [19].
The cathemeral nature of piping plovers allows for nutrient acquisition during both day and night, and to forage when diurnal avian predators are not active. Despite this flexibility, disturbances from both humans and predators can have negative impacts on chick development and predation. Anthropogenic disturbance, such as recreation and ORV (off road vehicles) use, generally during the day, has been shown impact feeding in the Atlantic population and result in decreased chick body condition and survival [20]. Later hatching piping plovers (i.e., those from second nest attempts) were shown to experience lower survival due to poorer body condition and were less likely to successfully breed in the subsequent season [17]. Several studies [17,18,20] underscore the importance sufficient invertebrate prey, and undisturbed foraging time to capture such prey throughout the breeding season to assure successful recruitment of piping plovers. Understanding how plovers feed throughout both day and night is essential in management aimed at decreasing disturbance.
In addition to nutrient acquisition, decreasing depredation rates is an essential strategy for continued success of the Great Lakes population of piping plovers [19]. Protective nest exclosures decrease the chances of egg predation and depredation of adults while incubating eggs [15], yet avian predators pose a significant challenge for population growth. Gulls and corvids prey on chicks; and perhaps the greatest threat to the population overall, merlins (Falco columbarius), prey on adults, chicks, and fledglings [19,21].
Current management strategies include the intense monitoring of all breeding pairs and nests, which includes regular (daily if possible) confirmation of adult and chick presence and assessment of possible threats. Actions include erecting nest exclosures upon locating a nest, collection and raising abandoned eggs in captivity if abandonment occurs, addressing predation issues, and individually marking all individuals. However, depredation occurs, and nest abandonments continue. While disturbance and predation can be identified and documented during the daytime when monitors are present, incidences at night are unknown. Additionally, the nighttime activity of piping plovers themselves is largely unknown.
Piping plovers forage and breed along the sparsely vegetated beaches of the Great Lakes. They are visual predators, utilizing their eye site to locate invertebrates along the beach surface or to glean insects from vegetation [22]. These shorebirds will normally feed near any wet substrate along a beach [23]. Breeding pairs will often forage within a defined territory typically averaging 473 ± 53 m in either direction of a nest [24]. Breeding stage and high parental care drive foraging behaviors [18,25].
A combination of foraging behavior, sparse vegetation along breeding habitat, and proximity to potential predators, such as merlins, require plovers to partition time spent between feeding and watching for predators. Furthermore, behavioral changes throughout three breeding phases (courtship, incubation, and chick rearing) necessitate a fine balance between acquiring energy and avoiding predation. On arrival to breeding grounds, females must select quality foraging areas to quickly prepare for breeding activities [18]. During incubation, foraging decreases [26], and during chick rearing, they must acquire energy to migrate while remaining alert to predation risk toward chicks and themselves.
Cathemeral activity is common in for plovers and other shorebirds [10,11,12,13] and nocturnal foraging may be associated with reduced predation risk and an increased abundance of invertebrate food sources [13]. Tidal cycles were the most important factor determining amount of feeding in Atlantic Coast piping plovers [26] and have been shown to determine feeding frequency in other shorebirds as well [22,23,25]. Piping plovers in the Great Plains moved more at night than during the daytime [27], yet bar-tailed godwits (Limosa lipponica) foraged in smaller territories along the French Atlantic Coast at night [11].
Within this research, we set out to characterize the cathemeral behavior of Piping plovers nesting along Michigan’s Lake Superior shoreline with special interest in foraging behaviors and impacts of anthropogenic disturbance on those behaviors. We expected to find variation in time devoted to different behaviors among breeding phases and potential for anthropogenic disturbance would increase throughout the summer as tourism increased.
2. Materials and Methods
2.1. Study Species and Sites
Within the Great Lakes population, only four breeding locations are found along Lake Superior: Apostle Islands National Lakeshore in Wisconsin, Grand Marais, Vermilion, and Whitefish Point in Michigan’s Upper Peninsula. The breeding population of piping plovers in Michigan’s eastern Upper Peninsula along Lake Superior was once pivotal in recovery when the population was small but has since come to represent a small regional pocket of breeding activity that annually consists of 6–10 breeding pairs [28] and is 190–230 km north of the major breeding population at Sleeping Bear Dunes National Seashore which represented 33 of the total 67 breeding pairs in 2018 [29].
The Lake Superior shoreline represents a unique breeding habitat for Great Lakes piping plovers. Ice out can be up to a month after areas on Lake Michigan, where the majority of the population nests, forcing birds to nest later in the breeding season. This delay puts individual chicks at a presumable disadvantage in time available to acquire energy for migration [17]. These locations are remote and see sporadic levels of human activity varying from little in a season, to a few locals taking a daily walk along the beach, to surges in tourists visiting for a day. Finally, these sites were identified as particularly prone to merlin depredation [19]. The uniqueness of these breeding locations, and the fact that they consistently fledge young each year despite potentially nesting later in the season and having high predation risk during the day indicates that nighttime activity may be essential in their success.
This study was performed during the 2018 breeding season where five pairs nested at three locations, Vermilion (N46.763080, W-85.150961), Whitefish Point (N46.769686, W-84.959219), and Grand Marais (N46.676257, W-85.983728) Michigan, USA (Figure 1). The three locations represent known breeding areas along Michigan’s Lake Superior shoreline and contain sparsely vegetated beach with cobble substrate. Vermilion is a remote nature preserve with relatively few visitors (estimate 0–30/day) during the breeding season compared to the other sites and had one breeding pair that made two nest attempts. Whitefish Point is a part of the Seney National Wildlife Refuge and site of the Whitefish Point Shipwreck Museum which experiences light to heavy tourist activity (estimate 50–2000/day) throughout the breeding season and had one breeding pair. Grand Marais is a small town with a residential beach having both public and private access and experiences local residential use in addition to varying tourist activity (estimate 0–200 people/day) throughout the breeding season and had three breeding pairs.
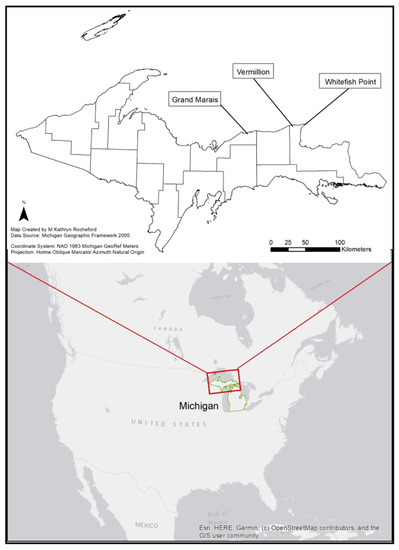
Figure 1.
Regional map of North America, highlighting Michigan in green, and showing breeding sites of piping plovers along Michigan’s Lake Superior Shoreline.
2.2. Video Collection
Knowing we would have a low sample size, we used video to conduct an adapted version of focal animal sampling [30] of piping plovers in two-minute segments [26], where behaviors of all plovers captured in video were tallied. Video filming was performed using a Bestt Guarder Digital Night Vision NV-800 camera mounted to a tripod (1.3 m above the ground). Sessions began May 29th (date chosen based on first arrival of a breeding pair at Vermilion) and occurred at least once per week in paired day/night sessions, ending 7 August 2018. Sunrise varied from 05:46 to 06:29 and sunset varied from 21:02 to 21:38. Once filming dates were set, a beginning daylight observation time was chosen that would correspond 12 h later to another session to conduct night observations at least one hour after sunset and one hour before sunrise to assure darkness. If weather or availability of staff at Whitefish Point eliminated one observation period, we sampled at the next available opportunity. At each site we approached known nest locations and recorded behaviors of the first plover encountered. Upon locating the bird, the observer backed away to the optimal filming distance and allowed 30 min for normal behavior to be restored. Each filming session consisted of six videos with a duration of two minutes each (720 s). Videos were separated by five-minute increments allowing for a 42-min total observation period and providing sufficient opportunity for individual birds to alter behavior before and after any disturbance [26]. Quality of lens and ability to identify behaviors at night limited estimated distance to 20–30 m, sufficient to observe behaviors but avoided disturbing the bird. If an individual bird was feeding in a specific direction, observations were set for the bird to feed away from the camera also allowing for minimal disturbance. After each filming session, distance to the nest or assumed established nest location in the case of plovers in the courtship stage were recorded up to 300 m with a measuring tape and estimated beyond that distance. Artificial light was estimated on a scale of 0 (none detected) to 5 (enough light to simulate full moon).
2.3. Video Analysis
Videos were analyzed focusing on the following variables: feeding, alert, running or flying, aggression, incubation, copulation, and other, as well as noting disturbance due to human presence (birds changing behavior in response to human in video). Feeding was defined as actively searching for food. Alert was defined by a bird in a position with head raised searching for possible threats. Running or flying was defined by birds moving or flying not involved with feeding or aggression. Aggression was defined as any period in which the bird showed territorial dispute between conspecifics or other species. Incubation was defined as an adult sitting on the nest cup. Copulation included tilt displays, goose stepping, and copulation. Other was defined by activities such as loafing or maintenance, or where none of the above variables occurred. During feeding activity, numbers of pecks made at substrate were recorded. Videos were analyzed multiple times focusing on a single behavior each time. Each filming session was analyzed using all six, two-minute time trials, yielding a total of 720 plover seconds observed. In cases where multiple plovers were captured in video, individual behaviors of each plover were tallied, which resulted in greater than 720 plover seconds. We were not able to identify band combinations, or sex of all birds at night, especially those facing away from the camera, but could distinguish chicks from adults.
2.4. Data Analysis
Time budgets, expressed as percent of observed time (sec) dedicated to each behavioral variable, were calculated to standardize observations, to account for instances with more than one plover present, and to compare dedication of time related to each behavior collectively between day and night using a Chi square analysis [26]. Further scrutiny of the percent of time devoted to individual behaviors was performed using Wilcoxon rank sum test [31]. We analyzed each behavior separately and treated percent of time engaged in each behavior independent between day and night. Differences in time devoted to activities were evaluated within breeding stages for those behaviors with more than one observation in each day and night, and in such cases a Bonferroni correction to the P considered significant (0.05/number of comparisons) was applied [32]. Feeding, and efficiency of feeding, was of particular interest, therefore, seconds spent feeding within the normal observation period (720 sec), and pecks to the substrate, were evaluated using the Wilcoxon rank sum test. In those cases where more than one plover was observed feeding both time feeding and pecks to substrate were standardized to 720 sec observations. Distance from nest was non-normally distributed and therefore a permutation test appropriate for linear distance measurements [33] was used to analyze differences between day and nighttime distances. All statistical analysis was performed in R and considered significant at p = 0.05, except where Bonferroni correction was applied.
3. Results
Eleven piping plovers (representing 7.4% of the Great Lakes breeding population) established six nests along the eastern Lake Superior shoreline and fledged a total of eight chicks in 2018. We observed activities of eight different adults and four chicks throughout the three different locations. Overall activities as documented in time budgets varied significantly between day and night (p < 0.001; Table 1). Plovers devoted a greater percent of their time to feeding during the night (48.9% ± 8.6%) than day (22.8% ± 5.6%) (p = 0.023) and, conversely, spent a higher percentage of their time alert during the day (22.3% ± 6.6%) than at night (4.0% ± 1.5%) (p = 0.045; Table 1) (Figure 2). Differences in percent of time devoted to running/flying during the day (11.4% ± 3.0%) and night (7.7% ± 2.5%) and devoted to other activities during day (16.9% ± 7.0%) and night (6.7% ± 3.0%) did not differ (p = 0.169, and p = 0.052, respectively). Aggression was only observed at night during courtship and chick rearing and accounted for 0.5% (±0.3%) of overall nighttime activity. Additionally, within the courtship stage, copulation (including precopulatory events such as goosestepping and tilt displays) occupied 5.2% of time during day (one observation period) and 15.7% (±4.7%) of nighttime activities (two observation periods) of the one pair observed.

Table 1.
Mean (±SE), test, test statistic, degrees of freedom, calculated p, sample size (n), and correction to p (Bonferroni applied where appropriate resulting in value > 0.05) for all behaviors (combined) and individual behaviors observed, and distance from nest (Distance), of piping plovers nesting along Michigan’s Lake Superior shoreline in 2018.
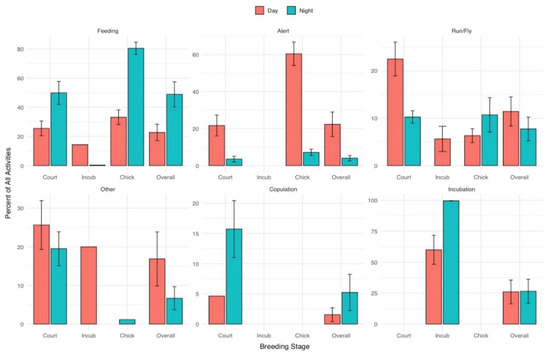
Figure 2.
Cumulative time budget (percent of recorded observation ± se) for behaviors observed (feeding, alert, running or flying, other, copulation, and incubation) from 12 piping plovers throughout three stages of the 2018 breeding season along Lake Superior, Michigan, USA.
We were unable to detect any significant differences in behaviors between day and night within breeding phases after applying Bonferroni corrections (Table 1).
Piping plovers spent more time engaged in foraging activity at night (480.4 ± 130.4 s) than during the day (246.2 ± 105.5; p = 0.023), made similar numbers of pecks to substrate between day and night (174.8 ± 89.1 and 187.8 ± 96.6, p = 0.877), and devoted a higher percent of time to feeding at night (Figure 3), indicating that foraging efficiency may have been decreased at night. Again, we were unable to detect differences within breeding phases after application of the Bonferroni correction (Table 1). Distance the plovers were found from nests was greater during the day (206.78 m ± 61.72 m) than at night (30.37 m ± 4.29 m) (Permutation test, S = −3.21, p = 0.011) (Figure 4), low samples sizes within breeding phases did not allow further assessment.
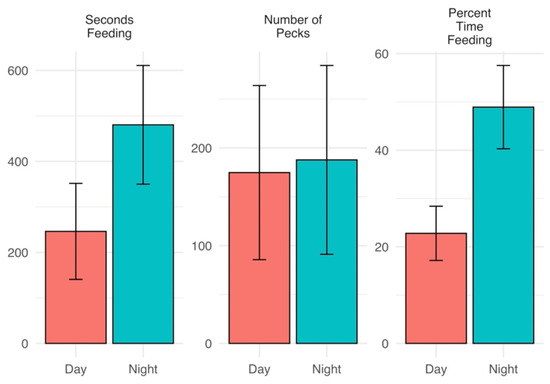
Figure 3.
Mean (±se) time spent feeding (seconds), numbers of pecks to substrate during feeding, and time budgeted to feeding (percent of observed time) for 12 piping plovers during the 2018 breeding season along Lake Superior, Michigan, USA.

Figure 4.
Mean distance from nest location where piping plovers were observed throughout three breeding phases during the 2018 breeding season along Lake Superior, Michigan, USA.
We did not observe any instances of disturbance due to humans within our videos and, thus, the level of activity in relation to disturbance was not assessed. Only one observation of artificial light above 0 was recorded, indicating our sites were devoid of artificial light, and further statistical analyses were not performed.
4. Discussion
During the 2018 breeding season we examined the cathemeral behavior of piping plovers by creating time budgets using seven different behaviors displayed by 12 plovers observed during both day and night. Overall, plovers spent significantly more time foraging during the night than during the day, but made similar numbers of pecks to the substrate during both night and day. They also spent more time alert and were observed further from nests during the day than at night. We also observed that breeding phase specific behaviors, such as copulation, accounted for more time at night than during the day, and we recorded more incubation at night than during the day, although likely an artifact of sampling design.
The time piping plovers devoted to foraging at night was greater than that devoted to foraging during the day, despite observing little foraging activity at night during incubation. The greatest variability in numbers of plovers feeding at night in New Jersey was primarily related to tidal stage, and then breeding stage for two of three sites, but primarily associated with breeding stage in the third [26]. Being a freshwater body, Lake Superior does not experience tidal stages like the oceans and foraging opportunities along the shoreline are impacted by wave action generated by weather patterns and much less predictable.
Foraging along Lake Superior was greatest during courtship and chick rearing stages as was previously along the Atlantic coast [26]. During the courtship stage, piping plovers and other shorebirds in general, devote time to establish their breeding territories and mates. Foraging at this time was particularly important for females needing to attain breeding condition [18], however our camera did not have the resolution to determine sex of plovers at night in all cases so we could not evaluate this. We expected foraging activity to decrease with incubation since, ideally, each adult is spending half of their time devoted to incubation. Our methodology also made daytime observations of foraging during the incubation phase more likely than at night due to the ability to see greater distance and increasing probability of seeing a plover during daylight when approaching the nest area. With limited visibility at night and walks of up to 2 km from access point to the nest area, the probability of encountering a non-incubating plover first was greater during the daytime skewing behavior at night during incubation. Further study focusing on nighttime behaviors of non-incubating plovers is warranted. Predictably, foraging was greatest during the chick stage, partly due to the adults need to acquire energy stores for migration and the fact that we included activities of chicks in need of energy to fledge and then migrate in our observations.
Piping Plovers allocated more time, and a higher percentage of their time, to foraging at night, yet did not make more pecks to the substrate at night. As visual foragers [34] adapted to seeing in both day and night [14], plovers may have a harder time locating, and, thus, spend more time searching for, prey at night. This may be more pronounced in the Great Lakes, where diets consist of arthropods [22], on the substrate rather than benthic invertebrates found in marine environments [34]. We did not analyze the number of pecks to substrate in relation to moon phase as we did not also measure nighttime cloud cover and ambient light near the beach substrate, but, as a visual forager, it seems that efficiency may increase with moonlight. Similarly, common redshanks (Tringa totanus) exhibited more visual than tactile foraging under bright moonlight and fed more visually where artificial light sources were present than where absent [13].
Birds may alter foraging activity in response to predation risk [35] and a factor likely leading to the increased feeding at night, associated increased time alert during day, and evolutionary force driving cathemeral activity [2], is reduced predation risk. Merlins, diurnal hunting raptors, have negatively impacted the Great Lakes piping plover population over the past two decades especially at Michigan breeding sites along Lake Superior [19], and accounted (likely) for a depredation event at Vermilion as they were reported by monitors just prior to the depredation event. Foraging at night when merlins are not actively hunting them, even though less efficient, likely provides much needed respite from their primary predator. Decreased predation risk also explains the observations of increased copulatory activity at night where conspicuous behaviors are not as easily observed.
Time spent alert was greatest during the day of the chick rearing stage and was nearly triple that of the courtship stage. During the day, piping plover pairs exhibited patterns of alertness and vigilance where both parents monitor each other’s behavior [9]. Both parents staying alert would allow for a quicker response in the case that a mate needs assistance.
Breeding territory typically establishes how far from a nest an individual will forage, and individuals from our study stayed in smaller territories than reported previously. Increased copulatory activity at night, which occurs near the nest location, along with aforementioned methodological factors, may account for some of this variation as we likely oversampled nearer nest locations at night. Individuals with chicks were observed within 50 m of a nest location both day and night. Characteristics of sites with chicks (Whitefish Point and Grand Marais) lend themselves to relatively small territories, as both are fairly narrow. For example, from the nest location at Whitefish Point, birds could actively feed on either north facing or east facing shoreline within 50 m of the nest location. Adults with chicks could range widely east to west at Grand Marais, yet were most often found near nest locations. Vermilion offers the widest and longest beaches, yet two separate (assumed) depredation events, a snowy owl (Bubo scandiacus) taking the first female during the egg laying period, and a merlin taking the second female during incubation, prevented analysis of foraging during chick rearing.
Anthropogenic disturbances were not observed in 2018 during this study due to the remote locations. Whitefish Point represented the most human activity but directs visitors away from birds with psychological barriers (signs indicating area closed with string between them to mark the boundary of closed area) surrounding the entire known historical breeding area. Grand Marais represented the only site where human effects on foraging behavior were likely, but little occurred during our observations.
Overall, we have documented the cathemeral behavior of piping plovers breeding along Michigan’s Lake Superior but must acknowledge some limitations of our study. As a one-year study in remote locations with limited access and personnel, we have a small sample size and fairly narrow scope of data collected, as we focused on estimating time budgets. Furthermore, with few individuals among three sites, differences in behavior of plovers at individual sites may have skewed overall variation observed. While monitors look for, and report presence of, predators such as merlins to appropriate officials, their presence was not part of our data set. They were reported at all three sites, but our data collection did not take into account whether or not they were present at the times we observed plovers. Food availability along beaches was also not assessed. Additionally, changes to housing for piping plover monitors in 2019 prevented nighttime observations at Grand Marais and limitations due to the pandemic limited opportunities in 2020 and 2021; therefore, we presented the data available from one year which saw very little human disturbance. Recently, “Yooperlites” (rocks containing fluorescent sodalite) were discovered and promoted along Lake Superior at Vermilion and Whitefish Point. Piping plover monitors reported ATV’s with fluorescent light bars mounted to them in the area and found tracks adjacent to a nest in 2019. This discovery has brought more visitors to the beaches at night and may pose a problem in the future. Due to this, a curfew of 10 pm was imposed on visitors at Vermilion during the 2021 breeding season.
5. Conclusions
Within this study we used a relatively inexpensive night vision system to record behaviors of piping plovers. We add to the growing base of evidence showing how active piping plovers are at night, and the importance of nighttime foraging where they may be less efficient but can avoid predation by merlins. This may be especially true for those nesting along Lake Superior as the shorter breeding season necessitates rapid energy consumption. Perhaps anecdotally at this point, we showed the increased devotion of time to copulation at night during the courtship stage, which warrants further exploration and provides another reason for incorporating protection at night into management strategies.
Author Contributions
Conceptualization and Methodology, R.W. and J.G.; Software, Validation, Formal Analysis, R.W. and J.G.; Investigation and data acquisition, R.W.; Resources, R.W. and J.G.; Data Curation, R.W.; Writing—Original Draft Preparation, R.W.; Writing—Review & Editing, J.G.; Visualization, J.G.; Supervision, J.G.; Project Administration, and Funding Acquisition, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
Plover monitors, who observed plovers every day of the breeding season, were supported by USFWS Cooperative Agreement F18AC00683.
Institutional Review Board Statement
Plover monitoring activities for 2018 were reviewed by the Algoma University Animal Care Committee approval number 2018-SRG-001.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the corresponding author.
Acknowledgments
We want to thank Daniel Derschum (USF&WS) and Michael Gray for field assistance during nighttime observations and Michela Curtis and Tanner Fowler, the Grand Marais plover monitors, for assistance in locating plovers and sharing local knowledge. Thank you to Seney National Wildlife Refuge, especially Greg McClellan, for assistance with access to Whitefish Point to conduct observations. Shannon Rowell-Garvon from Algoma University, provided valuable advice in the early planning stages and thanks to M. Kathryn Rocheford for creating the site map. Finally, we want to thank the Academic Editor and three anonymous reviewers for helpful comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tattersall, I. The concept of cathemerality: History and definition. Folia Primatol. 2006, 77, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Curtis, D.J.; Rasmussen, M.A. The evolution of cathemerality in Primates and other mammals: A comparative and chronoecological approach. Folia Primatol. 2006, 77, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Razanaparany, P.T.; Sato, H. Abiotic Factors Affecting the Cathemeral Activity of Eulemur fulvus in the Dry Deciduous Forest of North-Western Madagascar. Folia Primatol. 2020, 91, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.L.; Veilleux, C.C.; Louis, E.E.; Herrera, J.P.; Hiramatsu, C.; Frankel, D.C.; Irwin, M.T.; Melin, A.D.; Bradley, B.J. Less is more: Lemurs (Eulemur spp.) may benefit from loss of trichromatic vision. Behav. Ecol. Sociobiol. 2019, 73, 22. [Google Scholar] [CrossRef]
- Grignolio, S.; Brivio, F.; Apollonio, M.; Frigato, E.; Tettamanti, F.; Filli, F.; Bertolucci, C. Is nocturnal activity compensatory in chamois? A study of activity in a cathemeral ungulate. Mamm. Biol. 2018, 93, 173–181. [Google Scholar] [CrossRef]
- Castro-Sa, M.J.; Dias-Silva, R.H.; Barnett, A.A. Cathemeral activity by brown-throated three-toed sloths (Bradypus variegatus) in central Amazonian flooded igapó forests. Can. J. Zool. 2021, 99, 832–838. [Google Scholar] [CrossRef]
- Merke, F.R.; Mosbech, A. Diurnal and nocturnal feeding strategies in Common Eiders. Waterbirds 2008, 31, 580–586. [Google Scholar]
- Robert, M.; McNeil, R.; Leduc, A. Conditions and significance of night feeding in shorebirds and other water birds in a tropical lagoon. Auk 1989, 106, 94–101. [Google Scholar] [CrossRef]
- Dodd, S.L.; Colwell, M.A. Environmental correlates of diurnal and nocturnal foraging patterns of nonbreeding shorebirds. Wilson Bull. 1998, 110, 182–189. [Google Scholar]
- Rojas, L.M.; McNeil, R.; Cabana, T.; Lachapelle, P. Diurnal and nocturnal visual capabilities in shorebirds as a function of their feeding strategies. Brain Behav. Evol. 1999, 53, 29–43. [Google Scholar] [CrossRef]
- Jourdan, C.; Fort, J.; Pinaud, D.; Delaporte, P.; Gernigon, J.; Lachaussée, N.; Bocher, P. Nycthemeral Movements of Wintering Shorebirds Reveal Important Differences in Habitat Uses of Feeding Areas and Roosts. Estuar. Coast. 2021, 44, 1454–1468. [Google Scholar] [CrossRef]
- Thomas, R.J.; Székely, T.; Powell, R.F.; Cuthill, I.C. Eye size, foraging methods and the timing of foraging in shorebirds. Funct. Ecol. 2006, 20, 157–165. [Google Scholar] [CrossRef]
- Dwyer, R.G.; Bearhop, S.; Campbell, H.A.; Bryant, D.M. Shedding light on light: Benefits of anthropogenic illumination to a nocturnally foraging shorebird. J. Anim. Ecol. 2013, 82, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.I.; Ross, C.F. Eye shape and activity pattern in birds. J. Zool. 2007, 271, 437–444. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. Recovery Plan for the Great Lakes Piping Plover; Fort Snelling: St. Paul, MN, USA, 2003. [Google Scholar]
- Shubel, S.; (University of Minnesota Great Lakes Piping Plover Field Team, Pellston, MI, USA). Great Lakes Piping Plover Call Newsletter, 2021. Unpublished work. 2021. [Google Scholar]
- Saunders, S.P.; Arnold, T.W.; Roche, E.A.; Cuthbert, F.J. Age specific survival and recruitment of piping plovers Charadrius melodus in the Great Lakes region. J. Avian Biol. 2014, 45, 437–449. [Google Scholar] [CrossRef]
- Cohen, J.B.; Fraser, J.D. Piping Plover foraging distribution and prey abundance in the pre-laying period. Wilson J. Ornithol. 2010, 122, 578–582. [Google Scholar] [CrossRef]
- Saunders, S.P.; Cuthbert, F.J.; Zipkin, E.F. Evaluating population viability and efficiacy of conservation management using integrated population models. J. Appl. Ecol. 2017, 55, 1380–1392. [Google Scholar] [CrossRef]
- DeRose-Wilson, A.L.; Hunt, K.L.; Monk, J.D.; Catlin, D.H.; Karpantry, S.M.; Fraser, J.D. Piping Plover chick survival negatively correlated with beach recreation. J. Wildl. Manag. 2018, 82, 1608–1616. [Google Scholar] [CrossRef]
- Roche, E.A.; Arnold, T.W.; Cuthbert, F.J. Apparent nest abandonment as evidence of breeding-season mortality in Great Lakes Piping Plovers (Charadrius melodus). Auk 2010, 127, 402–410. [Google Scholar] [CrossRef]
- Cuthbert, F.J.; Scholtens, B.; Wemmer, L.C.; McLain, R. Gizzard contents of Piping Plover chicks in northern Michigan. Wilson Bull. 1999, 111, 121–123. [Google Scholar]
- Burger, J. Foraging behavior and the effect of human disturbance on the Piping Plover (Charadrius melodus). J. Coast. Res. 1991, 7, 39–52. [Google Scholar] [CrossRef]
- Haffner, C.D.; Cuthbert, F.J.; Arnold, T.W. Space use by Great Lakes Piping Plovers during the breeding season. J. Field Ornithol. 2009, 80, 270–279. [Google Scholar] [CrossRef]
- Flemming, S.P.; Chiasson, R.D.; Smith, P.C.; Austin-Smith, P.J.; Bancroft, R.P. Piping Plover Status in Nova Scotia Related to Its Reproductive and Behavioral Responses to Human Disturbance (Estatus de Charadrius melodus en Nueva Escocia, Relacionado a su reproducción y respuestas de conducta a la perturbación humana). J. Field Ornithol. 1988, 59, 321–330. [Google Scholar]
- Staine, K.J.; Burger, J. Nocturnal foraging behavior of breeding Piping Plovers (Charadrius melodus) in New Jersey. Auk 1994, 111, 579–587. [Google Scholar]
- Sherfy, M.H.; Anteau, M.J.; Shaffer, T.L.; Sovada, M.A.; Stucker, J.H. Foraging Ecology of Least Terns and Piping Plovers Nesting on Central Platte River Sandpits and Sandbars; Open File Report 2012-1059; U.S. Geological Survey: Reston VA, USA, 2012. [Google Scholar] [CrossRef]
- Garvon, J.M.; (Lake Superior State University. Sault Ste. Marie MI, USA). Unpublished annual reports of grant related activities. Unpublished work. 2020. [Google Scholar]
- Shubel, S.; (University of Minnesota Great Lakes Piping Plover Field Team, Pellston MI, USA). Great Lakes Piping Plover Call newsletter, 2018. Unpublished work. 2018. [Google Scholar]
- Altmann, J. Observational study of behavior: Sampling methods. Behavior 1974, 49, 227–267. [Google Scholar] [CrossRef] [PubMed]
- Koprowski, J.L.; Corse, M.C. Time budgets, activity periods, and behavior of Mexican Fox Squirrels. J. Mammal. 2005, 86, 947–952. [Google Scholar] [CrossRef][Green Version]
- Divine, G.; Norton, H.J.; Hunt, R.; Dienemann, J. A review of analysis and sample size calculation considerations for Wilcoxon Tests. Anesth. Analg. 2013, 117, 699–710. [Google Scholar] [CrossRef]
- Zimmerman, G.M.; Goetz, H.; Mielke, P.W., Jr. Use of an improved statistical method from group comparisons to study effects of prairie fire. Ecology 1985, 66, 606–611. [Google Scholar] [CrossRef]
- Elliott-Smith, E.; Haig, S.M. Piping Plover (Charadrius melodus), version 1.0. In Birds of the World; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Houston, A.I.; McNamara, J.M.; Hutchinson, J.M.C. General results concerning the trade-off between gaining energy and avoiding predation. Phil. Trans. R. Soc. Lond. B 1993, 341, 375–397. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).