Artificial Light at Night, Higher Brain Functions and Associated Neuronal Changes: An Avian Perspective
Abstract
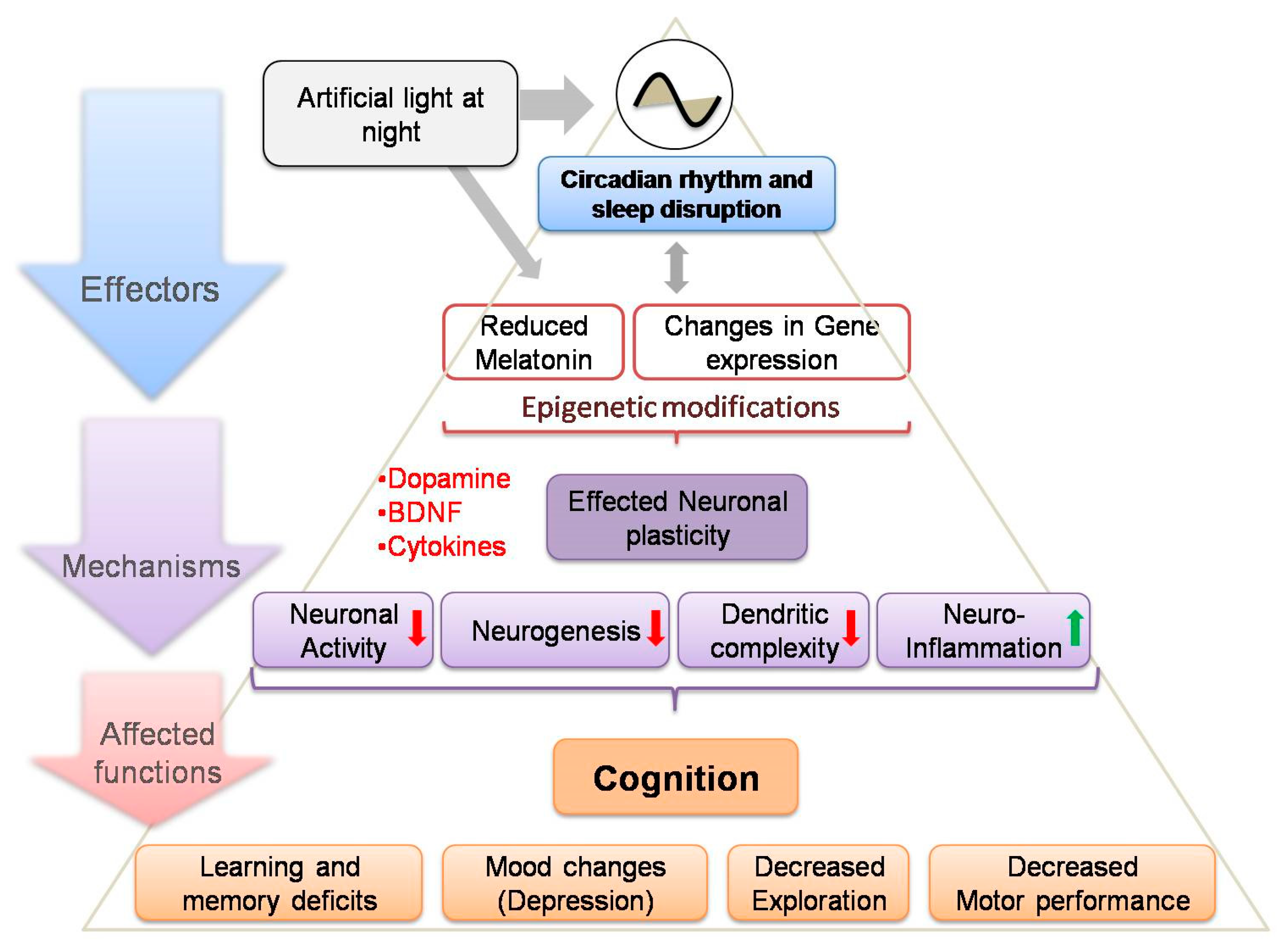
:Simple Summary
Abstract
1. Introduction
2. Review Methods
3. Artificial Light at Night—Circadian Misalignment and Sleep Disruption
4. Adverse Effects on Cognitive Functions
5. Melatonin Induced Modulation of Neuroplasticity
6. Melatonin and Epigenetics: A Possible Link to ALAN-Induced Cognitive Dysfunction
7. Conclusions and Future Direction
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef] [Green Version]
- Rich, C.; Longcore, T. (Eds.) Ecological Consequences of Artificial Night Lighting; Island Press: Washington, DC, USA, 2006. [Google Scholar]
- Navara, K.J.; Nelson, R.J. The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal Res. 2007, 43, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J.; Davies, T.W.; Nedelec, S.L.; Holt, L.A. Impacts of Artificial Light at Night on Biological Timings. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 49–68. [Google Scholar] [CrossRef]
- Dominoni, D.M.; Borniger, J.C.; Nelson, R.J. Light at night, clocks and health: From humans to wild organisms. Biol. Lett. 2016, 12, 20160015. [Google Scholar] [CrossRef] [Green Version]
- Lunn, R.M.; Blask, D.E.; Coogan, A.N.; Figueiro, M.G.; Gorman, M.R.; Hall, J.E.; Hansen, J.; Nelson, R.J.; Panda, S.; Smolensky, M.H.; et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 2017, 607, 1073–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempenaers, B.; Borgström, P.; Loës, P.; Schlicht, E.; Valcu, M. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 2010, 20, 1735–1739. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.; Valcu, M.; Kempenaers, B. Light pollution alters the phenology of dawn and dusk singing in common European songbirds. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140126. [Google Scholar] [CrossRef] [Green Version]
- McLaren, J.D.; Buler, J.J.; Schreckengost, T.; Smolinsky, J.A.; Boone, M.; van Loon, E.E.; Dawson, D.K.; Walters, E.L. Artificial light at night confounds broad-scale habitat use by migrating birds. Ecol. Lett. 2018, 21, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Raap, T.; Pinxten, R.; Eens, M. Artificial light at night disrupts sleep in female great tits (Parus major) during the nestling period, and is followed by a sleep rebound. Environ. Pollut. 2016, 215, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.Q.; Jong, M.; Grunsven, R.H.A.; Matson, K.D.; Haussmann, M.F.; Meerlo, P.; Visser, M.E.; Spoelstra, K. Restless roosts: Light pollution affects behavior, sleep, and physiology in a freeliving songbird. Glob. Chang. Biol. 2017, 23, 4987–4994. [Google Scholar] [CrossRef]
- Leveau, L.M. Artificial Light at Night (ALAN) Is the Main Driver of Nocturnal Feral Pigeon (Columba livia f. domestica) Foraging in Urban Areas. Animals 2020, 10, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenço, P.M. Southern Lapwings Vanelluschilensis may take advantage of artificial illumination duringnight foraging. Wader Study Group Bull. 2012, 119, 61. [Google Scholar]
- Stracey, C.M.; Wynn, B.; Robinson, S.K. Light pollution allows the northern mockingbird (Mimuspolyglottos) to feed nestlings after dark. Wilson J. Ornithol. 2014, 126, 366–369. [Google Scholar] [CrossRef]
- Sierro, A.; Erhardt, A. Light pollution hampers recolonization of revitalised European Nightjar habitats in the Valais (Swiss Alps). J. Ornithol. 2020, 160, 749–761. [Google Scholar] [CrossRef]
- Rodríguez, A.; Orozco-Valor, P.M.; Sarasola, J.H. Artificial light at night as a driver of urban colonization by an avian predator. Landsc. Ecol. 2020, 36, 17–27. [Google Scholar] [CrossRef]
- Taufique, S.T.; Kumar, V. Differential activation and tyrosine hydroxylase distribution in the hippocampal, pallial and midbrain brain regions in response to cognitive performance in Indian house crows exposed to abrupt light environment. Behav. Brain Res. 2016, 314, 21–29. [Google Scholar] [CrossRef]
- Taufique, S.K.T.; Prabhat, A.; Kumar, V. Illuminated night alters hippocampal gene expressions and induces depressive-like responses in diurnal corvids. Eur. J. Neurosci. 2018, 48, 3005–3018. [Google Scholar] [CrossRef]
- Jha, N.A.; Kumar, V. Effect of no-night light environment on behaviour, learning performance and personality in zebra finches. Anim. Behav. 2017, 132, 29–47. [Google Scholar] [CrossRef]
- Cassone, V.M. Avian circadian organization: A chorus of clocks. Front. Neuroendocrinol. 2013, 35, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Dominoni, D.M.; Partecke, J. Does light pollution alter daylength? A test using light loggers on free-ranging European blackbirds (Turdusmerula). Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140118. [Google Scholar] [CrossRef] [Green Version]
- de Jong, M.; Jeninga, L.; Ouyang, J.Q.; van Oers, K.; Spoelstra, K.; Visser, M.E. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol. Behav. 2016, 155, 172–179. [Google Scholar] [CrossRef]
- Miller, M.W. Apparent effects of light pollution on singing behavior of American robins. Condor 2006, 108, 130–139. [Google Scholar] [CrossRef]
- Foster, R.G.; Kreitzman, L. A Time to Reap. BioScience 2005, 55, 795–797. [Google Scholar]
- Dominoni, D.M.; de Jong, M.; van Oers, K.; O’Shaughnessy, P.J.; Blackburn, G.; Atema, E.; Mateman, C.A.; D’Amelio, P.B.; Trost, L.; Bellingham, M.; et al. Artificial light at night leads to circadian disruption in a songbird: Integrated evidence from behavioural, genomic and metabolomic data. bioRxiv 2021. [Google Scholar] [CrossRef]
- Batra, T.; Malik, I.; Prabhat, A.; Bhardwaj, S.K.; Kumar, V. Sleep in unnatural times: Illuminated night negatively affects sleep and associated hypothalamic gene expressions in diurnal zebra finches. Proc. R. Soc. B Boil. Sci. 2020, 287, 20192952. [Google Scholar] [CrossRef] [PubMed]
- Prabhat, A.; Jha, N.A.; Taufique, S.T.; Kumar, V. Dissociation of circadian activity and singing behaviour from gene expression rhythms in the hypothalamus, song control nuclei and cerebellum in diurnal zebra finches. Chronobiol. Int. 2019, 36, 1268–1284. [Google Scholar] [CrossRef]
- Prabhat, A.; Malik, I.; Jha, N.A.; Bhardwaj, S.K.; Kumar, V. Developmental effects of constant light on circadian behaviour and gene expressions in zebra finches: Insights into mechanisms of metabolic adaptation to aperiodic environment in diurnal animals. J. Photochem. Photobiol. B Biol. 2020, 211, 111995. [Google Scholar] [CrossRef] [PubMed]
- Batra, T.; Malik, I.; Kumar, V. Illuminated night alters behaviour and negatively affects physiology and metabolism in diurnal zebra finches. Environ. Pollut. 2019, 254, 112916. [Google Scholar] [CrossRef]
- Malik, I.; Batra, T.; Das, S.; Kumar, V. Light at night affects gut microbial community and negatively impacts host physiology in diurnal animals: Evidence from captive zebra finches. Microbiol. Res. 2020, 241, 126597. [Google Scholar] [CrossRef]
- Mishra, I.; Knerr, R.M.; Stewart, A.A.; Payette, W.I.; Richter, M.M.; Ashley, N.T. Light at night disrupts diel patterns of cytokine gene expression and endocrine profiles in zebra finch (Taeniopygia guttata). Sci. Rep. 2019, 9, 15833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renthlei, Z.; Trivedi, A.K. Effect of urban environment on pineal machinery and clock genes expression of tree sparrow (Passer montanus). Environ. Pollut. 2019, 255, 113278. [Google Scholar] [CrossRef]
- Yorzinski, J.L.; Chisholm, S.; Byerley, S.D.; Coy, J.R.; Aziz, A.; Wolf, J.A.; Gnerlich, A.C. Artificial light pollution increases nocturnal vigilance in peahens. PeerJ 2015, 3, e1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aulsebrook, A.E.; Connelly, F.; Johnsson, R.D.; Jones, T.M.; Mulder, R.A.; Hall, M.L.; Vyssotski, A.L.; Lesku, J.A. White and Amber Light at Night Disrupt Sleep Physiology in Birds. Curr. Biol. 2020, 30, 3657–3663.e5. [Google Scholar] [CrossRef]
- Romeo, S.; Viaggi, C.; Di Camillo, D.; Willis, A.W.; Lozzi, L.; Rocchi, C.; Capannolo, M.; Aloisi, G.; Vaglini, F.; Maccarone, R.; et al. Bright light exposure reduces TH-positive dopamine neurons: Implications of light pollution in Parkinson’s disease epidemiology. Sci. Rep. 2013, 3, 1395. [Google Scholar] [CrossRef]
- Taufique, S.T.; Prabhat, A.; Kumar, V. Constant light environment suppresses maturation and reduces complexity of new born neuron processes in the hippocampus and caudal nidopallium of a diurnal corvid: Implication for impairment of the learning and cognitive performance. Neurobiol. Learn. Mem. 2018, 147, 120–127. [Google Scholar] [CrossRef]
- Prabhat, A.; Kumar, M.; Kumar, A.; Kumar, V.; Bhardwaj, S.K. Effects of Night Illumination on Behavior, Body Mass and Learning in Male Zebra Finches. Birds 2021, 2, 381–394. [Google Scholar] [CrossRef]
- Bordnick, P.S.; Thyer, B.A.; Ritchie, B.W. Feather picking disorder and trichotillomania: An avian model of human psychopathology. J. Behav. Ther. Exp. Psych. 1994, 25, 189–196. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. Linking Molecules to Mood: New Insight Into the Biology of Depression. Am. J. Psychiatry 2010, 167, 1305–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moaraf, S.; Vistoropsky, Y.; Pozner, T.; Heiblum, R.; Okuliarová, M.; Zeman, M.; Barnea, A. Artificial light at night affects brain plasticity and melatonin in birds. Neurosci. Lett. 2020, 716, 134639. [Google Scholar] [CrossRef]
- Moaraf, S.; Heiblum, R.; Vistoropsky, Y.; Okuliarová, M.; Zeman, M.; Barnea, A. Artificial Light at Night Increases Recruitment of New Neurons and Differentially Affects Various Brain Regions in Female Zebra Finches. Int. J. Mol. Sci. 2020, 21, 6140. [Google Scholar] [CrossRef]
- Taufique, S.T.; Prabhat, A.; Kumar, V. Light at night affects hippocampal and nidopallial cytoarchitecture: Implication for impairment of brain function in diurnal corvids. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2019, 331, 149–156. [Google Scholar] [CrossRef]
- Sherwood, C.C.; Stimpson, C.D.; Raghanti, M.A.; Wildman, D.; Uddin, M.; Grossman, L.I.; Goodman, M.; Redmond, J.C.; Bonar, C.J.; Erwin, J.M.; et al. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc. Natl. Acad. Sci. USA 2006, 103, 13606–13611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freas, C.; Roth, T.C.; Ladage, L.D.; Pravosudov, V.V. Hippocampal neuron soma size is associated with population differences in winter climate severity in food-caching chickadees. Funct. Ecol. 2013, 27, 1341–1349. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The glia/neuron ratio: How it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia 2014, 62, 1377–1391. [Google Scholar] [CrossRef]
- Cotter, D.; Mackay, D.; Landau, S.; Kerwin, R.; Everall, I. Reduced Glial Cell Density and Neuronal Size in the Anterior Cingulate Cortex in Major Depressive Disorder. Arch. Gen. Psychiatry 2001, 58, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockmeier, C.A.; Mahajan, G.J.; Konick, L.C.; Overholser, J.C.; Jurjus, G.J.; Meltzer, H.Y.; Uylings, H.B.; Friedman, L.; Rajkowska, G. Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatry 2004, 56, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.-P.; Cao, J.; Tian, M.; Cui, M.-H.; Han, H.-L.; Yang, Y.-X.; Xu, L. Exposure to chronic constant light impairs spatial memory and influences long-term depression in rats. Neurosci. Res. 2007, 59, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, A.; Fujioka, T.; Tsuruta, R.; Izumi, T.; Kasaoka, S.; Maekawa, T. Effects of a constant light environment on hippocampal neurogenesis and memory in mice. Neurosci. Lett. 2011, 488, 41–44. [Google Scholar] [CrossRef]
- Tsuno, N.; Besset, A.; Ritchie, K. Sleep and depression. J. Clin. Psych. 2005, 66, 1254–1269. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Nelson, R.J. Timing of light exposure affects mood and brain circuits. Transl. Psychiatry 2017, 7, e1017. [Google Scholar] [CrossRef]
- Ulgezen, Z.N.; Käpylä, T.; Meerlo, P.; Spoelstra, K.; Visser, M.E.; Dominoni, D.M. The preference and costs of sleeping under light at night in forest and urban great tits. Proc. R. Soc. B Boil. Sci. 2019, 286, 20190872. [Google Scholar] [CrossRef]
- Borniger, J.C.; Weil, Z.M.; Zhang, N.; Nelson, R.J. Dim Light at Night Does Not Disrupt Timing or Quality of Sleep in Mice. Chrono Int. 2013, 30, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Dominoni, D.M.; Goymann, W.; Helm, B.; Partecke, J. Urban-like night illumination reduces melatonin release in European blackbirds (Turdusmerula): Implications of city life for biological timekeeping of songbirds. Front. Zool. 2013, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Dominoni, D.; Quetting, M.; Partecke, J. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominoni, D.M.; Teo, D.; Branston, C.J.; Jakhar, A.; Albalawi, B.F.A.; Evans, N.P. Feather, But Not Plasma, Glucocorticoid Response to Artificial Light at Night Differs between Urban and Forest Blue Tit Nestlings. Integr. Comp. Biol. 2021, 61, 1111–1121. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin: Clinical relevance. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 273–285. [Google Scholar] [CrossRef]
- Hardeland, R. Chronobiology of Melatonin beyond the Feedback to the Suprachiasmatic Nucleus—Consequences to Melatonin Dysfunction. Int. J. Mol. Sci. 2013, 14, 5817–5841. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Clough, S.J.; Dubocovich, M.L. Role of the MT1 and MT2 melatonin receptors in mediating depressive and anxiety-like behaviors in C3H/HeN mice. Genes Brain Behav. 2017, 16, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Rodriguez, G.; Ortíz-López, L.; Domínguez-Alonso, A.; Benítez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal Res. 2011, 50, 29–37. [Google Scholar] [CrossRef]
- Fredrich, M.; Hampel, M.; Seidel, K.; Christ, E.; Korf, H. Impact of melatonin receptor-signaling on Zeitgeber time-dependent changes in cell proliferation and apoptosis in the adult murine hippocampus. Hippocampus 2017, 27, 495–506. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Mayyas, F.A.; Khabour, O.F.; Salama, F.M.B.; Alhashimi, F.H.; Mhaidat, N.M. Chronic melatonin treat-ment prevents memory impairment induced by chronic sleep deprivation. Mol. Neurobiol. 2016, 53, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, H.Q.; Liang, X.Y.; Zhang, H.F.; Zhang, T.; Liu, F.E. Melatonin ameliorates cognitive impairment induced by sleep deprivation in rats: Role of oxidative stress, BDNF and CaMKII. Behav. Brain Res. 2013, 256, 72–81. [Google Scholar] [CrossRef]
- Brüning, A.; Hölker, F.; Franke, S.; Preuer, T.; Kloas, W. Spotlight on fish: Light pollution affects circadian rhythms of European perch but does not cause stress. Sci. Total Environ. 2015, 511, 516–522. [Google Scholar] [CrossRef]
- Brüning, A.; Hölker, F.; Franke, S.; Kleiner, W.; Kloas, W. Influence of light intensity and spectral composition of artificial light at night on melatonin rhythm and mRNA expression of gonadotropins in roach Rutilusrutilus. Fish Physiol. Biochem. 2018, 44, 1–12. [Google Scholar] [CrossRef]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High Sensitivity of Human Melatonin, Alertness, Thermoregulation, and Heart Rate to Short Wavelength Light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahar, S.; Sassone-Corsi, P. Circadian rhythms and memory formation: Regulation by chromatin remodelling. Front. Mol. Neurosci. 2012, 5, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, J.; Zhao, X. Genetics and Epigenetics in Adult Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018911. [Google Scholar] [CrossRef]
- Mews, P.; Calipari, E.S.; Day, J.; Lobo, M.K.; Bredy, T.; Abel, T. From circuits to chromatin: The emerging role of epigenetics in mental health. J. Neurosci. 2021, 41, 873–882. [Google Scholar] [CrossRef]
- Ma, D.K.; Jang, M.-H.; Guo, J.U.; Kitabatake, Y.; Chang, M.-L.; Pow-Anpongkul, N.; Flavell, R.A.; Lu, B.; Ming, G.-L.; Song, H. Neuronal Activity–Induced Gadd45b Promotes Epigenetic DNA Demethylation and Adult Neurogenesis. Science 2009, 323, 1074–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, B.; Christian, K.M.; He, C.; Jin, B.Y.P.; Ming, G.-L.; Song, K.M.C.G.-L.M.H. Epigenetic mechanisms in neurogenesis. Nat. Rev. Neurosci. 2016, 17, 537–549. [Google Scholar] [CrossRef]
- Vollmayr, B.; Mahlstedt, M.M.; Henn, F.A. Neurogenesis and depression: What animal models tell us about the link. Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ottenhof, T.; Rzeczkowska, P.A.; Niles, L.P. Epigenetic targets for melatonin: Induction of histone H3 hyperacetylation and gene expression in C17. 2 neural stem cells. J. Pineal Res. 2008, 45, 277–284. [Google Scholar] [CrossRef]
- Niles, L.P.; Pan, Y.; Kang, S.; Lacoul, A. Melatonin induces histone hyperacetylation in the rat brain. Neurosci. Lett. 2013, 541, 49–53. [Google Scholar] [CrossRef]
- Sarkar, A.; Chachra, P.; Kennedy, P.; Pena, C.J.; Desouza, L.A.; Nestler, E.J.; Vaidya, V.A. Hippocampal HDAC4 contributes to postnatal fluoxetine-evoked depression-like behavior. Neuropsychopharmacology 2014, 39, 2221–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwimmer, H.; Metzer, A.; Pilosof, Y.; Szyf, M.; Machnes, Z.M.; Fares, F.; Harel, O.; Haim, A. Light at night and melatonin have opposite effects on breast cancer tumors in mice assessed by growth rates and global DNA methylation. Chronobiol. Int. 2014, 31, 144–150. [Google Scholar] [CrossRef]
- Haim, A.; Zubidat, A.E. Artificial light at night: Melatonin as a mediator between the environment and epigenome. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.; Dann, P.; Chiaradia, A. Reducing light-induced mortality of seabirds: High pressure sodium lights decrease the fatal attraction of shearwaters. J. Nat. Conserv. 2017, 39, 68–72. [Google Scholar] [CrossRef]
- Cabrera-Cruz, S.A.; Smolinsky, J.A.; Buler, J.J. Light pollution is greatest within migration passage areas for nocturnally-migrating birds around the world. Sci. Rep. 2018, 8, 3261. [Google Scholar] [CrossRef]
- Loss, S.R.; Will, T.; Loss, S.S.; Marra, P.P. Bird–building collisions in the United States: Estimates of annual mortality and species vulnerability. Condor 2014, 116, 8–23. [Google Scholar] [CrossRef] [Green Version]
- Griffith, S.C.; Ton, R.; Hurley, L.L.; McDiarmid, C.S.; Pacheco-Fuentes, H. The Ecology of the Zebra Finch Makes It a Great Laboratory Model but an Outlier amongst Passerine Birds. Birds 2021, 2, 60–76. [Google Scholar] [CrossRef]
- Fatal Light Awareness Program, Canada. Available online: https://flap.org/ (accessed on 8 December 2021).
- Lights Out Program, Audubon Society, USA. Available online: https://www.audubon.org/about (accessed on 8 December 2021).
| Species | Light Environment | Affected Behavioural Phenotype | Molecular Correlates | Study |
|---|---|---|---|---|
| American Robins (Turdus migratorius) | ALAN | - Advances the morning chorus into the night | Miller [23] | |
| European Robin (Erithacus rubecula) Eurasian Blackbird (Turdus merula) Song Thrush (Turdus philomelos) Great Tits (Parus major) Blue Tits (Cyanistes caeruleus) Common Chaffinch (Fringilla coelebs) | dLAN | - Alters the phenology of dawn and dusk singing (Earlier in the year than before) | Da Silva et al. [8] | |
| Blackbirds | dLAN (~0.3 lux) | - Early onset of activity and night restlessness | Dominoni and Partecke [22] | |
| Blackbirds | dLAN (~0.3 lux) | - Increase in pre-dawn activity | Decreased nocturnal melatonin levels | Dominoni et al. [54] |
| Blackbirds | dLAN (~0.3 lux) | - Advances seasonal testicular growth. | Dominoni et al. [55] | |
| Great Tits | dLAN (~ 1.6 lux) | - Sleep deficits | Raap et al. [10] | |
| Great Tits | Different doses of dLAN (~0.05, 0.15, 0.5, 1.5 or 5 lux) | - Dose-dependent increase in nocturnal activity | Dose-dependent reduction in nocturnal melatonin levels | deJong et al. [23] |
| Great Tits | dLAN (~8 lux) of different wavelength | - Increase in nocturnal activity - Sleep deficits | Decreased plasma oxalic acid Reduced telomere length (cellular aging) | Ouyang et al. [11] |
| Great Tits | dLAN (~0.1, 0.5, 1.5 and 5 lux) | - Advanced wake-up time | Shift in bmal1 expression Shift in metabolite expressions Desynchronization of metabolic and immune genes | Dominoni et al. [25] |
| Blue Tits (Cyanistes caeruleus) | dLAN (2 lux) | Affects feather glucocorticoid levels | Dominoni et al. [56] | |
| Indian Peafowl (Pavo cristatus) | dLAN (~0.75 lux) | - Increased nocturnal vigilance - Sleep loss | Yorzinski et al. [33] | |
| European Nightjars (Caprimulgus europaeus) | dLAN | - Increased foraging opportunity - Changes in habitat selection | Sierro and Erhardt [15] | |
| Burrowing Owl (Athene cunicularia) | dLAN | - Increased foraging opportunity - Nest habitat selection near light source | Rodríguez et al. [16] | |
| House Crow (Corvus splendens) | LL (~150 lux) | - Activity rhythm disruption -Learning and memory deficits | Reduced neuronal activity in HP and NC Decreased expression of tyrosine hydroxylase in the mid-brain | Taufique and Kumar [17] |
| House Crow | LL (~150 lux) | - Learning and memory deficits | Decreased neurogenesis and dendritic complexity in HP and NC | Taufique et al. [36] |
| House Crow | dLAN (~6 lux) | - Increase in nocturnal activity - Sleep deprivation - Depressive-like | Decreased expression of BDNF, IL1β, TNFR1, NR4A2 in HPs Increased HDAC4 expression and histone H3 acetylation of BDNF gene in HP Decreased neurogenesis in HP Decreased levels of nocturnal melatonin | Taufique et al. [18] * Behavioural phenotypes were rescued by elimination of dLAN |
| House Crow | LL (~150 lux) and dLAN (~6 lux) | Decreased neuronal soma size Reduced glia–neuron ratio | Taufique et al. [42] | |
| Zebra Finch (Taeniopygia guttata) | LL (~5 lux) | - Disturbed activity rhythm - Learning deficits | Jha and Kumar [19] | |
| Zebra Finch (male) | LL (~150 lux) | - Disrupted activity and singing behaviour (30% of individuals) - Decline in song quality, reduced amplitude and song production | Loss of rhythm in the expression of clock genes in hypothalamus | Prabhat et al. [27] |
| Zebra Finch (female) | LL (~150 lux) | - Fattening, weight gain, and lipid accumulation in the liver | Loss of rhythm in expression of clock genes in hypothalamus and peripheral tissues | Prabhat et al. [28] |
| Zebra Finch | LL (~400 lux) and dLAN (~3 lux) | - | Loss of melatonin and corticosterone diurnal pattern Altered diurnal pattern of cytokines in the brain | Mishra et al. [31] |
| Zebra Finch | dLAN (~5 lux) | - Induced night-time feeding and perch-hopping - Sleep deprivation - Learning and memory deficits - Increased neophobia | Prabhat et al. [37] | |
| Zebra Finch | dLAN (~5 lux) | - Sleep deficits | Decreased plasma oxalate levels Decreased tlr4, il-b, nos gene expression, and attenuated achm3 mRNA levels Changes in gene expression of Ca2+ dependent sleep-inducing pathway | Batra et al. [26] |
| Zebra Finch | dLAN (~5 lux) | - Increased night-time activity - Nocturnal feeding - Body fattening and weight gain | Increased levels of plasma glucose Decreased levels of thyroxine and triglycerides Altered metabolic genes expression (sirt1, g6pc, and foxo1) | Batra et al. [29] |
| Zebra Finch | LL (~150 lux) and dLAN (~5 lux) | - Body fattening and weight gain - Hepatic lipid accumulation | Changes in gut microbiome with a decline in Lactobacillus richness | Malik et al. [30] * Phenotype was rescued by Lactobacillus supplement |
| Zebra Finch | dLAN | Increased neuronal recruitment reduced neuronal density in the hippocampus Decreased nocturnal melatonin levels | Moaraf et al. [40,41] | |
| Domestic Pigeons (Columba livia domestica) and Australian Magpies (Cracticus tibicen tyrannica) | dLAN (~9.6 and 18.89) | - reduced sleep duration and fragmentation - slow-wave activity during non-REM sleep - reduced REM sleep | Aulsebrook et al. [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taufique, S.K.T. Artificial Light at Night, Higher Brain Functions and Associated Neuronal Changes: An Avian Perspective. Birds 2022, 3, 38-50. https://doi.org/10.3390/birds3010003
Taufique SKT. Artificial Light at Night, Higher Brain Functions and Associated Neuronal Changes: An Avian Perspective. Birds. 2022; 3(1):38-50. https://doi.org/10.3390/birds3010003
Chicago/Turabian StyleTaufique, S. K. Tahajjul. 2022. "Artificial Light at Night, Higher Brain Functions and Associated Neuronal Changes: An Avian Perspective" Birds 3, no. 1: 38-50. https://doi.org/10.3390/birds3010003
APA StyleTaufique, S. K. T. (2022). Artificial Light at Night, Higher Brain Functions and Associated Neuronal Changes: An Avian Perspective. Birds, 3(1), 38-50. https://doi.org/10.3390/birds3010003






