Nesting Success and Nesting Height in the Critically Endangered Medium Tree Finch (Camarhynchus pauper)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Site and Study Species
2.2. Male Age
2.3. Nest Monitoring
2.4. Mist-Netting
2.5. Statistical Analysis
3. Results
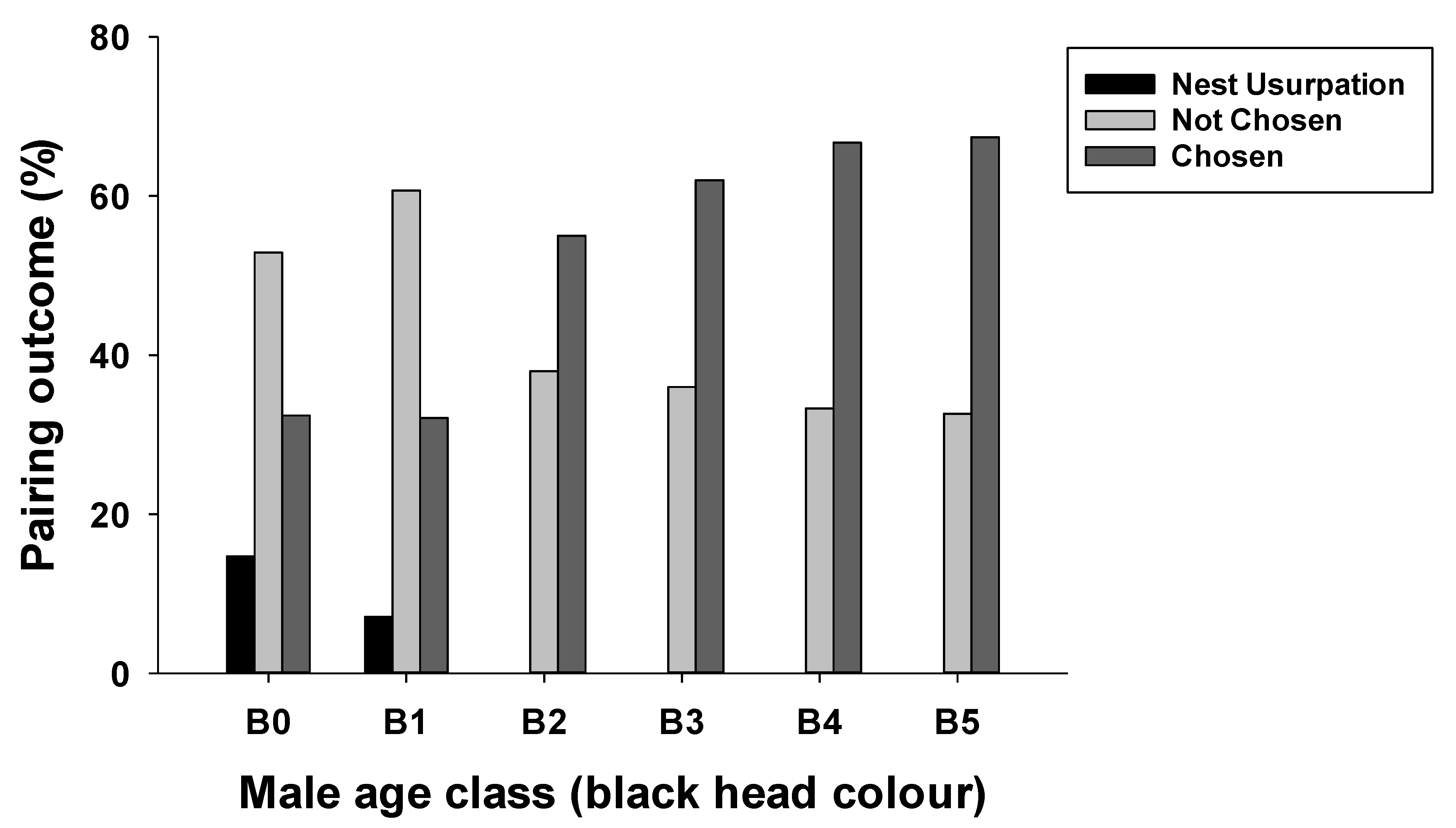
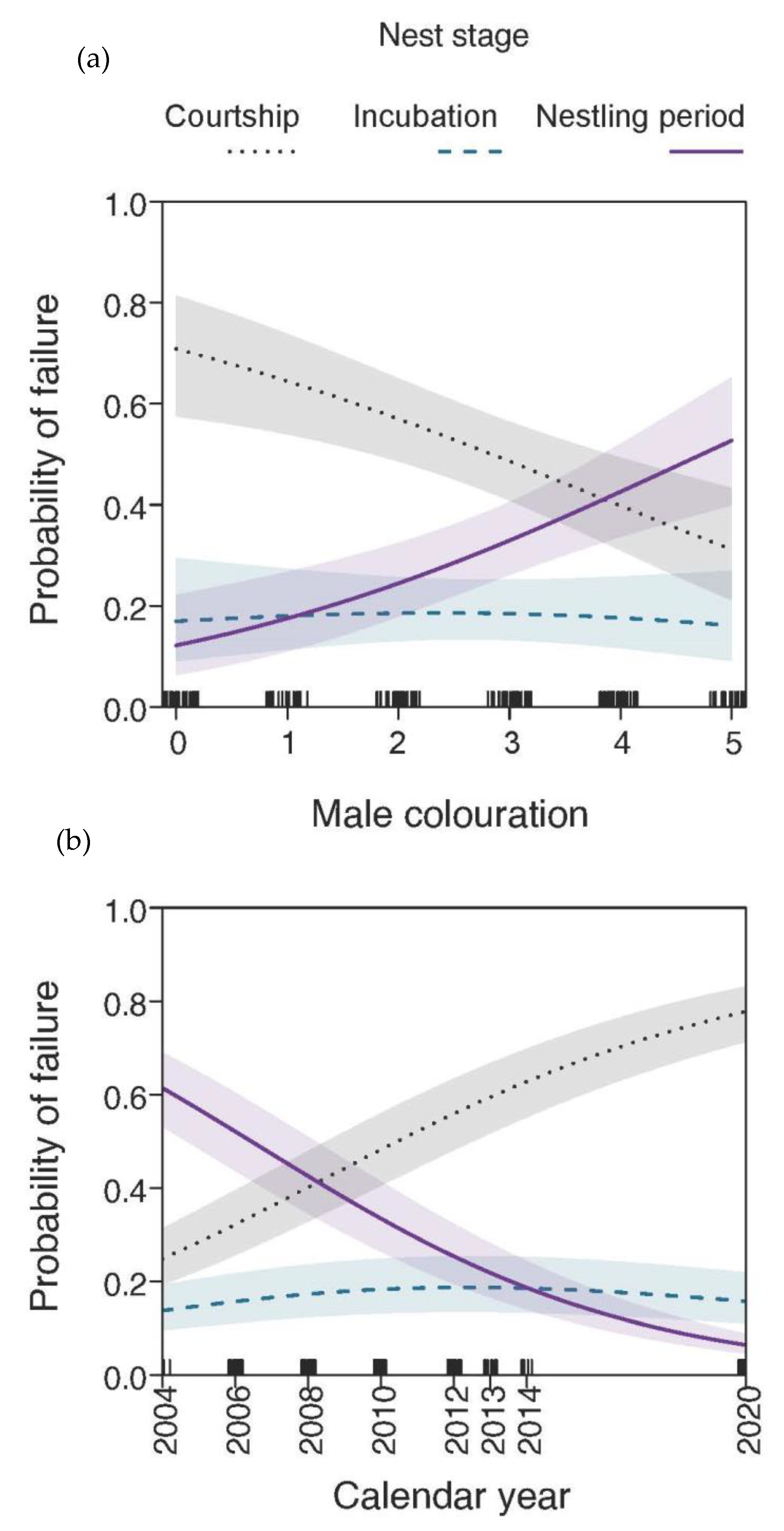
3.1. Chosen and Unchosen Males and the Stage of Nest Failure
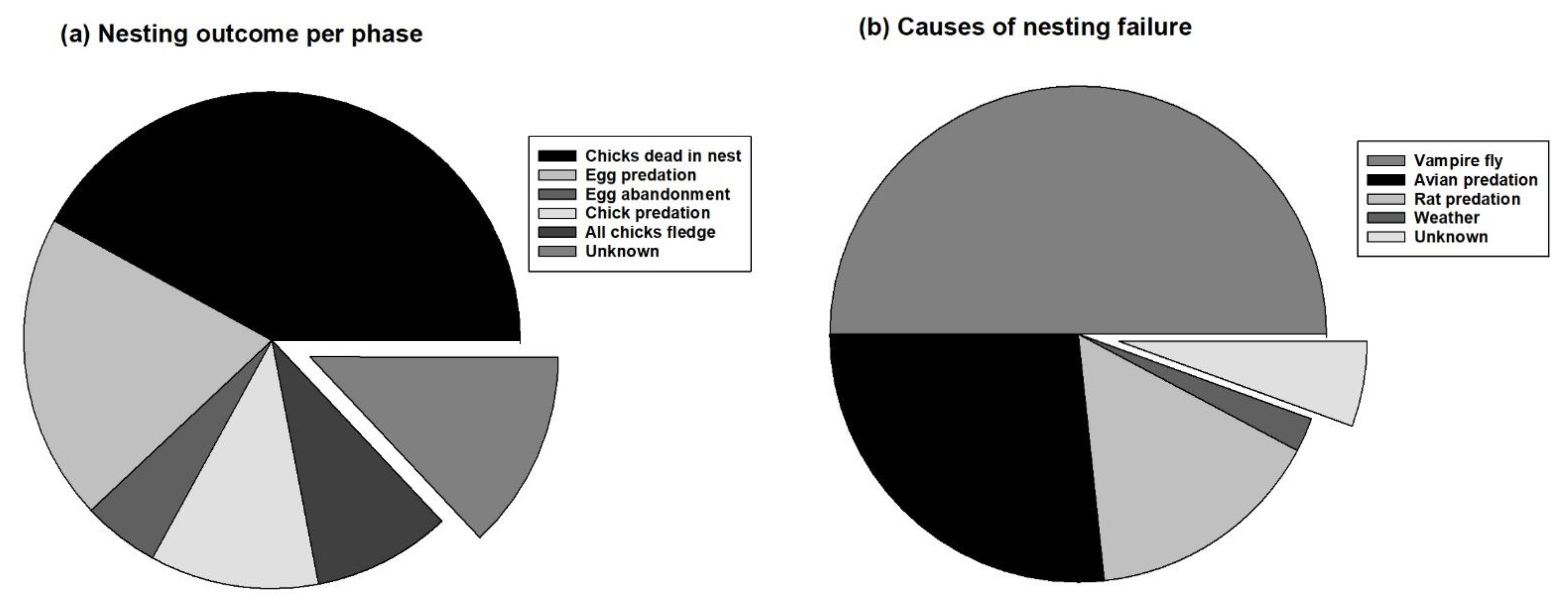
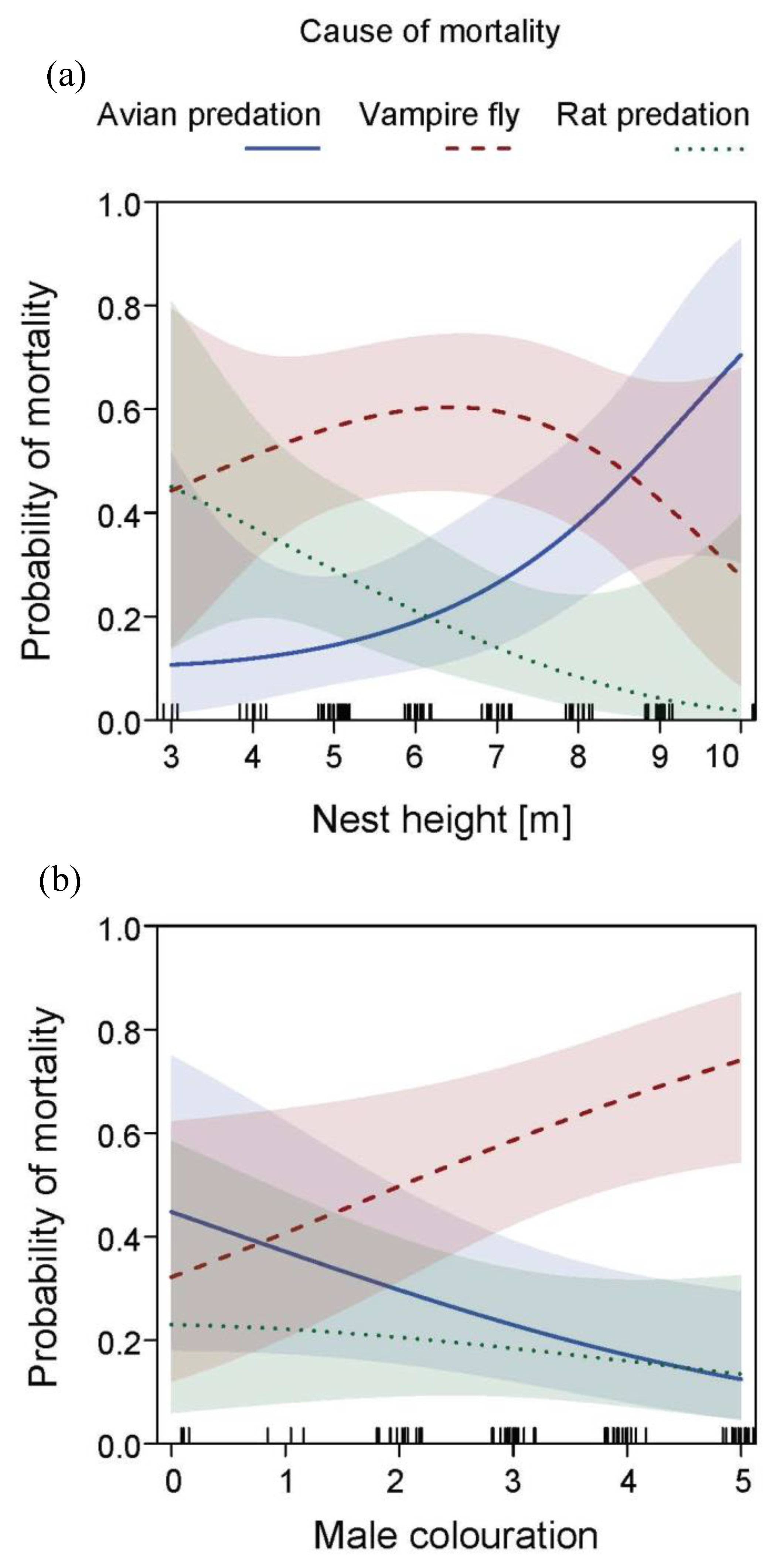
3.2. Causes of Nesting Failure
3.3. Effects of Male Age and Nesting Height on Nesting Outcome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, P.M.; Owens, I.P. Evolutionary Ecology of Birds: Life Histories, Mating Systems and Extinction. Ecology 2002, 83. [Google Scholar] [CrossRef]
- Glen, A.S.; Atkinson, R.; Campbell, K.J.; Hagen, E.; Holmes, N.D.; Keitt, B.S.; Parkes, J.P.; Saunders, A.; Sawyer, J.; Torres, H. Eradicating multiple invasive species on inhabited islands: The next big step in island restoration? Biol. Invasions 2013, 15, 2589–2603. [Google Scholar] [CrossRef]
- Holmes, N.D.; Spatz, D.R.; Oppel, S.; Tershy, B.; Croll, D.A.; Keitt, B.; Genovesi, P.; Burfield, I.J.; Will, D.J.; Bond, A.L.; et al. Globally important islands where eradicating invasive mammals will benefit highly threatened vertebrates. PLoS ONE 2019, 14, e0212128. [Google Scholar] [CrossRef] [Green Version]
- Loiseau, C.; Melo, M.; Lobato, E.; Beadell, J.S.; Fleischer, R.C.; Reis, S.; Doutrelant, C.; Covas, R. Insularity effects on the assemblage of the blood parasite community of the birds from the Gulf of Guinea. J. Biogeogr. 2017, 44, 2607–2617. [Google Scholar] [CrossRef]
- Bliard, L.; Paquet, M.; Robert, A.; Dufour, P.; Renoult, J.P.; Grégoire, A.; Crochet, P.-A.; Covas, R.; Doutrelant, C. Examining the link between relaxed predation and bird coloration on islands. Biol. Lett. 2020, 16, 20200002. [Google Scholar] [CrossRef] [Green Version]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 2001; Volume 1. [Google Scholar]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Whittaker, R.J.; Triantis, K.A.; Ladle, R.J. A general dynamic theory of oceanic island biogeography. J. Biogeogr. 2008, 35, 977–994. [Google Scholar] [CrossRef]
- Grant, P.R.; Grant, B.R. How and Why Species Multiply; Princeton University Press: Princeton, NJ, USA, 2020. [Google Scholar]
- Harper, G.A.; Bunbury, N. Invasive rats on tropical islands: Their population biology and impacts on native species. Glob. Ecol. Conserv. 2015, 3, 607–627. [Google Scholar] [CrossRef] [Green Version]
- Koop, J.A.H.; Kim, P.S.; Knutie, S.A.; Adler, F.; Clayton, D.H. An introduced parasitic fly may lead to local extinction of Darwin’s finch populations. J. Appl. Ecol. 2016, 53, 511–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, L.P.; Fessl, B.; Hernán Vargas, F.; Farrington, H.L.; Francesca Cunninghame, H.; Mueller, J.C.; Nemeth, E.; Christian Sevilla, P.; Petren, K. Slow motion extinction: Inbreeding, introgression, and loss in the critically endangered mangrove finch (Camarhynchus heliobates). Conserv. Genet. 2017, 18, 159–170. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Cassey, P.; Duncan, R.P.; Evans, K.L.; Gaston, K.J. Avian Extinction and Mammalian Introductions on Oceanic Islands. Science 2004, 305, 1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, R.P.; Blackburn, T.M. Causes of extinction in island birds. Anim. Conserv. 2007, 10, 149–150. [Google Scholar] [CrossRef]
- Bulgarella, M.; Quiroga, M.A.; Heimpel, G.E. Additive negative effects of Philornis nest parasitism on small and declining Neotropical bird populations. Bird Conserv. Int. 2019, 29, 339–360. [Google Scholar] [CrossRef]
- Covas, R. Evolution of reproductive life histories in island birds worldwide. Proc. R. Soc. B Biol. Sci. 2012, 279, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G. Do avian species survive better on islands? Biol. Lett. 2021, 17, 20200643. [Google Scholar] [CrossRef] [PubMed]
- Hromada, M.; Antczak, M.; Tryjanowski, P. Females prefer extra-pair males that are older and better hunters. Eur. J. Ecol. 2015, 1, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Wappl, C.; Cimadom, A.; Filek, N.; Heyer, E.; Tebbich, S. Under adverse conditions, older small tree finch males (Camarhynchus parvulus) produce more offspring than younger males. Ethology 2020, 126, 966–975. [Google Scholar] [CrossRef]
- Potti, J. Causes and consequences of age-assortative pairing in pied flycatchers (Ficedula hypoleuca). Etología 2000, 8, 29–36. [Google Scholar]
- Black, J.M.; Owen, M. Reproductive Performance and Assortative Pairing in Relation to Age in Barnacle Geese. J. Anim. Ecol. 1995, 64, 234–244. [Google Scholar] [CrossRef]
- Brooks, R.; Kemp, D.J. Can older males deliver the good genes? Trends Ecol. Evol. 2001, 16, 308–313. [Google Scholar] [CrossRef]
- Tripet, F.; Richner, H. Host Responses to Ectoparasites: Food Compensation by Parent Blue Tits. Oikos 1997, 78, 557–561. [Google Scholar] [CrossRef]
- Causton, C.E.; Peck, S.B.; Sinclair, B.J.; Roque-Albelo, L.; Hodgson, C.J.; Landry, B. Alien insects: Threats and implications for conservation of Galápagos Islands. Ann. Entomol. Soc. Am. 2006, 99, 121–143. [Google Scholar] [CrossRef]
- Fessl, B.; Couri, M.S.; Tebbich, S. Philornis downsi Dodge & Aitken, new to the Galapagos Islands (Diptera, Muscidae). Stud. Dipterol. 2001, 8, 317–322. [Google Scholar]
- Heyer, E.; Cimadom, A.; Wappl, C.; Tebbich, S. Parental Care in the Small Tree Finch Camarhynchus parvulus in Relation to Parasitism and Environmental Factors. Ibis 2021, 163, 137–149. [Google Scholar] [CrossRef]
- O’Connor, J.A.; Robertson, J.; Kleindorfer, S. Darwin’s finch begging intensity does not honestly signal need in parasitised nests. Ethology 2014, 120, 228–237. [Google Scholar] [CrossRef]
- Knutie, S.A.; Owen, J.P.; McNew, S.M.; Bartlow, A.W.; Arriero, E.; Herman, J.M.; DiBlasi, E.; Thompson, M.; Koop, J.A.; Clayton, D.H. Galápagos mockingbirds tolerate introduced parasites that affect Darwin’s finches. Ecology 2016, 97, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, S. Nesting success in Darwin’s small tree finch, Camarhynchus parvulus: Evidence of female preference for older males and more concealed nests. Anim. Behav. 2007, 74, 795–804. [Google Scholar] [CrossRef]
- Marzluff, J.M. Do pinyon jays alter nest placement based on prior experience? Anim. Behav. 1988, 36, 1–10. [Google Scholar] [CrossRef]
- Chalfoun, A.D.; Martin, T.E. Facultative nest patch shifts in response to nest predation risk in the Brewer’s sparrow: A “win-stay, lose-switch” strategy? Oecologia 2010, 163, 885–892. [Google Scholar] [CrossRef]
- Vanderwerf, E.A. Evolution of Nesting Height in an Endangered Hawaiian Forest Bird in Response to a Non-Native Predator. Conserv. Biol. 2012, 26, 905–911. [Google Scholar] [CrossRef]
- Kleindorfer, S.; Peters, K.J.; Custance, G.; Dudaniec, R.Y.; O’Connor, J.A. Changes in Philornis infestation behavior threaten Darwin’s finch survival. Curr. Zool. 2014, 60, 542–550. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, J.A.; Sulloway, F.J.; Robertson, J.; Kleindorfer, S. Philornis downsi parasitism is the primary cause of nestling mortality in the critically endangered Darwin’s medium tree finch (Camarhynchus pauper). Biodivers. Conserv. 2010, 19, 853–866. [Google Scholar] [CrossRef]
- O’Connor, J.A.; Dudaniec, R.Y.; Kleindorfer, S. Parasite infestation and predation in Darwin’s small ground finch: Contrasting two elevational habitats between islands. J. Trop. Ecol. 2010, 26, 285–292. [Google Scholar] [CrossRef]
- Kleindorfer, S.; Peters, K.J.; Hohl, L.; Sulloway, F.J. Flight behaviour of an introduced parasite affects its Galapagos Island hosts: Philornis downsi and Darwin’s finches. In Biological Invasions and Animal Behaviour; Judith, S., Weis, D.S., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 158–179. [Google Scholar]
- Peters, K.J.; Kleindorfer, S. Avian population trends in Scalesia forest on Floreana Island (2004–2013): Acoustical surveys cannot detect hybrids of Darwin’s tree finches Camarhynchus spp. Bird Conserv. Int. 2018, 28, 319–335. [Google Scholar] [CrossRef]
- Dvorak, M.; Nemeth, E.; Wendelin, B.; Herrera, P.; Mosquera, D.; Anchundia, D.; Sevilla, C.; Tebbich, S.; Fessl, B. Conservation status of landbirds on Floreana: The smallest inhabited Galápagos Island. J. Field Ornithol. 2017, 88, 132–145. [Google Scholar] [CrossRef]
- Common, L.K.; O’Connor, J.A.; Dudaniec, R.Y.; Peters, K.J.; Kleindorfer, S. Evidence for rapid downward fecundity selection in an ectoparasite (Philornis downsi) with earlier host mortality in Darwin’s finches. J. Evol. Biol. 2020, 33, 524–533. [Google Scholar] [CrossRef] [Green Version]
- Holmgren, M.; Scheffer, M.; Ezcurra, E.; Gutiérrez, J.R.; Mohren, G.M.J. El Niño effects on the dynamics of terrestrial ecosystems. Trends Ecol. Evol. 2001, 16, 89–94. [Google Scholar] [CrossRef]
- Thomsen, S.K.; Mazurkiewicz, D.M.; Stanley, T.R.; Green, D.J. El Niño/Southern Oscillation-driven rainfall pulse amplifies predation by owls on seabirds via apparent competition with mice. Proc. Royal Soc. B 2018, 285, 20181161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleindorfer, S.; Dudaniec, R.Y. Host-parasite ecology, behavior and genetics: A review of the introduced fly parasite Philornis downsi and its Darwin’s finch hosts. BMC Zool. 2016, 1, 1. [Google Scholar] [CrossRef]
- Wiedenfeld, D.A.; Jiménez, G.U.; Fessl, B.; Kleindorfer, S.; Carlos Valarezo, J. Distribution of the introduced parasitic fly Philornis downsi (Diptera, Muscidae) in the Galápagos Islands. Pac. Conserv. Biol. 2007, 13, 14–19. [Google Scholar] [CrossRef]
- Common, L.K.; Sumasgutner, P.; Sumasgutner, S.C.; Colombelli-Négrel, D.; Dudaniec, R.Y.; Kleindorfer, S. Temporal and spatial variation in sex-specific abundance of the avian vampire fly (Philornis downsi). Parasitol. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, S.; Common, L.K.; O’Connor, J.A.; Garcia-Loor, J.; Katsis, A.C.; Dudaniec, R.Y.; Colombelli-Négrel, D.; Adreani, N.M. Female in-nest attendance predicts the number of ectoparasites in Darwin’s finch species. Proc. R. Soc. London Ser. B Biol. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Langton, A.; Kleindorfer, S. Minimum longevity and age-related male plumage in Darwin’s finches on Floreana Island. J. Ornithol. 2019, 160, 351–361. [Google Scholar] [CrossRef]
- Kleindorfer, S. The ecology of clutch size variation in Darwin’s Small Ground Finch Geospiza fuliginosa: Comparison between lowland and highland habitats. Ibis 2007, 149, 730–741. [Google Scholar] [CrossRef]
- Brown, K.P. Predation at nests of two New Zealand endemic passerines; implications for bird community restoration. Pac. Conserv. Biol. 1997, 3, 91–98. [Google Scholar] [CrossRef]
- Colombelli-Négrel, D.; Robertson, J.; Kleindorfer, S. A new audio-visual technique for effectively monitoring nest predation and the behaviour of nesting birds. Emu-Austral Ornithol. 2009, 109, 83–88. [Google Scholar] [CrossRef]
- Thompson III, F.R.; Burhans, D.E. Predation of songbird nests differs by predator and between field and forest habitats. J. Wildl. Manag. 2003, 67, 408–416. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Croissant, Y. Estimation of Random Utility Models in R: The mlogit Package. J. Stat. Softw. 2020, 95, 1–41. [Google Scholar] [CrossRef]
- Venables, W.; Ripley, B. Random and mixed effects. In Modern Applied Statistics with S; Springer: Berlin/Heidelberg, Germany, 2002; pp. 271–300. [Google Scholar]
- Sarkar, D. Lattice: Multivariate Data Visualization with R.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Fox, J. Effect displays in R for Generalised Linear Models. J. Stat. Softw. 2003, 8, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Pasch, B.; Bolker, B.M.; Phelps, S.M. Interspecific Dominance Via Vocal Interactions Mediates Altitudinal Zonation in Neotropical Singing Mice. Am. Nat. 2013, 182, E161–E173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuur, A.F.; Ieno, E.N. A protocol for conducting and presenting results of regression-type analyses. Methods Ecol. Evol. 2016, 7, 636–645. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Practical Use of the Information-Theoretic Approach. In Model Selection and Inference: A Practical Information-Theoretic Approach; Burnham, K.P., Anderson, D.R., Eds.; Springer: New York, NY, USA, 1998; pp. 75–117. [Google Scholar]
- Colombelli-Négrel, D.; Kleindorfer, S. Nest height, nest concealment, and predator type predict nest predation in superb fairy-wrens (Malurus cyaneus). Ecol. Res. 2009, 24, 921–928. [Google Scholar] [CrossRef]
- Kleindorfer, S.; Fessl, B.; Hoi, H. Avian nest defence behaviour: Assessment in relation to predator distance and type, and nest height. Anim. Behav. 2005, 69, 307–313. [Google Scholar] [CrossRef]
- Latif, Q.S.; Heath, S.K.; Rotenberry, J.T. How avian nest site selection responds to predation risk: Testing an ‘adaptive peak hypothesis’. J. Anim. Ecol. 2012, 81, 127–138. [Google Scholar] [CrossRef]
- Bollmer, J.L.; Whiteman, N.K.; Cannon, M.D.; Bednarz, J.C.; de Vries, T.; Parker, P.G. Population Genetics of the Galápagos Hawk (Buteo galapagoensis): Genetic Monomorphism Within Isolated Populations. Auk 2005, 122, 1210–1224. [Google Scholar] [CrossRef]
- Faaborg, J. Potential for restocking Galapagos hawks on islands where they have been extirpated. Not. De Galapagos 1984, 39, 28–30. [Google Scholar]
- Christian, E.J. Demography and Conservation of the Floreana Racer (Pseudalsophis biserialis biserialis) on Gardner-By-Floreana and Champion Islets, Galápagos Islands, Ecuador. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2017. [Google Scholar]
- Zaher, H.; Yánez-Muñoz, M.H.; Rodrigues, M.T.; Graboski, R.; Machado, F.A.; Altamirano-Benavides, M.; Bonatto, S.L.; Grazziotin, F.G. Origin and hidden diversity within the poorly known Galápagos snake radiation (Serpentes: Dipsadidae). System. Biodivers. 2018, 16, 614–642. [Google Scholar] [CrossRef]
- Ortiz-Catedral, L.; Christian, E.; Skirrow, M.J.; Rueda, D.; Sevilla, C.; Kirtana, K.; Reyes, E.M.; Daltry, J.C. Diet of six species of Galapagos terrestrial snakes (Pseudalsophis spp.) inferred from fecal samples. Herpetol. Notes 2019, 12, 701–704. [Google Scholar]
- Rosenberg, D.K.; Wilson, M.H.; Cruz, F. The distribution and abundance of the smooth-billed ani Crotophaga ani (L.) in the Galapagos Islands, Ecuador. Biol. Conserv. 1990, 51, 113–123. [Google Scholar] [CrossRef]
- Cooke, S.C.; Anchundia, D.; Caton, E.; Haskell, L.E.; Jäger, H.; Kalki, Y.; Mollá, Ó.; Rodríguez, J.; Schramer, T.D.; Walentowitz, A.; et al. Endemic species predation by the introduced smooth-billed ani in Galápagos. Biol. Invasions 2020, 22, 2113–2120. [Google Scholar] [CrossRef] [Green Version]
- Cooke, S.C.; Haskell, L.E.; van Rees, C.B.; Fessl, B. A review of the introduced smooth-billed ani Crotophaga ani in Galápagos. Biol. Conserv. 2019, 229, 38–49. [Google Scholar] [CrossRef]
- Guerrero, A.M.; Tye, A. Native and introduced birds of Galapagos as dispersers of native and introduced plants. Ornithol. Neotrop. 2011, 22, 207–217. [Google Scholar]
- Connett, L.; Guézou, A.; Herrera, H.W.; Carrión, V.; Parker, P.G.; Deem, S.L. Gizzard contents of the smooth-billed ani Crotophaga ani in Santa Cruz, Galápagos Islands, Ecuador. Galapagos Res. 2016, 68, 43–48. [Google Scholar]
- Jiménez-Uzcátegui, G.; Milstead, B.; Márquez, C.; Zabala, J.; Buitrón, P.; Llerena, A.; Salazar, S.; Fessl, B. Galapagos vertebrates: Endangered status and conservation actions. Galapagos Rep. 2006, 2007, 104–110. [Google Scholar]
- Harper, G.A.; Carrion, V. Introduced rodents in the Galápagos: Colonisation, removal and the future. In Island Invasives: Eradication and Management; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; IUCN: Gland, Switzerland, 2011; pp. 63–66. [Google Scholar]
- Wiedenfeld, D.A.; Jiménez-Uzcátegui, G. Critical problems for bird conservation in the Galápagos Islands. Cotinga 2008, 29, 22–27. [Google Scholar]
- Schulwitz, S.; Castaño, P.A.; Mosquera, D.; Chugcho, M.; Campbell, K.J.; Johnson, J.A. Floreana Island re-colonization potential of the Galápagos short-eared owl (Asio flammeus galapagoensis). Conserv. Genet. 2018, 19, 193–205. [Google Scholar] [CrossRef]
- Iudica, C.A.; Andrade, L.F.; Sheffield, S.R. Galapagos Short-Eared Owl (Asio Flammeus Galapagoensis) Preying Upon a Marine Iguana (Amblyrhynchus cristatus) on Isla Isabela, Galapagos Islands, Ecuador. J. Raptor. Res. 2021, 55, 124–126. [Google Scholar] [CrossRef]
- De Groot, R. Origin, status and ecology of the owls in Galapagos. Ardea 1983, 71, 167–182. [Google Scholar]
- Jones, H.P.; Holmes, N.D.; Butchart, S.H.; Tershy, B.R.; Kappes, P.J.; Corkery, I.; Aguirre-Muñoz, A.; Armstrong, D.P.; Bonnaud, E.; Burbidge, A.A. Invasive mammal eradication on islands results in substantial conservation gains. Proc. Natl. Acad. Sci. USA 2016, 113, 4033–4038. [Google Scholar] [CrossRef] [Green Version]
- Travers, T.; Lea, M.-A.; Alderman, R.; Terauds, A.; Shaw, J. Bottom-up effect of eradications: The unintended consequences for top-order predators when eradicating invasive prey. J. Appl. Ecol. 2021, 58, 801–811. [Google Scholar] [CrossRef]
- Hanson, C.; Campbell, K. Floreana Island Ecological Restoration: Rodent and Cat Eradication Feasibility Analysis; Island Conservation: Santa Cruz, CA, USA, 2013. [Google Scholar]
- Rodríguez, J.; Fessl, B. Population Viability Analysis to Assess the Impact of Non-Target Mortality of the Lava Lizard and Seven Land Bird Species Due to an Eradication Program of Rodents and Cats in Floreana Island, Galápagos; Conservation Breeding Specialist Group (IUCN/SSC/CBSG-Mesoamerica) and Charles Darwin Foundation for Island Conservation: Santa Cruz, Ecuador, 2016. [Google Scholar]
- Russell, J.C.; Holmes, N.D. Tropical island conservation: Rat eradication for species recovery. Biol. Conserv. 2015, 185, 1–7. [Google Scholar] [CrossRef]
- Le Corre, M.; Danckwerts, D.K.; Ringler, D.; Bastien, M.; Orlowski, S.; Morey Rubio, C.; Pinaud, D.; Micol, T. Seabird recovery and vegetation dynamics after Norway rat eradication at Tromelin Island, western Indian Ocean. Biol. Conserv. 2015, 185, 85–94. [Google Scholar] [CrossRef]
- Oppel, S.; Bond, A.L.; Brooke, M.d.L.; Harrison, G.; Vickery, J.A.; Cuthbert, R.J. Temporary captive population and rapid population recovery of an endemic flightless rail after a rodent eradication operation using aerially distributed poison bait. Biol. Conserv. 2016, 204, 442–448. [Google Scholar] [CrossRef]
- Kurle, C.M.; Zilliacus, K.M.; Sparks, J.; Curl, J.; Bock, M.; Buckelew, S.; Williams, J.C.; Wolf, C.A.; Holmes, N.D.; Plissner, J.; et al. Indirect effects of invasive rat removal result in recovery of island rocky intertidal community structure. Sci. Rep. 2021, 11, 5395. [Google Scholar] [CrossRef] [PubMed]
- Gollin, J.F.; Gorman, N.; Armstrong, D.P. Twenty years on: Changes in lizard encounter rates following eradication of rats from Kāpiti Island. N. Z. J. Ecol. 2021, 45, 3423. [Google Scholar] [CrossRef]
- Ortega, S.; Rodríguez, C.; Mendoza-Hernández, B.; Drummond, H. How removal of cats and rats from an island allowed a native predator to threaten a native bird. Biol. Invasions 2021. [Google Scholar] [CrossRef]
- Gibbs, J.P.; Cayot, L.J.; Tapia, A.W. Chapter 25-Beyond rescue to full recovery. In Galapagos Giant Tortoises; Gibbs, J.P., Cayot, L.J., Aguilera, W.T., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 503–509. [Google Scholar]
- Segal, R.D.; Whitsed, R.; Massaro, M. Review of the reporting of ecological effects of rodent eradications on Australian and New Zealand islands. Pac. Conserv. Biol. 2021. [Google Scholar] [CrossRef]
- Simmons, L.W. Correlates of male quality in the field cricket, Gryllus campestris L.: Age, size, and symmetry determine pairing success in field populations. Behav. Ecol. 1995, 6, 376–381. [Google Scholar] [CrossRef]
- Freeman-Gallant, C.R.; Taff, C.C.; Morin, D.F.; Dunn, P.O.; Whittingham, L.A.; Tsang, S.M. Sexual Selection, Multiple Male Ornaments, and Age- and Condition-Dependent Signalling in the Common Yellowthroat. Evolution 2010, 64, 1007–1017. [Google Scholar] [CrossRef]
- Grant, B.R. The significance of subadult plumage in Darwin’s finches, Geospiza fortis. Behav. Ecol. 1990, 1, 161–170. [Google Scholar] [CrossRef]
- Cox, W.A.; Thompson Iii, F.R.; Cox, A.S.; Faaborg, J. Post-fledging survival in passerine birds and the value of post-fledging studies to conservation. J. Wildl. Manag. 2014, 78, 183–193. [Google Scholar] [CrossRef]

| Coefficients | Estimate | SE | z-Value | p-Value | LR Χ2 | p (>Χ2) |
|---|---|---|---|---|---|---|
| (Intercept): 1 | 85.07 | 101.51 | 0.84 | 0.402 | 32.65 | <0.001 |
| (Intercept): 2 | 360.84 | 125.84 | 2.87 | 0.004 | ||
| Male age: 1 | 0.20 | 0.13 | 1.51 | 0.132 | 17.99 | <0.001 |
| Male age: 2 | 0.60 | 0.14 | 4.30 | <0.001 | ||
| Year: 1 | −0.04 | 0.05 | −0.85 | 0.395 | 20.77 | <0.001 |
| Year: 2 | −0.18 | 0.06 | −2.88 | 0.004 |
| Coefficients | Estimate | SE | z-Value | p-Value | LR Χ2 | p (>Χ2) |
|---|---|---|---|---|---|---|
| (Intercept): Vampire Fly | −0.53 | 0.66 | −0.81 | 0.420 | 19.14 | 0.004 |
| (Intercept): Rat predation | −0.91 | 0.84 | −1.08 | 0.279 | ||
| Nest height (linear): Vampire Fly | −9.38 | 4.70 | −1.99 | 0.046 | 13.37 | 0.010 |
| Nest height (linear): Rat predation | −20.47 | 7.55 | −2.71 | 0.007 | ||
| Nest height (quadratic): Vampire Fly | −3.34 | 4.76 | −0.70 | 0.483 | ||
| Nest height (quadratic): Rat predation | −3.94 | 6.60 | −0.60 | 0.551 | ||
| Male age: Vampire Fly | 0.42 | 0.19 | 2.20 | 0.028 | 5.65 | 0.059 |
| Male age: Rat predation | 0.15 | 0.25 | 0.60 | 0.548 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleindorfer, S.; Common, L.K.; Sumasgutner, P. Nesting Success and Nesting Height in the Critically Endangered Medium Tree Finch (Camarhynchus pauper). Birds 2021, 2, 427-444. https://doi.org/10.3390/birds2040032
Kleindorfer S, Common LK, Sumasgutner P. Nesting Success and Nesting Height in the Critically Endangered Medium Tree Finch (Camarhynchus pauper). Birds. 2021; 2(4):427-444. https://doi.org/10.3390/birds2040032
Chicago/Turabian StyleKleindorfer, Sonia, Lauren K. Common, and Petra Sumasgutner. 2021. "Nesting Success and Nesting Height in the Critically Endangered Medium Tree Finch (Camarhynchus pauper)" Birds 2, no. 4: 427-444. https://doi.org/10.3390/birds2040032
APA StyleKleindorfer, S., Common, L. K., & Sumasgutner, P. (2021). Nesting Success and Nesting Height in the Critically Endangered Medium Tree Finch (Camarhynchus pauper). Birds, 2(4), 427-444. https://doi.org/10.3390/birds2040032






