Morph Composition Matters in the Gouldian Finch (Chloebia gouldiae): Involvement of Red-Headed Birds Increases Vigilance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedures
2.2. Data Analysis
2.3. Ethical Note
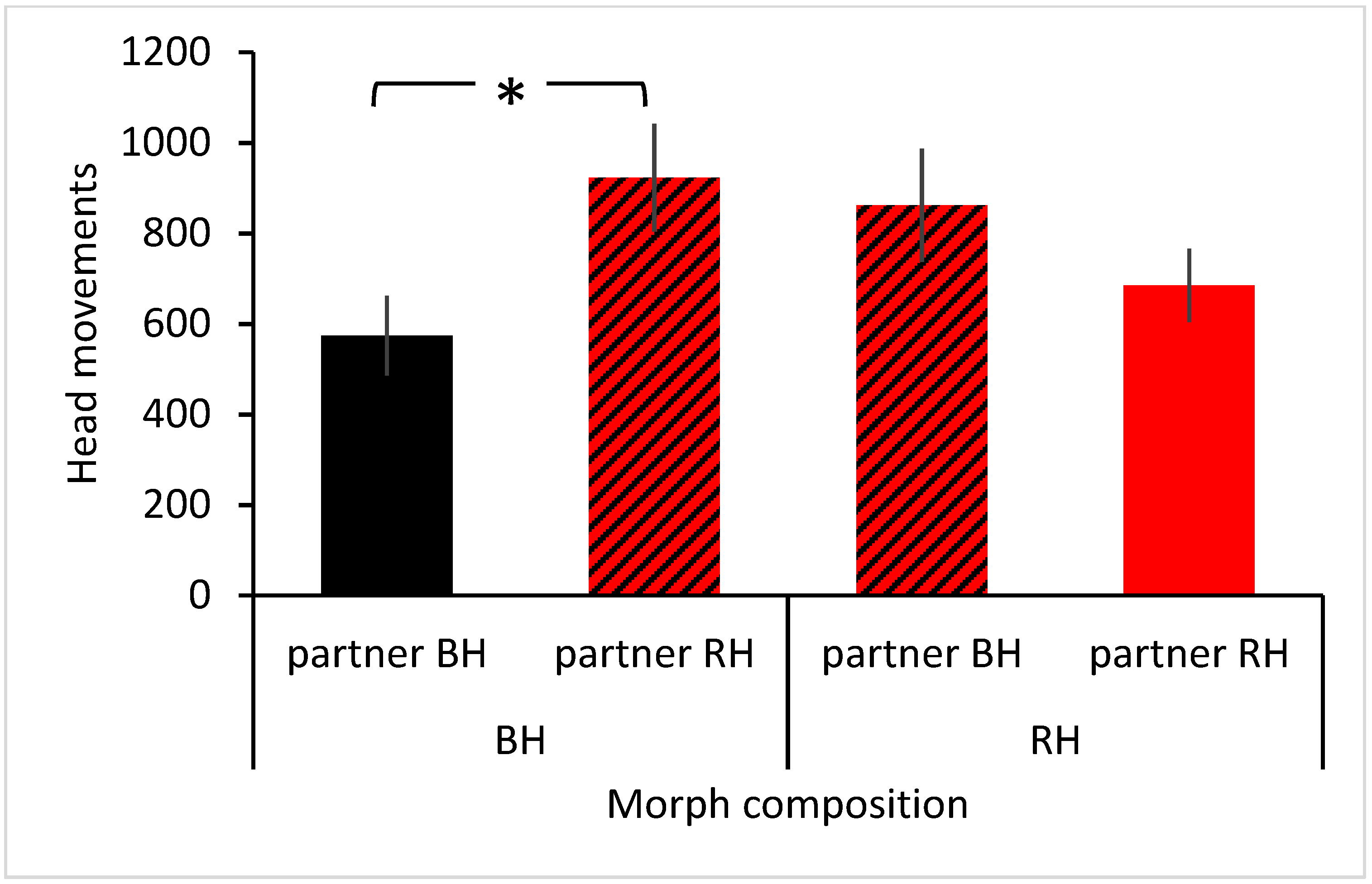
3. Results
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, D.J.; Stillman, R.A.; Smart, S.L.; Bullock, J.M.; Norris, K.J. Are the costs of routine vigilance avoided by granivorous foragers? Func. Ecol. 2011, 25, 617–627. [Google Scholar] [CrossRef]
- Watson, M.; Aebischer, N.J.; Cresswell, W. Vigilance and fitness in grey partridges Perdix perdix: The effects of group size and foraging-vigilance trade-offs on predation mortality. J. Anim. Ecol. 2007, 76, 211–221. [Google Scholar] [CrossRef]
- Fairbanks, B.; Dobson, F.S. Mechanisms of the group-size effect on vigilance in Columbian ground squirrels: Dilution versus detection. Anim. Behav. 2006, 73, 115–123. [Google Scholar] [CrossRef]
- Bertram, B.C.R. Living in groups: Predators and prey. In Behavioural Ecology: An Evolutionary Approach; Krebs, J.R., Davies, N.B., Eds.; Blackwell Scientific: Oxford, UK, 1978; pp. 64–96. [Google Scholar]
- Stojan-Dolar, M.; Haymann, E.W. Vigilance of mustached tamarins in single-species and mixed-species groups—The influence of group composition. Behav. Ecol. Sociobiol. 2010, 64, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, G. Animal Vigilance: Monitoring Predators and Competitors; Elsevier: London, UK, 2015. [Google Scholar]
- Ye, Y.; Jiang, Y.; Hu, C.; Liu, Y.; Qing, B.; Wang, C.; Fernández-Juricic, E.; Ding, C. What makes a tactile forager join mixed-species flocks? A case study with the endangered Crested Ibis (Nipponia nippon). Auk Ornithol. Adv. 2017, 134, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Stears, K.; Schmitt, M.H.; Wilmer, C.C.; Shrader, A.M. Mixed-species herding levels the landscape of fear. Proc. R. Soc. B 2020, 287, 20192555. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, N.B. Flocking preferences in relation to vigilance benefits and aggression costs in mixed-species shorebird flocks. Oikos 1989, 56, 91–98. [Google Scholar] [CrossRef]
- Favreau, F.-R.; Goldizen, A.W.; Fritz, H.; Blomberg, S.P.; Best, E.C.; Pays, O. Within-population differences in personality and plasticity in the trade-off between vigilance and foraging in kangaroos. Anim. Behav. 2014, 92, 175–184. [Google Scholar] [CrossRef]
- Schmitt, M.H.; Stears, K.; Shrader, A.M. Zebra reduce predation risk in mixed-species herds by eavesdropping on cues from giraffe. Behav. Ecol. 2016, 27, 1073–1077. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, N.B. The effects of mixed-species flocking on the vigilance of shorebirds: Who do they trust? Anim. Behav. 1984, 32, 986–993. [Google Scholar] [CrossRef]
- Periquet, S.; Valeix, M.; Loveridge, A.J.; Madzikanda, H.; Macdonald, D.W.; Fritz, H. Individual vigilance of African herbivores while drinking: The role of immediate predation risk and context. Anim. Behav. 2010, 79, 665–671. [Google Scholar] [CrossRef]
- Beauchamp, G. Predator attack patterns influence vigilance in a virtual experiment. Behav. Ecol. Sociobiol. 2020, 74, 49. [Google Scholar] [CrossRef]
- Pascal, J.; Senar, J.C. Antipredator behavioural compensation of proactive personality trait in male Eurasian siskins. Anim. Behav. 2014, 90, 297–303. [Google Scholar] [CrossRef]
- Cresswell, W.; Quinn, J.L.; Whittingham, M.J.; Butler, S. Good foragers can also be good at detecting predators. Proc. R. Soc. B 2003, 270, 1069–1076. [Google Scholar] [CrossRef] [Green Version]
- Barros, M.; Alencar, C.; de Souza Silva, M.A.; Tomaz, C. Changes in experimental conditions alter anti-predator vigilance and sequence predictability in captive marmosets. Behav. Proc. 2008, 77, 351–356. [Google Scholar] [CrossRef]
- Shonfield, J. The effect of familiarity on vigilance behaviour in grey squirrels. McGill Sci. Undergrad. Res. J. 2011, 6, 45–49. [Google Scholar]
- Coolen, I.; Giraldeau, L.-A. Incompatibility between antipredatory vigilance and scrounger tactic in nutmeg mannikins, Lonchura punctulate. Anim. Behav. 2003, 66, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Robinson, B.; Merrill, E.H. Foraging vigilance trade-offs in a partially migratory population: Comparing migrants and residents on a sympatric range. Anim. Behav. 2013, 85, 849–856. [Google Scholar] [CrossRef]
- Randler, C. Vigilance of mallards in the presence of greylag geese. J. Field Ornithol. 2004, 75, 404–408. [Google Scholar] [CrossRef]
- Fernandez-Juricic, E.; Gall, M.D.; Dolan, T.; O’Rourke, C.; Thomas, S.; Lynch, J. Visual systems and vigilance behaviour of two ground-foraging avian prey species: White-crowned sparrows and California towhees. Anim. Behav. 2011, 81, 705–713. [Google Scholar] [CrossRef]
- Fernandez-Juricic, E. Sensory basis of vigilance behavior in birds: Synthesis and future prospects. Behav. Proc. 2012, 89, 143–152. [Google Scholar] [CrossRef]
- Jones, K.A.; Krebs, J.R.; Whittingham, M.J. Vigilance in the third dimension: Head movement not scan duration varies in response to different predator models. Anim. Behav. 2007, 74, 1181–1187. [Google Scholar] [CrossRef]
- Fernandez-Juricic, E.; Beauchamp, G.; Treminio, R.; Hoover, M. Making heads turn: Association between head movements during vigilance and perceived predation risk in brown-headed cowbird flocks. Anim. Behav. 2011, 82, 573–577. [Google Scholar] [CrossRef]
- Krebs, H.; Weyers, P.; Macht, M.; Weijers, H.-G.; Janke, W. Scanning behavior of rats during eating under stressful noise. Physiol. Behav. 1997, 62, 151–154. [Google Scholar] [CrossRef]
- Lazarus, J. The early warning function of flocking in birds: An experimental study with captive Quelea. Anim. Behav. 1979, 27, 855–865. [Google Scholar] [CrossRef]
- Griesser, M. Nepotistic vigilance behavior in Siberian jay parents. Behav. Ecol. 2003, 14, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Arenz, C.L.; Leger, D.W. Antipredator vigilance of juvenile and adult thirteen-lined ground squirrels and the role of nutritional need. Anim. Behav. 2000, 59, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Monclus, R.; Roedel, H.G. Influence of different individual traits on vigilance behaviour in European rabbits. Ethology 2009, 115, 758–766. [Google Scholar] [CrossRef]
- Beauchamp, G. The effect of age on vigilance: A longitudinal study with a precocial species. Behaviour 2018, 155, 1011–1024. [Google Scholar] [CrossRef]
- Lea, A.J.; Blumstein, D.T. Age and sex influence marmot antipredator behavior during periods of heightened risk. Behav. Ecol. Sociobiol. 2011, 65, 1525–1533. [Google Scholar] [CrossRef] [Green Version]
- Rieucau, G.; Blanchard, P.; Martin, J.G.A.; Favreau, F.-R.; Goldizen, A.W.; Pays, O. Investigating differences in vigilance tactic use within and between the sexes in Eastern grey kangaroos. PLoS ONE 2010, 7, e44801. [Google Scholar] [CrossRef] [Green Version]
- Mathot, K.J.; van den Hout, P.J.; Piersma, T.; Kempenaers, B.; Reale, D.; Dingemanse, N.J. Disentangling the roles of frequency-vs. state-dependence in generating individual differences in behavioural plasticity. Ecol. Lett. 2011, 14, 1254–1262. [Google Scholar] [CrossRef]
- Roche, E.A.; Brown, C.R. Among-individual variation in vigilance at the nest in colonial cliff swallows. Wilson J. Ornithol. 2013, 125, 685–695. [Google Scholar] [CrossRef]
- Galeotti, P.; Rubolini, D.; Dunn, P.O.; Fasola, M. Colour polymorphism in birds: Causes and functions. J. Evol. Biol. 2003, 16, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Pryke, S.R.; Griffith, S.C. Red dominates black: Agonistic signalling among head morphs in the colour polymorphic Gouldian finch. Proc. R. Soc. B 2006, 273, 949–957. [Google Scholar] [CrossRef] [Green Version]
- Boerner, M.; Krueger, O. Aggression and fitness differences between plumage morphs in the common buzzard (Buteo buteo). Behav. Ecol. 2008, 20, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Hurtado-Gonzales, J.L.; Baldassarre, D.T.; Uy, J.A.C. Interaction between female mating preferences and predation may explain the maintenance of rare males in the pentamorphic fish Poecilia parae. J. Evol. Biol. 2010, 23, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Mettke-Hofmann, C. The effect of head colour and age on personality traits in a social setting. Ethology 2012, 118, 906–916. [Google Scholar] [CrossRef]
- Costanzo, A.; Romano, A.; Ambrosinia, R.; Parolinia, M.; Rubolinia, D.; Capriolia, M.; Cortia, M.; Canovac, L.; Saino, N. Barn swallow antipredator behavior covaries with melanic coloration and predicts survival. Behav. Ecol. 2018, 29, 1472–1480. [Google Scholar] [CrossRef]
- Huhta, E.; Rytkonen, S.; Solonen, T. Plumage brightness of prey increases predation risk: An among-species comparison. Ecology 2003, 84, 1793–1799. [Google Scholar] [CrossRef]
- Karpestam, E.; Merilaita, S.; Forsman, A. Colour polymorphism protects prey individuals and populations against predation. Sci. Rep. 2016, 6, 22122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohls, P.; Koehnle, T.J. Responses of Eastern Gray squirrels (Sciurus carolinensis) to predator calls and their modulation by coat color. Am. Midl. Nat. 2017, 178, 226–236. [Google Scholar] [CrossRef]
- Scriba, M.F.; Rattenborg, N.C.; Dreiss, A.N.; Vyssotski, A.L.; Roulin, A. Sleep and vigilance linked to melanism in wild barn owls. J. Evol. Biol. 2014, 27, 2057–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dostine, P.L.; Johnson, G.C.; Franklin, D.C.; Zhang, Y.; Hempel, C. Seasonal use of savanna landscapes by the Gouldian finch, Erythrura gouldiae, in the Yinberrie Hills area, Northern Territory. Wildl. Res. 2001, 28, 445–458. [Google Scholar] [CrossRef]
- Brush, A.H.; Seifried, H. Pigmentation and feather structure in genetic variants of the Gouldian finch, Poephila gouldiae. Auk Ornithol. Adv. 1968, 85, 416–430. [Google Scholar] [CrossRef]
- Williams, L.J.; King, A.J.; Mettke-Hofmann, C. Colourful characters: Head-colour reflects personality in a social bird, the Gouldian finch (Erythrura gouldiae). Anim. Behav. 2012, 84, 159–165. [Google Scholar] [CrossRef]
- O’Reilly, A.O.; Hofmann, G.; Mettke-Hofmann, C. Gouldian finches are followers with black-headed females taking the lead. PLoS ONE 2019, 14, e0214531. [Google Scholar] [CrossRef]
- Pryke, S.R.; Astheimer, L.B.; Buttemer, W.A.; Griffith, S.C. Frequency-dependent physiological trade-offs between competing colour morphs. Biol. Lett. 2007, 3, 494–497. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, R.; Mettke-Hofmann, C. Ecological aspects of neophobia and neophilia in birds. Curr. Ornithol. 2001, 16, 119–178. [Google Scholar]
- Mettke-Hofmann, C.; Rowe, K.C.; Hayden, T.J.; Canoine, V. Effects of experience and object complexity on exploration in garden wearblers (Sylvia borin). J. Zool. 2006, 268, 405–413. [Google Scholar] [CrossRef]
- Inglis, I.R.; Langton, S.; Forkman, B.; Lazarus, J. An information primacy model of exploratory and foraging behaviour. Anim. Behav. 2001, 62, 543–557. [Google Scholar] [CrossRef] [Green Version]
- Grünberger, S.; Leisler, B. Auswirkung der Umwelterfahrung auf die Neophobie der Tannenmeise (Parus ater). J. Ornithol. 1993, 134, 352–355. [Google Scholar] [CrossRef]
- Morelli, F.; Benedetti, Y.; Diaz, M.; Grim, T.; Ibanez-Alamo, J.D.; Jokimaeki, J.; Kaisanlahti-Jokimaeki, M.-L.; Taette, K.; Marko, G.; Jiang, Y.; et al. Contagious fear: Escape behavior increases with flock size in European gregarious birds. Ecol. Evol. 2019, 9, 6096–6104. [Google Scholar] [CrossRef]
- Ruiz Rodriguez, M.; Aviles, J.M.; Cuervo, J.J.; Parejo, D.; Ruano, F.; Zamora-Munoz, C.; Sergio, F.; Lopez-Jimenez, L.; Tanferna, A.; Martın-Vivaldi, M. Does avian conspicuous colouration increase or reduce predation risk? Oecologia 2013, 173, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Brink, V.; Dolivo, V.; Falourd, X.; Dreiss, A.N.; Roulin, A. Melanic color-dependent antipredator behavior strategies in barn owl nestlings. Behav. Ecol. 2012, 23, 473–480. [Google Scholar] [CrossRef]
- Popp, J.W. Scanning behavior of finches in mixed-species groups. Condor 1988, 90, 510–512. [Google Scholar] [CrossRef]
- Cords, M. Vigilance and mixed-species association of some East African forest monkeys. Behav. Ecol. Sociobiol. 1990, 26, 297–300. [Google Scholar] [CrossRef]
- Goodale, E.; Ruxton, G.D.; Beauchamp, G. Predator eavesdropping in a mixed-species environment: How prey species may use grouping, confusion, and the cocktail party effect to reduce predator detection. Front. Ecol. Evol. 2019, 7, 141. [Google Scholar] [CrossRef] [Green Version]
- Pays, O.; Ekori, A.; Fritz, H. On the advantages of mixed-species groups: Impalas adjust their vigilance when associated with larger prey herbivores. Ethology 2014, 120, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, G. On how risk and group size interact to influence vigilance. Biol. Rev. 2019, 94, 1918–1934. [Google Scholar] [CrossRef]
- Pryke, S.R. Fiery red heads: Female dominance among head color morphs in the Gouldian finch. Behav. Ecol. 2007, 18, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, H.; Liu, Y.; Lloyd, H.; Li, J.; Zhang, Z.; Li, D. Saltmarsh vegetation and social environment influence flexible seasonal vigilance strategies for two sympatric migratory curlew species in adjacent coastal habitats. Avian Res. 2021, 12, 39. [Google Scholar] [CrossRef]

| Model | AIC | Diff 1 |
|---|---|---|
| Basic model 2 + age class × situation | 1018.619 | |
| Basic model + partner colour morph × situation | 1053.297 | 34.678 |
| Basic model + partner colour morph × situation + sex × situation | 1112.279 | 93.66 |
| Basic model + sex × situation | 1134.826 | 116.207 |
| Basic model + age class × situation + sex × situation | 1143.323 | 124.704 |
| Basic model | 1175.461 | 156.842 |
| Basic model + partner colour morph × situation + age class × situation | 1226.478 | 207.859 |
| Basic model + colour morph × situation | 1247.169 | 228.55 |
| Basic model + partner colour morph × situation + colour morph × situation | 1267.519 | 248.9 |
| Variables | F-Value | Df1 | Df2 | p |
|---|---|---|---|---|
| Corrected model | 13.927 | 13 | 67 | <0.001 |
| Situation | 67.804 | 2 | 67 | <0.001 |
| Colour morph | 0.058 | 1 | 67 | 0.810 |
| Age class | 0.448 | 2 | 67 | 0.641 |
| Colour morph × partner colour morph | 3.443 | 2 | 67 | 0.038 |
| Colour morph × sex | 0.896 | 2 | 67 | 0.413 |
| Age class × situation | 1.637 | 4 | 67 | 0.175 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mettke-Hofmann, C. Morph Composition Matters in the Gouldian Finch (Chloebia gouldiae): Involvement of Red-Headed Birds Increases Vigilance. Birds 2021, 2, 404-414. https://doi.org/10.3390/birds2040030
Mettke-Hofmann C. Morph Composition Matters in the Gouldian Finch (Chloebia gouldiae): Involvement of Red-Headed Birds Increases Vigilance. Birds. 2021; 2(4):404-414. https://doi.org/10.3390/birds2040030
Chicago/Turabian StyleMettke-Hofmann, Claudia. 2021. "Morph Composition Matters in the Gouldian Finch (Chloebia gouldiae): Involvement of Red-Headed Birds Increases Vigilance" Birds 2, no. 4: 404-414. https://doi.org/10.3390/birds2040030
APA StyleMettke-Hofmann, C. (2021). Morph Composition Matters in the Gouldian Finch (Chloebia gouldiae): Involvement of Red-Headed Birds Increases Vigilance. Birds, 2(4), 404-414. https://doi.org/10.3390/birds2040030






