Climate Change and the Spatiotemporal Variation in Survival of a Long-Distance Migrant (White Stork, Ciconia ciconia) across Western Europe
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
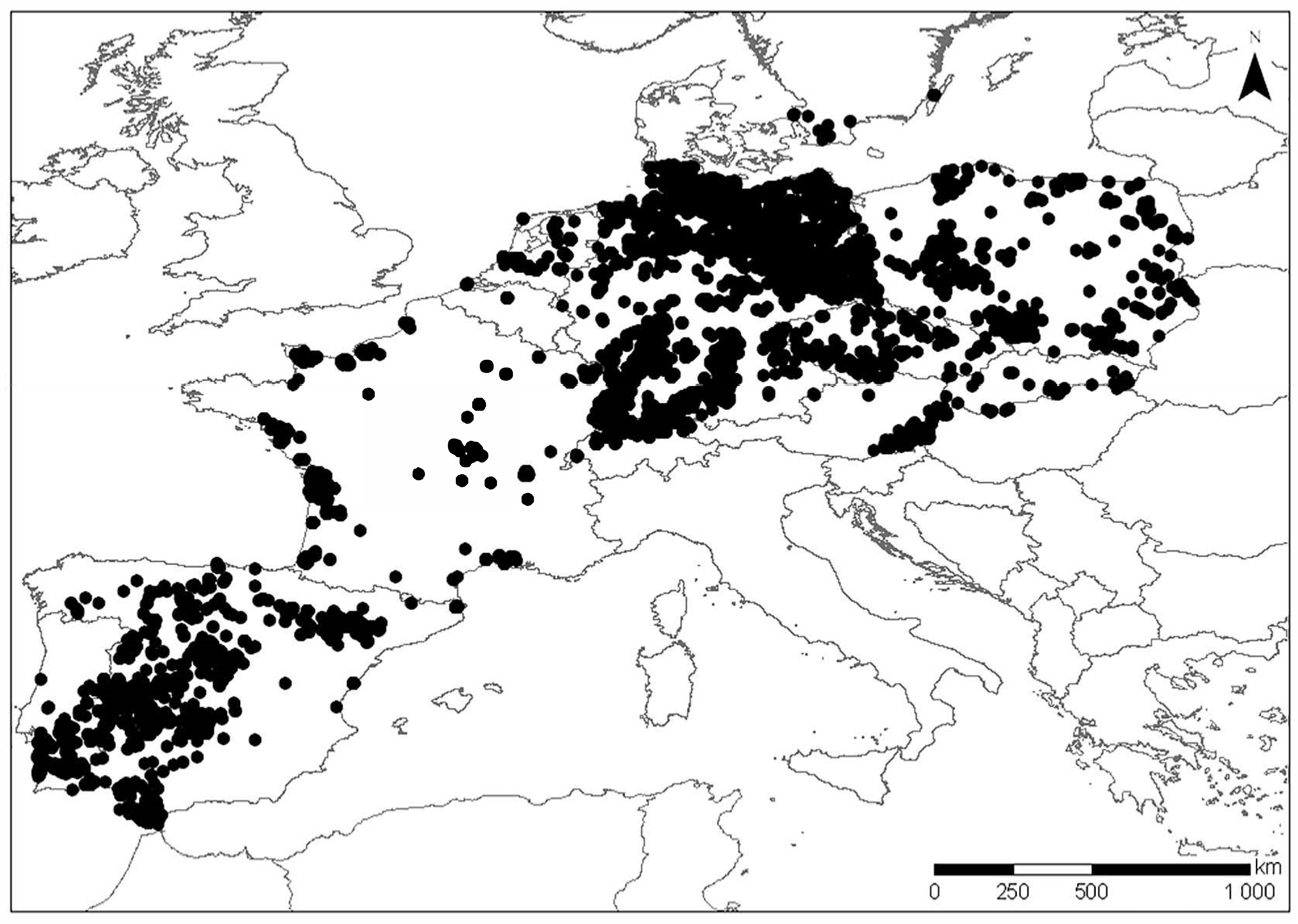
2.2. Ringing Data
2.3. Analyses
2.4. Predictors of Survival
2.5. Recovery Rates
2.6. Models
3. Results
3.1. Description of the Best Fit Model
3.2. Parameters in the Best Fit Model: Additive and Interaction Terms
3.3. Beta Estimates of the Model Predictors
3.4. Recapture Probabilities
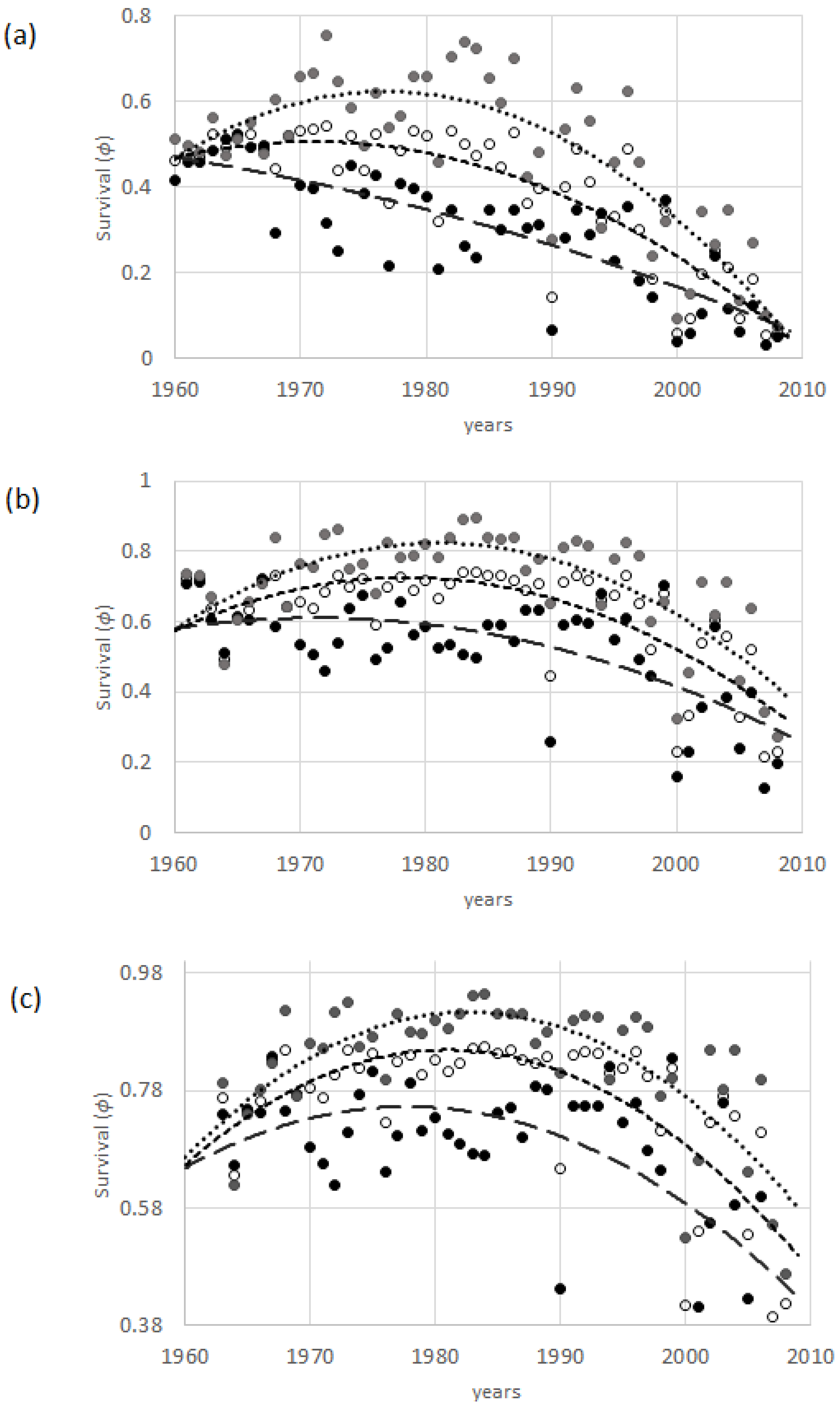
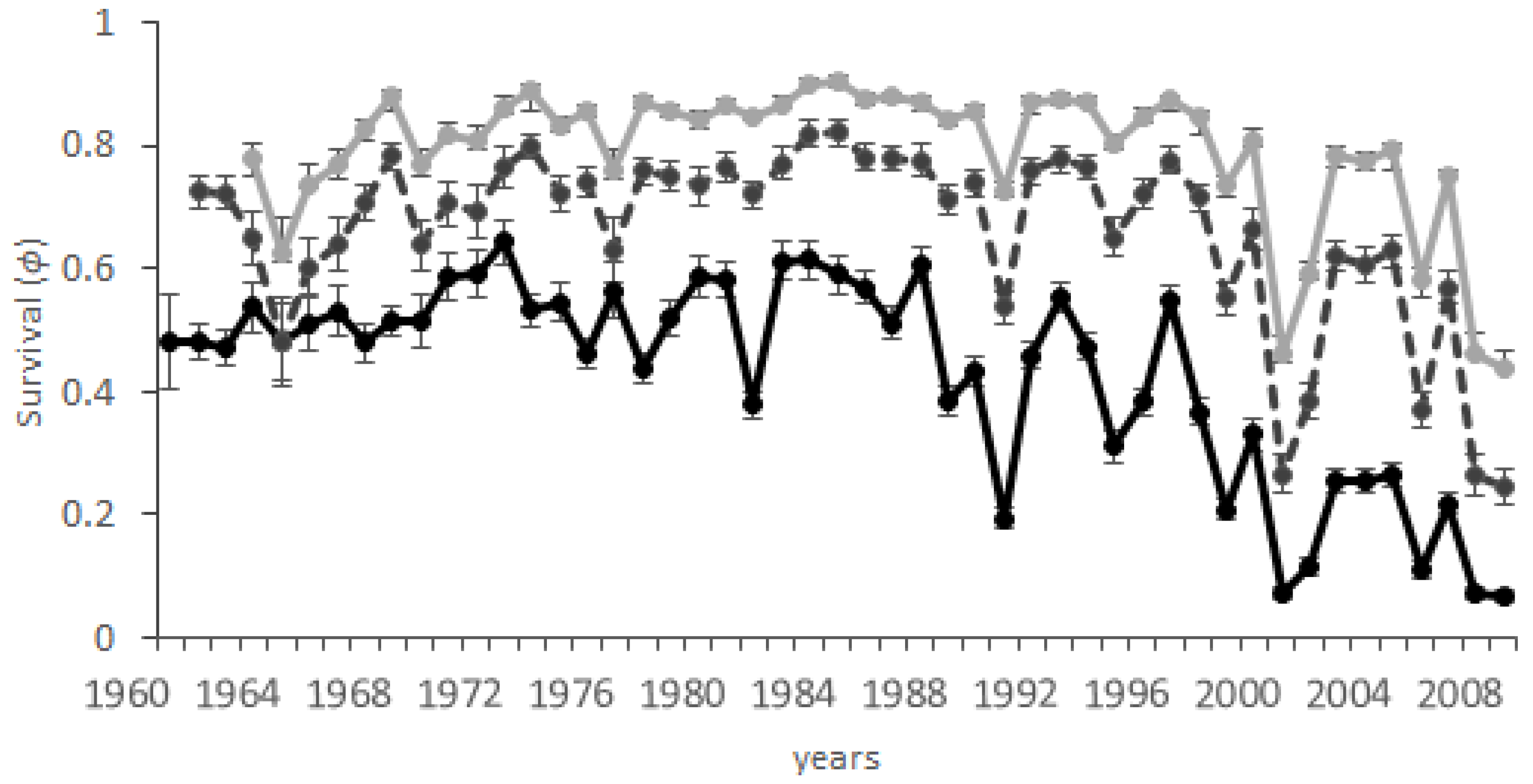
3.5. Differences among Age Classes
4. Discussion
4.1. Latitude and Asynchrony in Survival
4.2. Latitude and Land Use Changes
4.3. Age-Dependent Survival
4.4. Consequences at the Population Level
4.5. Other Factors and Caveats beyond Climate Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Battisti, C.; Poeta, G.; Fanelli, G. The Concept of Disturbance. In An Introduction to Disturbance Ecology Environmental Science and Engineering; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Oreskes, N. The Scientific Consensus on Climate Change. Science 2004, 306, 1686. [Google Scholar] [CrossRef] [Green Version]
- Radchuk, V.; Reed, T.; Teplitsky, C.; van de Pol, M.; Charmantier, A.; Hassall, C.; Adamík, P.; Adriaensen, F.; Ahola, M.P.; Arcese, P.; et al. Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 2019, 10, 3109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211. [Google Scholar] [CrossRef] [PubMed]
- Battisti, C.; Poeta, G.; Fanelli, G. Nomenclature and Taxonomy of Threats. In An Introduction to Disturbance Ecology Environmental Science and Engineering; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Salafsky, N.; Salzer, D.; Stattersfield, A.J.; Hilton-Taylor, C.; Neugarten, R.; Butchart, S.H.M.; Collen, B.; Cox, N.; Master, L.L.; O'Connor, S.; et al. A Standard Lexicon for Biodiversity Conservation: Unified Classifications of Threats and Actions. Conserv. Biol. 2008, 22, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S.J.; White, M.D.; Newell, G.R. How useful are species distribution models for managing biodiversity under future climates? Ecol. Soc. 2010, 15, 8. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Sparks, T.H.; Profus, P. Uphill Shifts in the Distribution of the White Stork Ciconia ciconia in Southern Poland: The Importance of Nest Quality. Divers. Distrib. 2005, 11, 219–223. [Google Scholar] [CrossRef]
- Halley, J.M.; Van Houtan, K.S.; Mantua, N. How survival curves affect populations’ vulnerability to climate change. PLoS ONE 2018, 13, e0203124. [Google Scholar] [CrossRef]
- Morelli, F.; Benedetti, Y.; Ibáñez-Álamo, J.D.; Tryjanowski, P.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.-L.; Pérez-Contreras, T.; Sprau, P.; Suhonen, J.; Yosef, R.; et al. Insurance for the future? Potential avian community resilience in cities across Europe. Clim. Chang. 2020, 159, 195–214. [Google Scholar] [CrossRef] [Green Version]
- Ahola, M.P.; Laalsonen, T.; Eeva, T.; Lehikoinen, A. Climate change can alter competitive relationships between resident and migratory birds. J. Anim. Ecol. 2007, 76, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Keith, D.A.; Akçakaya, H.R.; Thuiller, W.; Midgley, G.F.; Pearson, R.G.; Phillips, S.J.; Regan, H.M.; Araújo, M.B.; Rebelo, T.G. Predicting extinction risks under climate change: Coupling stochastic population models with dynamic bioclimatic habitat models. Biol. Lett. 2008, 4, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Saether, B.-E.; Bakke, O. Avian Life History Variation and Contribution of Demographic Traits to the Population Growth Rate. Ecology 2000, 81, 642–653. [Google Scholar] [CrossRef] [Green Version]
- Menu, S.; Gauthier, G.; Reed, A. Changes in survival rates and population dynamics of greater snow geese over a 30-year period: Implications for hunting regulations. J. Appl. Ecol. 2002, 39, 91–102. [Google Scholar] [CrossRef]
- Ozgul, A.; Armitage, K.B.; Blumstein, D.T.; Oli, M.K. Spatiotiemporal variation in survival rates: Implications for population dynamics of yellow-bellied marmots. Ecology 2006, 87, 1027–1037. [Google Scholar] [CrossRef] [Green Version]
- Colchero, F.; Jones, O.R.; Conde, D.A.; Hodgson, D.; Zajitschek, F.; Schmidt, B.R.; Malo, A.F.; Alberts, S.C.; Becker, P.H.; Bouwhuis, S.; et al. The diversity of population responses to environmental change. Ecol. Lett. 2019, 22, 342–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC. Climate Change 2007: Synthesis Report; IPCC: Geneva, Switzerland, 2007; 104p. [Google Scholar]
- WMO; World Climate Data and Monitoring Programme. The Role of Climatological Normals in a Changing Climate; WCDMP-No. 61; WMO-TD/No. 1377; World Meteorological Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Sim, I.M.W.; Rebecca, G.W.; Ludwig, S.C.; Grant, M.C.; Reid, J.M. Characterizing demographic variation and contributions to population growth rate in a declining population. J. Anim. Ecol. 2011, 80, 159–170. [Google Scholar] [CrossRef]
- Van De Pol, M.; Ens, B.J.; Heg, D.; Brouwer, L.; Krol, J.; Maier, M.; Exo, K.-M.; Oosterbeek, K.; Lok, T.; Eising, C.M.; et al. Do changes in the frequency, magnitude and timing of extreme climatic events threaten the population viability of coastal birds? J. Appl. Ecol. 2010, 47, 720–730. [Google Scholar] [CrossRef]
- Fiedler, W.; Bairlein, F.; Köppen, U. Using large-scale data from ringed birds for the investigation of effects of climate change on migrating birds: Pitfalls and prospects. Adv. Ecol. Res. 2004, 35, 49–67. [Google Scholar]
- Sparks, T.; Tryjanowski, P. Patterns of spring arrival dates differ in two hirundines. Clim. Res. 2007, 35, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Keller, V.; Herrando, S.; Voříšek, P.; Franch, M.; Kipson, M.; Milanesi, P.; Martí, D.; Anton, M.; Klvanová, A.; Kalyakin, M.V.; et al. European Breeding Bird Atlas 2: Distribution, Abundance and Change; European Bird Census Council: Barcelona, Spain, 2020. [Google Scholar]
- BirdLife International. Species Factsheet: Ciconia Ciconia. 2021. Available online: http://www.birdlife.org (accessed on 10 September 2015).
- Gordo, O.; Sanz, J.J.; Lobo, J.M. Environmental and geographical constraints on common swift and barn swallow spring arrival patterns throughout the Iberian Peninsula. J. Biogeogr. 2007, 34, 1065–1076. [Google Scholar] [CrossRef]
- Nevoux, M.; Barbraud, J.-C.; Barbraud, C. Nonlinear impact of climate on survival in a migratory white stork population. J. Anim. Ecol. 2008, 77, 1143–1152. [Google Scholar] [CrossRef]
- Martín, B.; Onrubia, A.; Ferrer, M. Responses to climate change in the migration timing differ between adult and juvenile White Storks across western Europe. Clim. Res. 2016, 69, 9–23. [Google Scholar] [CrossRef]
- Jerzak, E.; Shephard, J.; Aquirre, J.; Shamoun-Baranes, J.; Tryjanowski, P.; Góra, Z.; Wilharm, G.; Skiebe, E.; Kasprzak, M.; Bochenski, M.; et al. The White Stork: Studies in biology. In The White Stork: Studies in Biology, Ecology and Conservation; Oficyna Wydawnicza UZ: Lublin, Poland, 2016; pp. 209–218. [Google Scholar]
- Schaub, M.; Kania, W.; Köppen, U. Variation of primary production during winter induces synchrony in survival rates in migratory white storks Ciconia ciconia. J. Anim. Ecol. 2005, 74, 656–666. [Google Scholar] [CrossRef]
- Kania, W. Movements of Polish White Storks Ciconia ciconia—An analysis of ringing results. In The White Stork in Poland: Studies in Biology, Ecology and Conservation; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2006; pp. 313–358. [Google Scholar]
- Carrascal, L.M.; Bautista, L.M.; Lázaro, E. Geographical variation in the density of the white stork Ciconia ciconia in Spain: Influence of habitat structure and climate. Biol. Conserv. 1993, 65, 83–87. [Google Scholar] [CrossRef]
- Janss, G.; Ferrer, M. Mitigation of Raptor Electrocution on Steel Power Poles. Wildl. Soc. Bull. 1999, 27, 263–273. [Google Scholar]
- Du Feu, C.R.; Joys, A.C.; Clark, J.A.; Fiedler, W.; Downie, I.S.; Van Noordwijk, A.J. EURING Data Bank Geographical Index 2009. Available online: http://www.euring.org/edb (accessed on 30 May 2017).
- Jiguet, F.; Devictor, V.; Ottvall, R.; Van Turnhout, C.; Van der Jeugd, H.; Lindström, Å. Bird population trends are linearly affected by climate change along species thermal ranges. Proc. R. Soc. B Biol. Sci. 2010, 277, 3601–3608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberhardt, L.L. A paradigm for population analysis of long-lived vertebrates. Ecology 2002, 83, 2841–2854. [Google Scholar] [CrossRef]
- Del Hoyo, J.; Elliot, A.; Sargatal, J. European White Stork. In Handbook of the Birds of the World; Lynx Edicions: Barcelona, Spain, 1992; pp. 460–461. [Google Scholar]
- Moller, A.; Nuttall, R.; Piper, S.; Szép, T.; Vickers, E. Migration, moult and climate change in barn swallows Hirundo rustica in South Africa. Clim. Res. 2011, 47, 201–205. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2021-2. 2021. Available online: https://www.iucnredlist.org (accessed on 27 October 2021).
- Leslie, P.H. The Intrinsic Rate of Increase and the Overlap of Successive Generations in a Population of Guillemots (Uria aalge Pont.). J. Anim. Ecol. 1966, 35, 291–301. [Google Scholar] [CrossRef]
- Hijmans, R.; Williams, E.; Vennes, C.; Package “Geosphere” (Manual). 35p. 2010. Available online: http://cran.r-project.org/web/packages/geosphere/geosphere.pdf (accessed on 23 October 2021).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Imboden, C.; Imboden, D. Formel für orthodrome und loxodrome bei der berechnung von richtung und distanz zwischen beringungs- und wiederfundort. Vogelwarte 1972, 26, 336–346. [Google Scholar]
- Lebreton, J.-D.; Burnham, K.P.; Clobert, J.; Anderson, D.R. Modeling Survival and Testing Biological Hypotheses Using Marked Animals: A Unified Approach with Case Studies. Ecol. Monogr. 1992, 62, 67–118. [Google Scholar] [CrossRef] [Green Version]
- Pollock, K.H.; Nichols, J.D.; Brownie, C.; Hines, J.E. Statistical Inference for Capture-Recapture Experiments. Wildl. Monogr. 1990, 3–97. [Google Scholar]
- Cooch, E.; White, G. Program MARK: Analysis of Data from Marked Individuals, “A Gentle Introduction” (11th ed.). 2012. Available online: http://www.cnr.colostate.edu/*gwhite/mark/mark.html (accessed on 28 August 2013).
- Barbraud, C.; Barbraud, J.-C.; Barbraud, C. Population dynamics of the White Stork Ciconia ciconia in western France. IBIS 1999, 141, 469–479. [Google Scholar] [CrossRef]
- Kanyamibwa, S.; Bairlein, F.; Schierer, A. Comparison of Survival Rates between Populations of the White Stork Ciconia ciconia in Central Europe. Ornis Scand. (Scand. J. Ornithol.) 1993, 24, 297–302. [Google Scholar] [CrossRef]
- Vergara, P.; Aguirre, J.I.; Fernández-Cruz, M. Arrival date, age and breeding success in white stork Ciconia ciconia. J. Avian Biol. 2007, 38, 573–579. [Google Scholar] [CrossRef]
- Chernetsov, N.; Chromik, W.; Dolata, P.T.; Profus, P.; Tryjanowski, P. Sex-Related Natal Dispersal of White Storks (Ciconia ciconia) in Poland: How Far and Where to? (Hатаʌьная дисперсия Ciconia ciconia Пoʌьше зависит oт пoʌа: как даʌекo и куда?). Auk 2006, 123, 1103–1109. [Google Scholar] [CrossRef]
- Holmgren, M.; Stapp, P.; Dickman, C.R.; Gracia, C.; Graham, S.; Gutiérrez, J.R.; Hice, C.; Jaksic, F.; Kelt, D.A.; Letnic, M.; et al. Extreme climatic events shape arid and semiarid ecosystems. Front. Ecol. Environ. 2006, 4, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Li, Y.; Hu, J.; Yang, X.; Sheng, S.; Liu, M. Evaluating the difference between the normalized difference vegetation index and net primary productivity as the indicators of vegetation vigor assessment at landscape scale. Environ. Monit. Assess. 2012, 184, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.; van Etten, J. Package “Raster”: Geographic Analysis and Modeling with Raster Data (Manual). R Package Version 1.9-5. 2011. Available online: http://cran.r-project.org/web/packages/raster/raster.pdf (accessed on 25 October 2021).
- Kruszyk, R.; Ciach, M. White Storks, Ciconia ciconia, forage on rubbish dumps in Poland—A novel behaviour in population. Eur. J. Wildl. Res. 2010, 56, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Tortosa, F.; Caballero, J.; Reyes Lopez, J. Effect of rubbish dumps on breeding success in the White Stork in southern Spain. Waterbirds 2002, 25, 39–43. [Google Scholar] [CrossRef]
- Bossard, M.; Feranec, J.; Otahel, J. Corine Land Cover Technical Guide—Addendum 2000; European Environment Agency: Copenhagen, Denmark, 2000. [Google Scholar]
- Büttner, G.; Feranec, J.; Jaffrain, G. Corine Land Cover Update 2000: Technical Guideline; Technical report No 89; EEA: Copenhagen, Denmark, 2002. [Google Scholar]
- CEC. CORINE Land Cover Technical Guide; Office for Official Publications of the European Communities: Brussels, Belgium, 1994. [Google Scholar]
- Gilbert, N.I.; Correia, R.A.; Silva, J.P.; Pacheco, C.; Catry, I.; Atkinson, P.W.; Gill, J.A.; Franco, A.M.A. Are white storks addicted to junk food? Impacts of landfill use on the movement and behaviour of resident white storks (Ciconia ciconia) from a partially migratory population. Mov. Ecol. 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Schaub, M.; Pradel, R.; Lebreton, J.-D. Is the reintroduced white stork (Ciconia ciconia) population in Switzerland self-sustainable? Biol. Conserv. 2004, 119, 105–114. [Google Scholar] [CrossRef]
- Lerche-Jørgensen, M.; Korner-Nievergelt, F.; Tøttrup, A.P.; Willemoes, M.; Thorup, K. Early returning long-distance migrant males do pay a survival cost. Ecol. Evol. 2018, 8, 11434–11449. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from populations of marked animals. Bird Study 1999, 46, S120–S139. [Google Scholar] [CrossRef]
- Ockendon, N.; Baker, D.J.; Carr, J.A.; White, E.C.; Almond, R.E.A.; Amano, T.; Bertram, E.; Bradbury, R.; Bradley, C.; Butchart, S.H.M.; et al. Mechanisms underpinning climatic impacts on natural populations: Altered species interactions are more important than direct effects. Glob. Chang. Biol. 2014, 20, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Newton, I. The Migration Ecology of Birds; Academic Press: London, UK, 2008. [Google Scholar]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, B.; Onrubia, A.; Ferrer, M. Effects of climate change on the migration behavior of the Common Buzzard (Buteo buteo). Clim. Res. 2014, 60, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Gordo, O.; Tryjanowski, P.; Kosicki, J.Z.; Fulín, M. Complex phenological changes and their consequences in the breeding success of a migratory bird, the white stork Ciconia ciconia. J. Anim. Ecol. 2013, 82, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- EEA. Climate Change, Impacts and Vulnerability in Europe 2012. An Indicator-Based Report; EEA Report No 12/2012; European Environment Agency: Copenhagen, Denmark, 2012. [Google Scholar]
- Stenseth, N.C.; Mysterud, A.; Ottersen, G.; Hurrell, J.W.; Chan, K.-S.; Lima, M. Ecological Effects of Climate Fluctuations. Science 2002, 297, 1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. LIFE and Europe’s Wetlands: Restoring a Vital Ecosystem; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- Saether, B.-E.; Grøtan, V.; Tryjanowski, P.; Barbraud, C.; Engen, S.; Fulin, M. Climate and spatio-temporal variation in the population dynamics of a long distance migrant, the white stork. J. Anim. Ecol. 2006, 75, 80–90. [Google Scholar] [CrossRef]
- Flack, A.; Fiedler, W.; Blas, J.; Pokrovsky, I.; Kaatz, M.; Mitropolsky, M.; Aghababyan, K.; Fakriadis, I.; Makrigianni, E.; Jerzak, L.; et al. Costs of migratory decisions: A comparison across eight white stork populations. Sci. Adv. 2016, 2, e1500931. [Google Scholar] [CrossRef] [Green Version]
- Tavecchia, G.; Pradel, R.; Boy, V.; Johnson, A.R.; Cézilly, F. Sex- and Age-Related Variation in Survival and Cost of First Reproduction in Greater Flamingos. Ecology 2001, 82, 165–174. [Google Scholar] [CrossRef]
- Gaillard, J.-M.; Festa-Bianchet, M.; Yoccoz, N.G. Population dynamics of large herbivores: Variable recruitment with constant adult survival. Trends Ecol. Evol. 1998, 13, 58–63. [Google Scholar] [CrossRef]
- Martín, B.; Onrubia, A.; De la Cruz, A.; Ferrer, M. Recent trends of autumn counts at Iberian migration bottlenecks as a tool for monitoring continental populations of soaring birds in Europe. Biodivers. Conserv. 2016, 25, 295–309. [Google Scholar] [CrossRef]
- Boere, G.C.; Galbraith, C.A.; Stroud, D.A. Waterbirds around the World; The Stationery Office: Edinburgh, UK, 2006. [Google Scholar]
- Huntley, B.; Green, R.; Collingham, Y.; Willis, S. A Climatic Atlas of European Breeding Birds; Durham University: Durham, UK; The RSPB and Lynx Edicions: Barcelona, Spain, 2007. [Google Scholar]
- Vergara, P.; Aguirre, J.I.; Fernández-Cruz, M. Fidelidad a los sitios y fenología en la invernada de la cigüeña blanca (Ciconia ciconia) en la Comunidad de Madrid (1998–2002). In Anuario Ornitológico de Madrid 2003; Puente, J., Pérez-Tris, J., Bermejo, A., Martínez, M.-J., Eds.; SEO-Monticola: Madrid, Spain, 2004. [Google Scholar]
- Molina, B.; Del Moral, J.C. La Cigüeña Blanca en España. In VI Censo Internacional (2004); SEO/BirdLife: Madrid, Spain, 2004. [Google Scholar]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Ciscar, J.C.; Ibarreta, D.; Soria, A.; Dosio, A.; Toreti, A.; Ceglar, A.; Fumagalli, D.; Dentener, F.; Lecerf, R.; Zucchini, A.; et al. Climate Impacts in Europe: Final Report of the JRC PESETA III Project; Report No.: EUR 29427 EN; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- EEA. Key Observed and Projected Climate Change and Impacts for the Main Regions in Europe. 2017. Available online: https://www.eea.europa.eu/legal/copyright (accessed on 28 October 2021).
- Barlein, F. Population studies of white storks (Ciconia ciconia) in Europe. In Bird Population Studies; Perrins, C., Lebreton, J.-D., Eds.; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Both, C.; Van Turnhout, C.A.M.; Bijlsma, R.G.; Siepel, H.; Van Strien, A.J.; Foppen, R.P.B. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. B Biol. Sci. 2010, 277, 1259–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanyamibwa, S.; Schierer, A.; Pradel, R.; Lebreton, J.-D. Changes in Adult Annual Survival Rates in a Western-European Population of the White Stork Ciconia-Ciconia. IBIS 1990, 132, 27–35. [Google Scholar] [CrossRef]
- Kosicki, J.Z.; Kuzniak, S. Long-term population size and productivity dynamics of a local white stork Ciconia ciconia population in Wielkopolska. In The White Stork in Poland: Studies in Biology, Ecology and Conservation; Tryjanowski, P., Sparks, T.H., Jerzak, L., Eds.; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2006; pp. 23–33. [Google Scholar]
- Johst, K.; Brandl, R.; Pfeifer, R. Foraging in a Patchy and Dynamic Landscape: Human Land Use and the White Stork. Ecol. Appl. 2001, 11, 60–69. [Google Scholar] [CrossRef]
- Martín, B.; Alonso, J.C.; Martín, C.A.; Palacín, C.; Magaña, M.; Alonso, J. Influence of spatial heterogeneity and temporal variability in habitat selection: A case study on a great bustard metapopulation. Ecol. Model. 2012, 228, 39–48. [Google Scholar] [CrossRef]
- Gadenne, H.; Cornulier, T.; Eraud, C.; Barbraud, J.-C.; Barbraud, C. Evidence for density-dependent habitat occupancy at varying scales in an expanding bird population. Popul. Ecol. 2014, 56, 493–506. [Google Scholar] [CrossRef] [Green Version]
- Wuczyński, A.; Krogulec, G.; Jakubiec, Z.; Profus, P.; Neubauer, G. Population size and spatial distribution of the white stork Ciconia ciconia in Poland in 1958 with insights into long-term trends in regional and global population. Eur. Zool. J. 2021, 88, 525–539. [Google Scholar] [CrossRef]
| Model * | Description |
|---|---|
| {ϕ(a3–tma2/tma2/tma2+sahel+ylat+ndvi+dump+sahel:ylat+ndvi:ylat+dump:ylat)p(a3(ylat/ylat/ylat)+trend} | 3 age-classes for survival (a3), all age classes time-constrained, constrained by quadratic Northern temperature anomaly -tma-, and constrained by Sahel index (sahel; additive effect); additive effect on survival of the covariates (ylat, ndvi, and dump) and interaction terms between covariates (sahel:ylat, ndvi:ylat, dump:ylat). 3 age-classes for recapture, all age classes dependent on latitude in the ringing site (ylat); exponential temporal trend in recapture (additive effect) |
| {ϕ(a3–tma2/tma2/tma2+sahel+ylat+sahel:ylat+ndvi+dump+ndvi:ylat+dump:ylat)p(a3+ylat+trend)} | 3 age-classes for survival (a3), all age classes time-constrained, constrained by quadratic Northern temperature anomaly -tma-, and constrained by Sahel index (sahel; additive effect); additive effect on survival of the covariates (ylat, ndvi, and dump) and interaction terms between covariates (sahel:ylat, ndvi:ylat, dump:ylat). 3 age-classes for recapture, exponential temporal trend in recapture (additive effect), additive effect of latitude in the ringing site |
| {ϕ(a3–tma2/tma2/tma2+sahel+ylat+ndvi+dump+sahel:ylat+ndvi:ylat+dump:ylat)p(a3+trend)} | 3 age-classes for survival (a3), all age classes time-constrained (constrained by quadratic Northern temperature anomaly -tma- and constrained by Sahel index (sahel; additive effect); additive effect on survival of the covariates (ylat, ndvi, and dump) and interaction terms between covariates (sahel:ylat, ndvi:ylat, dump:ylat). 3 age-classes for recapture, exponential temporal trend in recapture (additive effect) |
| {ϕ(a3–tma2/tma2/tma2+sahel+ylat+ndvi+dump+ndvi:ylat+dump:ylat)p(a3+trend)} | 3 age-classes for survival (a3), all age classes time-constrained, constrained by quadratic Northern temperature anomaly -tma-, and constrained by Sahel index (sahel; additive effect); additive effect on survival of the covariates (ylat, ndvi, and dump) and interaction terms between covariates (ndvi:ylat, dump:ylat). 3 age-classes for recapture, exponential temporal trend in recapture (additive effect) |
| {ϕ(a3–tma2/tma2/tma2+sahel+ylat+ndvi+dump+ndvi:ylat)p(a3+trend)} | 3 age-classes for survival (a3), all age classes time-constrained, constrained by quadratic Northern temperature anomaly -tma-, and constrained by Sahel index (sahel; additive effect); additive effect on survival of the covariates (ylat, ndvi, and dump) and interaction term between covariates (ndvi:ylat). 3 age-classes for recapture, exponential temporal trend in recapture (additive effect) |
| {ϕ(a3–tma2/tma2/tma2+sahel+ylat+ndvi+dump)p(a3+trend)} | 3 age-classes for survival (a3), all age classes time-constrained, constrained by quadratic Northern temperature anomaly -tma-and time-constrained by Sahel index (sahel; additive effect); additive effect on survival of the covariates (ylat, ndvi, and dump). 3 age-classes for recapture, exponential temporal trend in recapture (additive effect) |
| {ϕ(a3–tma2/tma2/tma2+sahel+ylat)p(a3+trend)} | 3 age-classes for survival (a3), all age classes time-constrained, constrained by quadratic Northern temperature anomaly -tma-, and time-constrained by Sahel index (sahel; additive effect); additive effect on survival of the covariate ‘latitude’ (ylat). 3 age-classes for recapture, exponential temporal trend in recapture (additive effect) |
| {ϕ(a3–tma2/tma2/tma2+sahel)p(a3+trend)} | 3 age-classes for survival (a3), all age classes time-constrained, constrained by quadratic Northern temperature anomaly -tma-, and constrained by Sahel index (sahel; additive effect); latitudinal additive effect on survival (ylat). 3 age-classes for recapture, exponential temporal trend in recapture (additive effect) |
| {ϕ(a3–tma2/tma2/tma2)p(a3+trend)} | 3 age-classes for survival (a3), all age classes time-constrained, constrained by quadratic Northern temperature anomaly -tma-, and constrained by Sahel index (sahel; additive effect). 3 age-classes for recapture, exponential temporal trend in recapture (additive effect) |
| {(a3–././.)p(a3+trend)} | 3 age-classes for survival (a3), all age classes constant (././.) - juvenile (first)/ immature (second)/ adult (third)- through time |
| {ϕ(t)p(t)} | Standard CJS model; time dependence in survival and recapture |
| {(t)p(.)} | Time-dependent survival; constant recapture through time |
| {ϕ(.)p(.)} | Constant survival and recapture through time |
| Model | QAICc | Delta QAICc | wi | np | QDeviance |
|---|---|---|---|---|---|
| {ϕ(a3 tma2/tma2/tma2+sahel+ylat+sahel:ylat+ndvi+dump+ndvi:ylat+dump:ylat)p(a3(ylat/ylat/ylat)+trend)} | 21,569.35 | 0.00 | 1.00 | 22 | 21,525.32 |
| {ϕ(a3 tma2/tma2/tma2+sahel+ylat+sahel:ylat+ndvi+dump+ndvi:ylat+dump:ylat)p(a3+trend+ylat)} | 21,591.17 | 21.82 | 0.00 | 20 | 21,551.14 |
| {ϕ(a3 tma2/tma2/tma2+sahel+ylat+sahel:ylat+ndvi+dump+ndvi:ylat+dump:ylat)p(a3+trend)} | 21,636.69 | 67.34 | 0.00 | 19 | 21,598.66 |
| {ϕ(a3 tma2/tma2/tma2+sahel+ylat+ndvi+dump+ndvi:ylat+dump:ylat)p(a3+trend)} | 21,672.07 | 102.72 | 0.00 | 18 | 21,636.05 |
| {ϕ(a3 tma2/tma2/tma2+sahel+ylat+ndvi+dump+ndvi:ylat)p(a3+trend)} | 21,718.74 | 149.39 | 0.00 | 17 | 21,684.72 |
| {ϕ(a3 tma2/tma2/tma2+sahel+ylat+ndvi+dump)p(a3+trend)} | 21,831.62 | 262.26 | 0.00 | 16 | 21,799.60 |
| {ϕ(a3 tma2/tma2/tma2+sahel+ylat)p(a3+trend)} | 22,844.18 | 1274.83 | 0.00 | 15 | 22,814.16 |
| {ϕ(a3 tma2/tma2/tma2+sahel)p(a3+trend)} | 22,844.47 | 1275.12 | 0.00 | 14 | 22,816.45 |
| {ϕ(a3 tma2/tma2/tma2)p(a3+trend)} | 23,123.97 | 1554.61 | 0.00 | 13 | 23,097.95 |
| {ϕ(a3)p(a3+trend)} | 23,448.88 | 1879.53 | 0.00 | 7 | 23,434.88 |
| {ϕ(t)p(t)} | 23,541.65 | 1972.30 | 0.00 | 96 | 23,349.00 |
| {(t)p(.)} | 23,933.62 | 2364.26 | 0.00 | 50 | 23,833.44 |
| {ϕ(.)p(.)} | 24,952.57 | 3383.22 | 0.00 | 2 | 24,948.57 |
| Parameter | Beta | E.S. | Lower | Upper |
|---|---|---|---|---|
| Survival–ϕ- | ||||
| age 1 | –1.182 | 0.584 | –2.327 | –0.037 |
| tma (age 1) | –0.317 | 0.275 | –0.857 | 0.223 |
| tma2 (age 1) | –1.276 | 0.201 | –1.670 | –0.882 |
| age2 | –0.475 | 0.579 | –1.610 | 0.659 |
| tma (age 2) | 1.180 | 0.367 | 0.459 | 1.900 |
| tma2 (age 2) | –1.924 | 0.271 | –2.455 | –1.394 |
| age3 | 0.185 | 0.586 | –0.963 | 1.333 |
| tma (age 3) | 1.353 | 0.365 | 0.638 | 2.069 |
| tma2 (age 3) | –1.942 | 0.278 | –2.486 | –1.398 |
| sahel | 1.809 | 0.349 | 1.125 | 2.494 |
| ylat | 0.010 | 0.011 | –0.013 | 0.032 |
| sahel:ylat | –0.040 | 0.007 | –0.054 | –0.026 |
| ndvi | 2313.312 | 270.802 | 1782.540 | 2844.084 |
| ndvi:ylat | –53.876 | 5.684 | –65.016 | –42.736 |
| dump | –1.391 | 0.209 | –1.802 | –0.981 |
| dump:ylat | 0.051 | 0.005 | 0.042 | 0.060 |
| Recapture -p- | ||||
| age1 | -0.513 | 0.748 | -1.979 | 0.953 |
| ylat | -0.049 | 0.015 | -0.078 | -0.020 |
| age2 | 2.707 | 0.844 | 1.052 | 4.361 |
| ylat | -0.107 | 0.017 | -0.141 | -0.074 |
| age3 | 5.060 | 0.843 | 3.409 | 6.712 |
| ylat | -0.149 | 0.017 | -0.183 | -0.115 |
| exponential temporal trend | 3.580 | 0.417 | 2.763 | 4.396 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, B.; Onrubia, A.; Ferrer, M. Climate Change and the Spatiotemporal Variation in Survival of a Long-Distance Migrant (White Stork, Ciconia ciconia) across Western Europe. Birds 2021, 2, 362-380. https://doi.org/10.3390/birds2040027
Martín B, Onrubia A, Ferrer M. Climate Change and the Spatiotemporal Variation in Survival of a Long-Distance Migrant (White Stork, Ciconia ciconia) across Western Europe. Birds. 2021; 2(4):362-380. https://doi.org/10.3390/birds2040027
Chicago/Turabian StyleMartín, Beatriz, Alejandro Onrubia, and Miguel Ferrer. 2021. "Climate Change and the Spatiotemporal Variation in Survival of a Long-Distance Migrant (White Stork, Ciconia ciconia) across Western Europe" Birds 2, no. 4: 362-380. https://doi.org/10.3390/birds2040027
APA StyleMartín, B., Onrubia, A., & Ferrer, M. (2021). Climate Change and the Spatiotemporal Variation in Survival of a Long-Distance Migrant (White Stork, Ciconia ciconia) across Western Europe. Birds, 2(4), 362-380. https://doi.org/10.3390/birds2040027







