Does Habitat Diversity Modify the Dietary and Reproductive Response to Prey Fluctuations in a Generalist Raptor Predator, the Eurasian Buzzard Buteo buteo?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Methods
2.3. Data Analysis
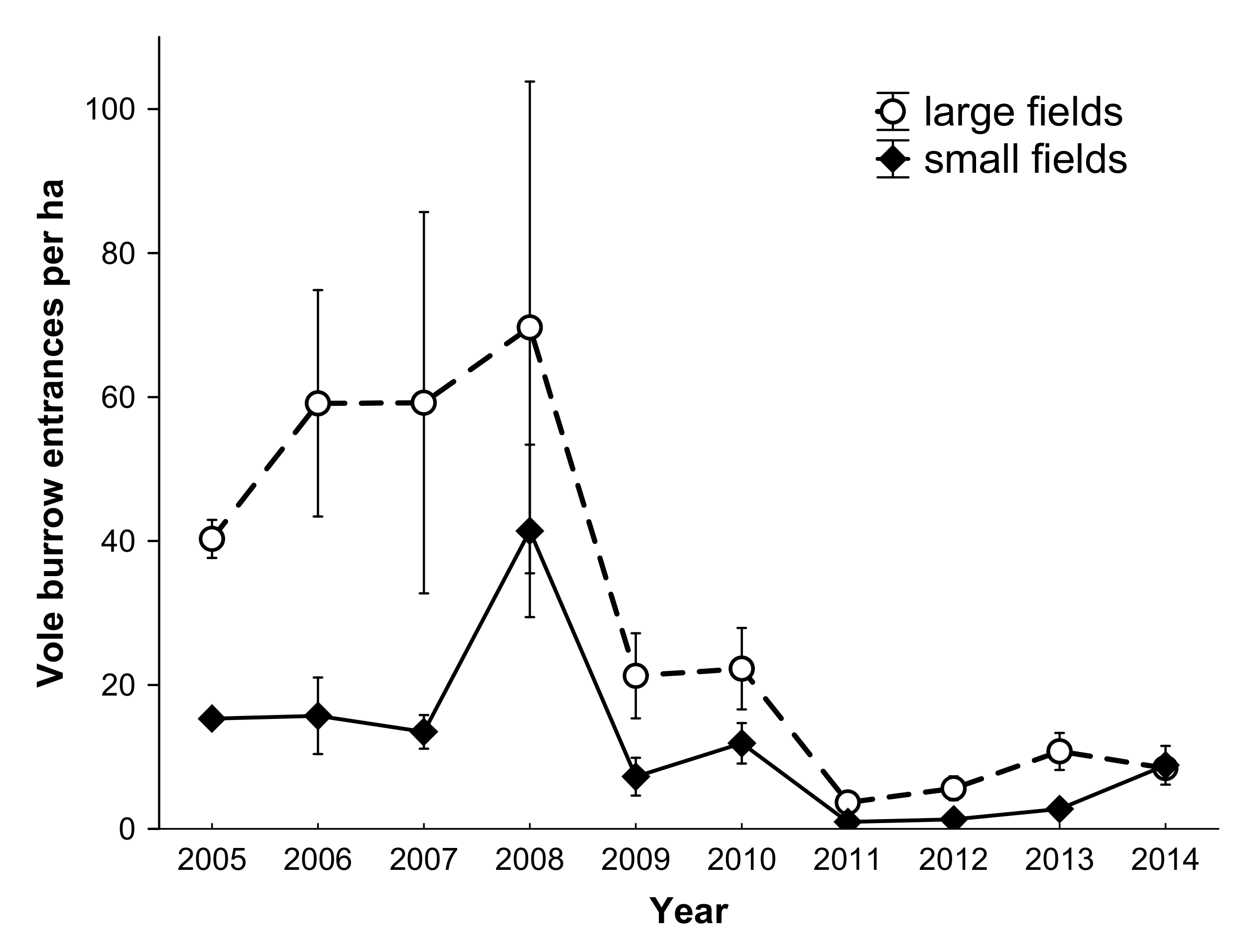
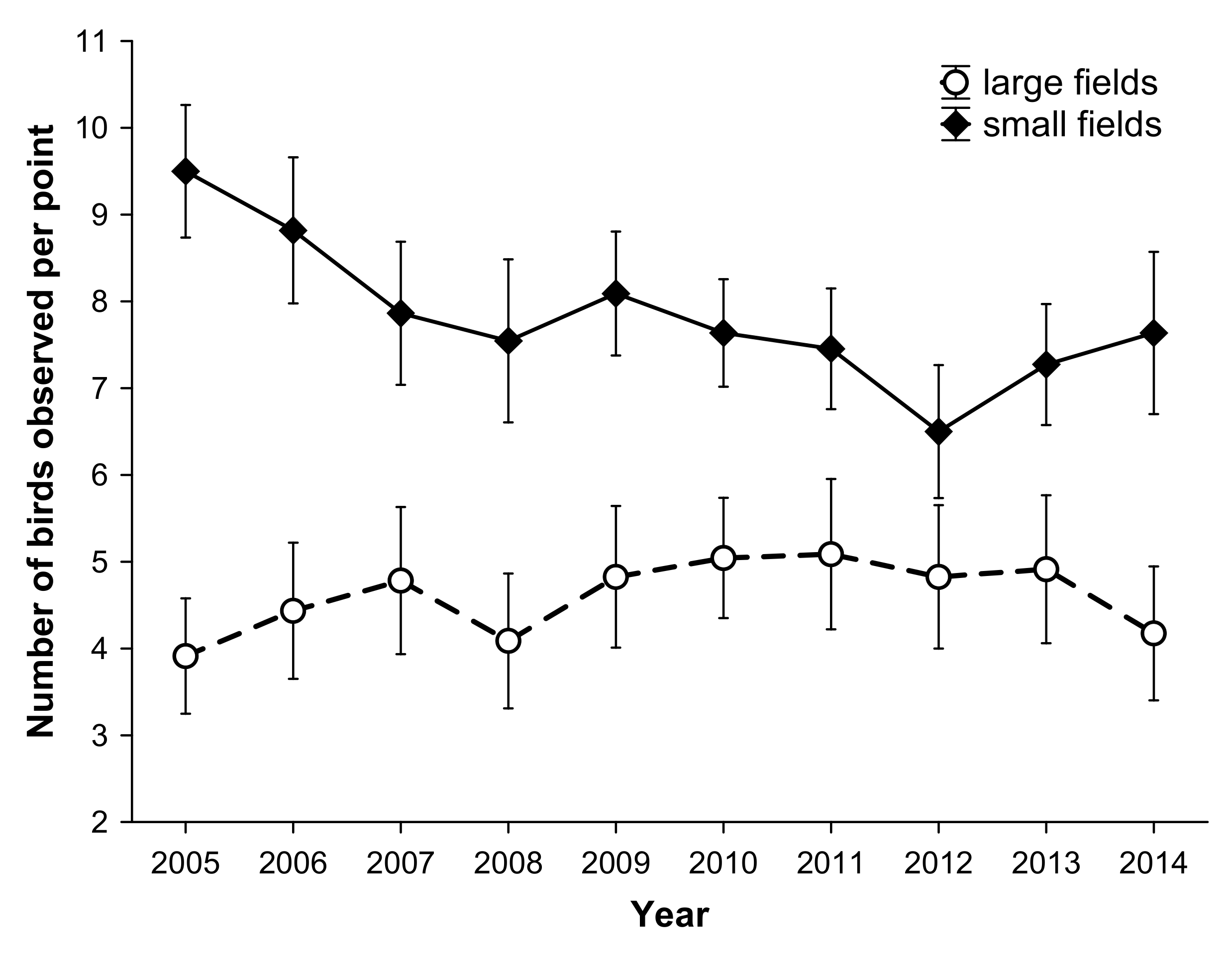
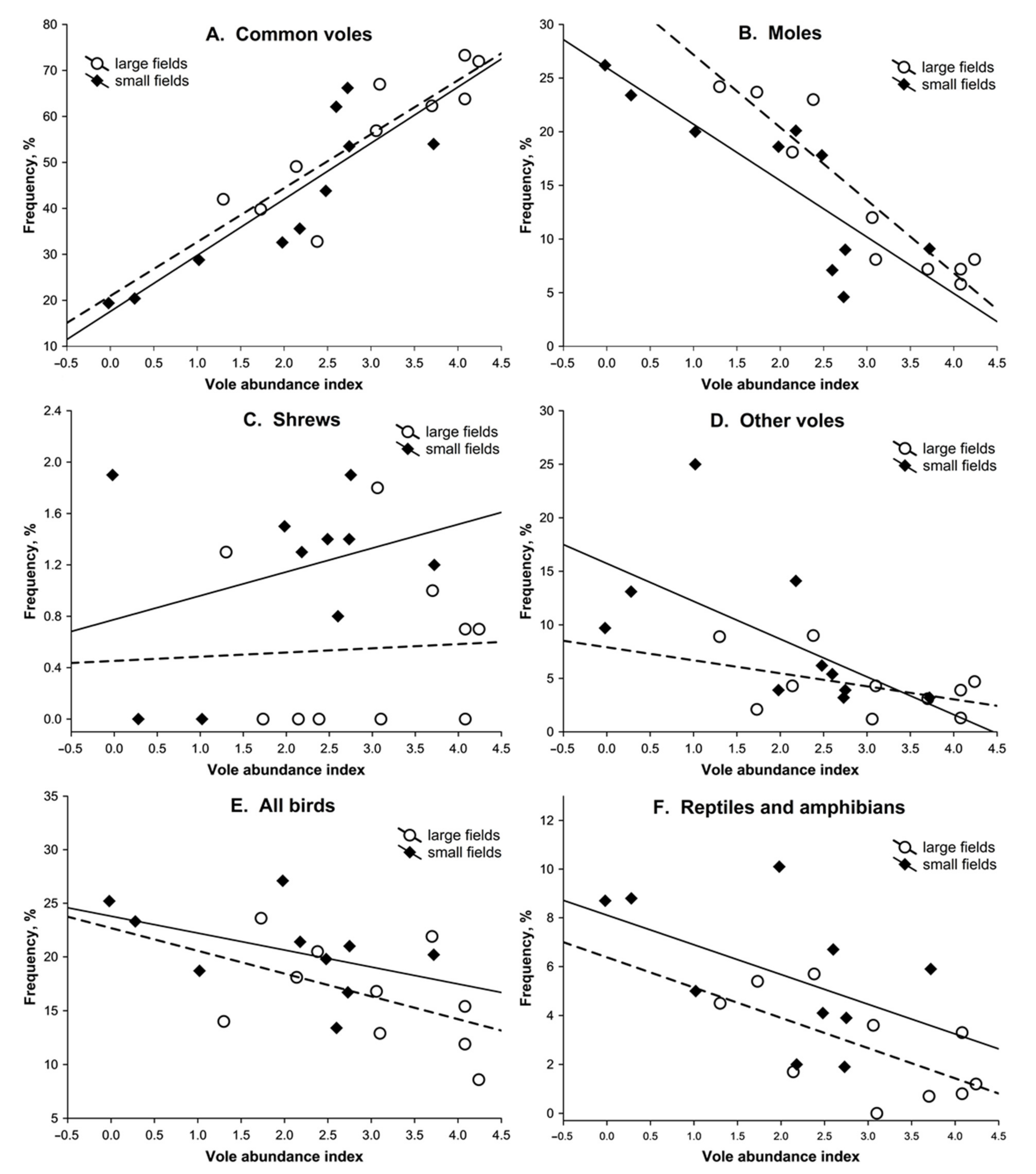
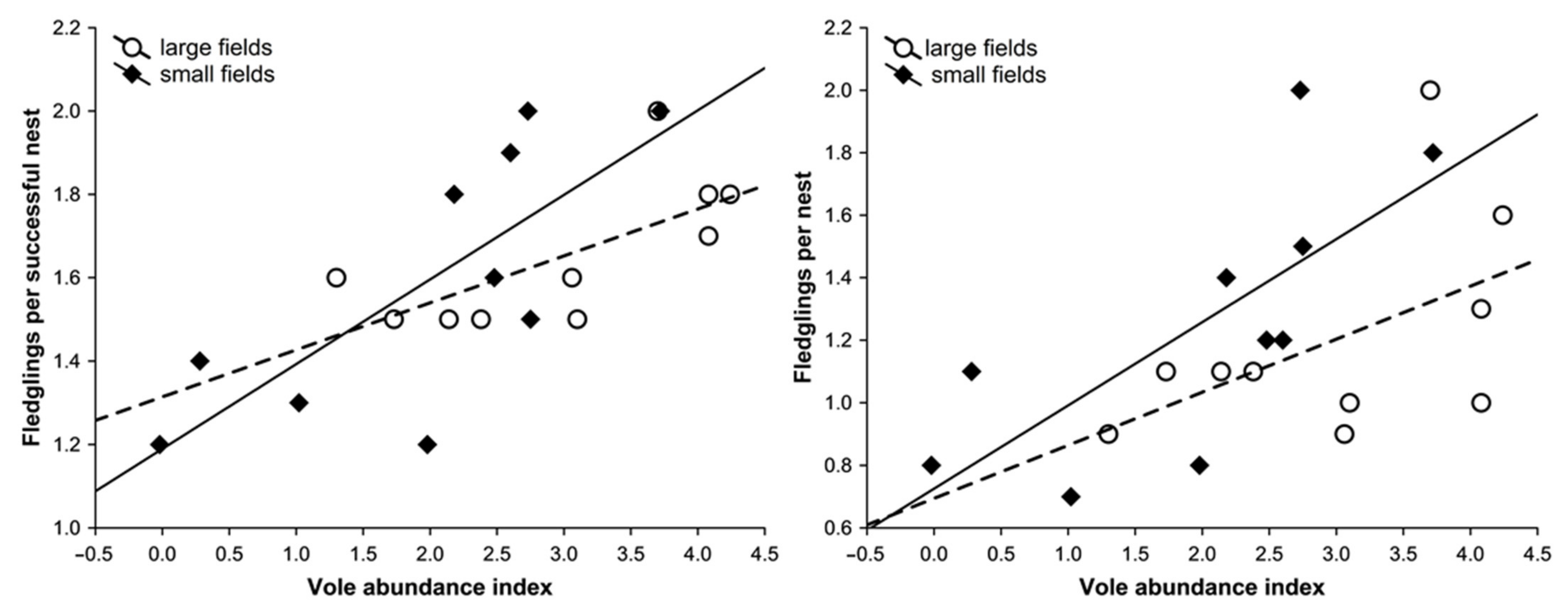
3. Results
3.1. Prey Abundance
3.2. Dietary Response
3.3. Reproductive Response
4. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Korpimäki, E.; Norrdahl, K. Predation of Tengmalm’s owls: Numerical responses, functional responses and dampening impact on population fluctuations of microtines. Oikos 1989, 54, 154–164. [Google Scholar] [CrossRef]
- Korpimäki, E.; Norrdahl, K. Numerical and functional responses of kestrels, short-eared owls, and long-eared owls to vole densities. Ecology 1991, 72, 814–826. [Google Scholar] [CrossRef]
- Nielsen, Ó.K. Gyrfalcon predation on ptarmigan: Numerical and functional responses. J. Anim. Ecol. 1999, 68, 1034–1050. [Google Scholar] [CrossRef]
- Redpath, S.M.; Thirgood, S.J. Numerical and functional responses in generalist predators: Hen harriers and peregrines on Scottish grouse moors. J. Anim. Ecol. 1999, 68, 879–892. [Google Scholar] [CrossRef]
- Salamolard, M.; Butet, A.; Leroux, A.; Bretagnolle, V. Responses of an avian predator to variation in prey density at a temperate latitude. Ecology 2000, 81, 2428–2441. [Google Scholar] [CrossRef]
- Gilg, O.; Sittler, B.; Sabard, B.; Hurstel, A.; Sané, R.; Delattre, P.; Hanski, I. Functional and numerical responses of four lemming predators in high arctic Greenland. Oikos 2006, 113, 193–216. [Google Scholar] [CrossRef]
- Andersson, M.; Erlinge, S. Influence of predation on rodent populations. Oikos 1977, 29, 591–597. [Google Scholar] [CrossRef]
- Hanski, I.; Hansson, L.; Henttonen, H. Specialist predators, generalist predators, and the microtine rodent cycle. J. Anim. Ecol. 1991, 60, 353–367. [Google Scholar] [CrossRef]
- Hanski, I.; Henttonen, H.; Korpimäki, E.; Oksanen, L.; Turchin, P. Small-rodent dynamics and predation. Ecology 2001, 82, 1505–1520. [Google Scholar] [CrossRef]
- Angelstam, P.; Lindström, E.; Widén, P. Role of predation in short-term population fluctuations of some birds and mammals in Fennoscandia. Oecologia 1984, 62, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Norrdahl, K.; Korpimäki, E. Do predators limit the abundance of alternative prey? Experiments with vole-eating avian and mammalian predators. Oikos 2000, 91, 528–540. [Google Scholar] [CrossRef]
- Terraube, J.; Arroyo, B.; Madders, M.; Mougeot, F. Diet specialization and foraging efficiency under fluctuating vole abundance: A comparison between generalist and specialist avian predators. Oikos 2011, 120, 234–244. [Google Scholar] [CrossRef]
- Korpimäki, E.; Krebs, C.J. Predation and population cycles of small mammals. BioScience 1996, 46, 754–764. [Google Scholar]
- Korpimäki, E.; Norrdahl, K.; Rinta-Jaskari, T. Responses of stoat and least weasels to fluctuating food abundances: Is the low phase of the vole cycle due to mustelid predation? Oecologia 1991, 88, 552–561. [Google Scholar] [CrossRef]
- Korpela, K.; Helle, P.; Henttonen, H.; Korpimäki, E.; Koskela, E.; Ovaskainen, O.; Pietiäinen, H.; Sundell, J.; Valkama, J.; Huitu, O. Predator-vole interactions in northern Europe: The role of small mustelids. Proc. R. Soc. B 2014, 281, 20142119. [Google Scholar] [CrossRef] [PubMed]
- Korpimäki, E. Gradients in population fluctuations of Tengmalm’s owl Aegolius funereus in Europe. Oecologia 1986, 69, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Erlinge, S.; Göransson, G.; Hansson, L.; Högstedt, G.; Liberg, O.; Nilsson, I.N.; Nilsson, T.; von Schantz, T.; Sylvén, M. Predation as a regulating factor on small rodent populations in southern Sweden. Oikos 1983, 40, 36–52. [Google Scholar] [CrossRef]
- Hansson, L.; Henttonen, H. Gradient in density variation of small rodents: The importance of latitude and snow cover. Oecologia 1985, 67, 394–402. [Google Scholar] [CrossRef]
- Erlinge, S. Predation and noncyclicity in a microtine population in southern Sweden. Oikos 1987, 50, 347–352. [Google Scholar] [CrossRef]
- Klemola, T.; Tanhuanpää, M.; Korpimäki, E.; Ruohomäki, K. Specialist and generalist natural enemies as an explanation for geographical gradients in population cycles of northern herbivores. Oikos 2002, 99, 83–94. [Google Scholar] [CrossRef]
- Mebs, T. Zur Biologie und Populationsdynamik des Mäusebussards (Buteo buteo). J. Ornith. 1964, 105, 247–306. [Google Scholar] [CrossRef]
- Goszczyński, J.; Piłatowski, T. Diet of common buzzards (Buteo buteo L.) and goshawks (Accipiter gentilis L.) in the nesting period. Ekologia Polska 1986, 34, 655–667. [Google Scholar]
- Jędrzejewski, W.; Szymura, A.; Jędrzejewska, B. Reproduction and food of the buzzard Buteo buteo in relation to the abundance of rodents and birds in Białowieża National Park, Poland. Ethol. Ecol. Evol. 1994, 6, 179–190. [Google Scholar] [CrossRef]
- Reif, V.; Tornberg, R.; Jungell, S.; Korpimäki, E. Diet variation of common buzzards in Finland supports the alternative prey hypothesis. Ecography 2001, 24, 267–274. [Google Scholar] [CrossRef]
- Šotnár, K.; Obuch, J. Feeding ecology of a nesting population of the common buzzard (Buteo buteo) in the Upper Nitra Region, Central Slovakia. Slovak Raptor J. 2009, 3, 13–20. [Google Scholar] [CrossRef]
- Sidorovich, A.A.; Ivanovskij, V.; Sidorovich, V.E.; Solovej, I.A. Landscape-related variation in the diet composition of the common buzzard (Buteo buteo) in Belarus. Slovak Raptor J. 2016, 10, 65–74. [Google Scholar] [CrossRef]
- Ryszkowski, L.; Goszczyński, J.; Truszkowski, J. Trophic relationships of the common vole in cultivated fields. Acta Theriol. 1973, 18, 125–165. [Google Scholar] [CrossRef]
- Mackin-Rogalska, R.; Nabagło, L. Geographical variation in cyclic periodicity and synchrony in the common vole, Microtus arvalis. Oikos 1990, 59, 343–348. [Google Scholar] [CrossRef]
- Delattre, P.; De Sousa, B.; Fichet-Calvet, E.; Quéré, J.P.; Giraudoux, P. Vole outbreaks in a landscape context: Evidence from a six year study of Microtus arvalis. Lands. Ecol. 1999, 14, 401–412. [Google Scholar] [CrossRef]
- Tkadlec, E.; Stenseth, N.C. A new geographical gradient in vole population dynamics. Proc. R. Soc. B 2001, 268, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Reif, V.; Jungell, S.; Korpimäki, E.; Tornberg, R.; Mykrä, S. Numerical response of common buzzards and predation rate of main and alternative prey under fluctuating food conditions. Ann. Zool. Fenn. 2004, 41, 599–697. [Google Scholar]
- Panek, M. Numerical responses of an avian predator to prey fluctuations in a temperate latitude: Breeders vs. entire population. Popul. Ecol. 2016, 58, 549–555. [Google Scholar] [CrossRef]
- Wuczyński, A. Habitat use and hunting behaviour of Common Buzzards Buteo buteo wintering in south-western Poland. Acta Ornithol. 2005, 40, 147–154. [Google Scholar] [CrossRef]
- Ryszkowski, L. Structure and function of the mammal community in an agricultural landscape. Acta Zool. Fenn. 1982, 169, 45–59. [Google Scholar]
- Panek, M.; Hušek, J. The effect of oilseed rape occurrence on main prey abundance and breeding success of the common buzzard Buteo buteo. Bird Study 2014, 61, 457–464. [Google Scholar] [CrossRef][Green Version]
- Newton, I. Population Ecology of Raptors; Poyser: Berkhamsted, UK, 1979. [Google Scholar]
- Jędrzejewska, B.; Jędrzejewski, W. Predation in Vertebrate Communities. The Białowieża Forest as a Case Study; Springer: Berlin, Germany, 1998. [Google Scholar]
- Pucek, Z. Keys to Vertebrates of Poland. Mammals; PWN: Warszawa, Poland, 1981. [Google Scholar]
- Mackin-Rogalska, R.; Adamczewska-Andrzejewska, K.; Nabagło, L. Common vole numbers in relation to the utilization of burrow systems. Acta Theriol. 1986, 31, 17–44. [Google Scholar] [CrossRef]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A.; Mustoe, S.H. Bird Census Techniques, 2nd ed.; Academic Press: London, UK, 2000. [Google Scholar]
- Fuller, R.J.; Hinsley, S.A.; Swetnam, R.D. The relevance of non-farmland habitats, uncropped areas and habitat diversity to the conservation of farmland birds. Ibis 2004, 146, 22–31. [Google Scholar] [CrossRef]
- Sanderson, F.J.; Kloch, A.; Sachanowicz, K.; Donald, P.F. Predicting the effects of agricultural change on farmland bird populations in Poland. Agric. Ecosyst. Environ. 2009, 129, 37–42. [Google Scholar] [CrossRef]
- Siriwardena, G.M.; Cooke, I.R.; Sutherland, W.J. Landscape, cropping and field boundary influences on bird abundance. Ecography 2012, 35, 162–173. [Google Scholar] [CrossRef]
- Fahrig, L.; Girard, J.; Duro, D.; Pasher, J.; Smith, A.; Javorek, S.; King, D.; Lindsay, K.F.; Mitchell, S.; Tischendorf, L. Farmlands with smaller crops fields have higher within-field biodiversity. Agric. Ecosyst. Environ. 2015, 200, 219–234. [Google Scholar] [CrossRef]
- Wuczyński, A. Farmland bird diversity in contrasting agricultural landscapes of southwestern Poland. Landsc. Urban Plan. 2016, 148, 108–119. [Google Scholar] [CrossRef]
- Evans, K.L. The potential for interactions between predation and habitat change to cause population declines of farmland birds. Ibis 2004, 146, 1–13. [Google Scholar] [CrossRef]
- Whittingham, M.J.; Evans, K.L. The effects of habitat structure on predation risk of birds in agricultural landscapes. Ibis 2004, 146, 210–220. [Google Scholar] [CrossRef]
- Gorini, L.; Linnell, J.D.; May, R.; Panzacchi, M.; Boitani, L.; Odden, M.; Nilsen, E.B. Habitat heterogeneity and mammalian predator-prey interactions. Mammal Rev. 2012, 42, 55–77. [Google Scholar] [CrossRef]
- Panek, M. Landscape structure, predation of red foxes on grey partridges, and their spatial relations. Cent. Eur. J. Biol. 2013, 8, 1119–1126. [Google Scholar] [CrossRef]
- Warfe, D.M.; Barmuta, L.A. Habitat structural complexity mediates the foraging success of multiple predator species. Oecologia 2004, 141, 171–178. [Google Scholar] [CrossRef]
- Chalfoun, A.D.; Martin, T.E. Habitat structure mediates predation risk for sedentary prey: Experiments tests of alternative hypotheses. J. Anim. Ecol. 2009, 78, 497–503. [Google Scholar] [CrossRef]
- Goszczyński, J. Density and productivity of common buzzard Buteo buteo and goshawk Accipiter gentilis populations in Rogów, Central Poland. Acta Ornithol. 1997, 32, 149–155. [Google Scholar]
- Selås, V. Breeding density and brood size of common buzzard Buteo buteo in relation to snow cover in spring. Ardea 2001, 89, 471–479. [Google Scholar]
- Sim, I.M.W.; Cross, A.V.; Lamacraft, D.L.; Pain, D.J. Correlates of common buzzard Buteo buteo density and breeding success in the West Midlands. Bird Study 2001, 48, 317–329. [Google Scholar] [CrossRef]
- Panek, M.; Kamieniarz, R. Vole fluctuations, red fox responses, predation on fawns, and roe deer dynamics in a temperate latitude. Mamm. Res. 2017, 62, 341–349. [Google Scholar] [CrossRef]

| Prey Category | Small Fields (n = 1917) | Large Fields (n = 1979) | Total (n = 3896) |
|---|---|---|---|
| Frequency, % | |||
| Moles | 12.9 | 11.5 | 12.2 |
| Shrews | 1.2 | 0.5 | 0.8 |
| Common voles | 46.0 | 59.7 | 53.1 |
| Other voles | 8.0 | 5.0 | 6.4 |
| Mice | 3.4 | 3.8 | 3.6 |
| Other mammals | 3.1 | 2.2 | 2.6 |
| Small birds (passerines) | 16.8 | 13.4 | 15.1 |
| Medium and large birds | 3.2 | 1.8 | 2.5 |
| Reptiles and amphibians | 5.4 | 2.1 | 3.7 |
| H’ | 1.67 | 1.36 | 1.52 |
| Prey Category | Field Type (df = 1, 17) | Vole Index (df = 1, 17) | Interaction (df = 1, 16) |
|---|---|---|---|
| Moles | F = 4.97, P = 0.040 | F = 57.10, P < 0.001 | F = 0.92, P > 0.05 |
| Shrews | F = 4.51, P = 0.049 | F = 0.69, P > 0.05 | F = 0.28, P > 0.05 |
| Common voles | F = 0.30, P > 0.05 | F = 49.78, P < 0.001 | F = 0.02, P > 0.05 |
| Other voles | F = 0.73, P > 0.05 | F = 6.66, P = 0.019 | F = 1.44, P > 0.05 |
| Mice | F = 1.08, P > 0.05 | F = 0.37, P > 0.05 | F = 0.002, P > 0.05 |
| Other mammals | F = 0.39, P > 0.05 | F = 0.99, P > 0.05 | F = 0.38, P > 0.05 |
| Small birds (passerines) | F = 0.72, P > 0.05 | F = 2.43, P > 0.05 | F = 0.08, P > 0.05 |
| Medium and large birds | F = 1.89, P > 0.05 | F = 4.21, P > 0.05 | F = 0.02, P > 0.05 |
| Reptiles and amphibians | F = 2.84, P > 0.05 | F = 7.38, P = 0.015 | F = 0.001, P > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panek, M. Does Habitat Diversity Modify the Dietary and Reproductive Response to Prey Fluctuations in a Generalist Raptor Predator, the Eurasian Buzzard Buteo buteo? Birds 2021, 2, 114-126. https://doi.org/10.3390/birds2010008
Panek M. Does Habitat Diversity Modify the Dietary and Reproductive Response to Prey Fluctuations in a Generalist Raptor Predator, the Eurasian Buzzard Buteo buteo? Birds. 2021; 2(1):114-126. https://doi.org/10.3390/birds2010008
Chicago/Turabian StylePanek, Marek. 2021. "Does Habitat Diversity Modify the Dietary and Reproductive Response to Prey Fluctuations in a Generalist Raptor Predator, the Eurasian Buzzard Buteo buteo?" Birds 2, no. 1: 114-126. https://doi.org/10.3390/birds2010008
APA StylePanek, M. (2021). Does Habitat Diversity Modify the Dietary and Reproductive Response to Prey Fluctuations in a Generalist Raptor Predator, the Eurasian Buzzard Buteo buteo? Birds, 2(1), 114-126. https://doi.org/10.3390/birds2010008






