Long-Term Trends of Hazel Grouse (Tetrastes bonasia) in the Bohemian Forest (Šumava), Czech Republic, 1972–2019
Simple Summary
Abstract
1. Introduction
2. Methods
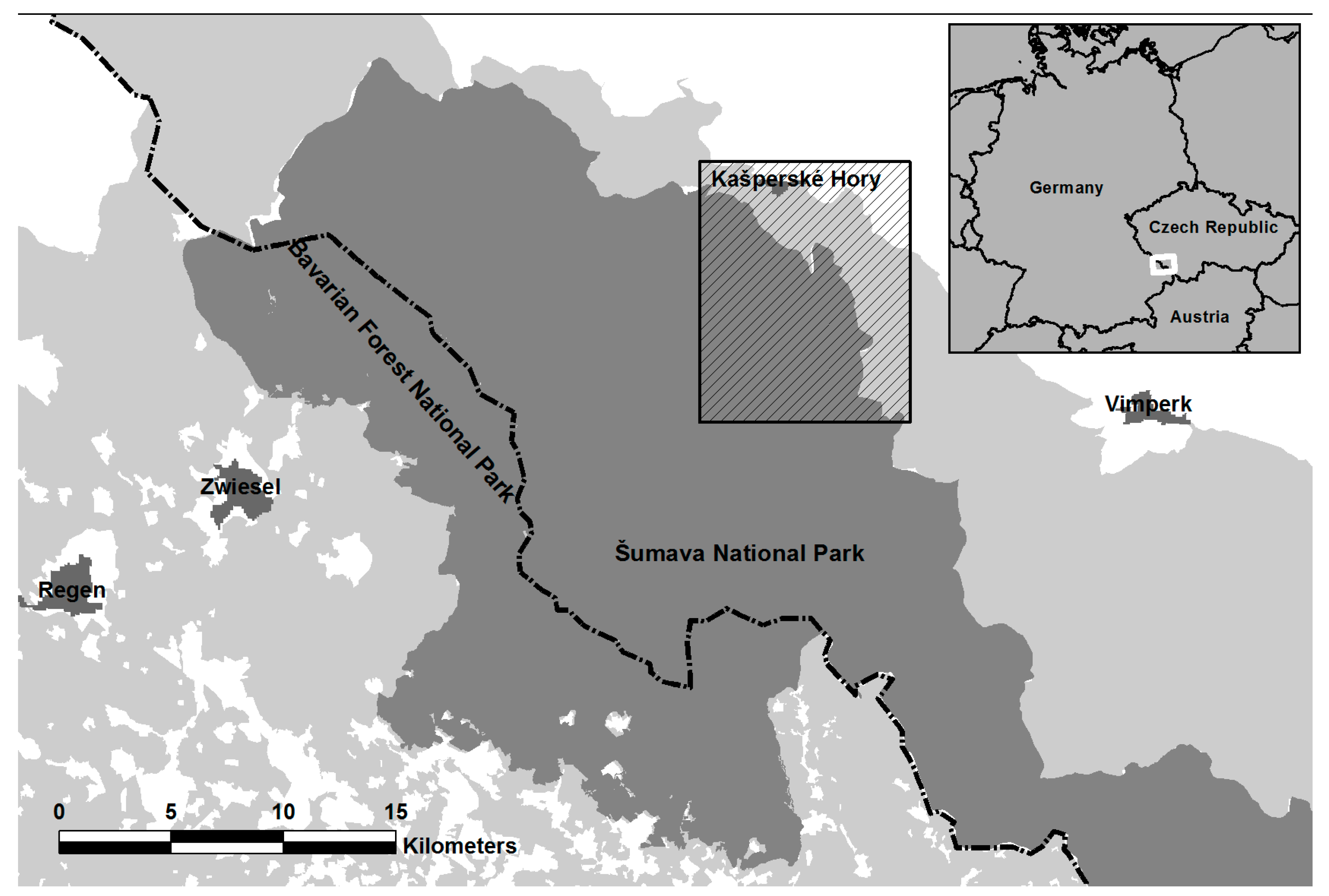
2.1. Study Area
2.2. Detection of Hazel Grouse Occupancy
2.3. Statistical Methods
3. Results
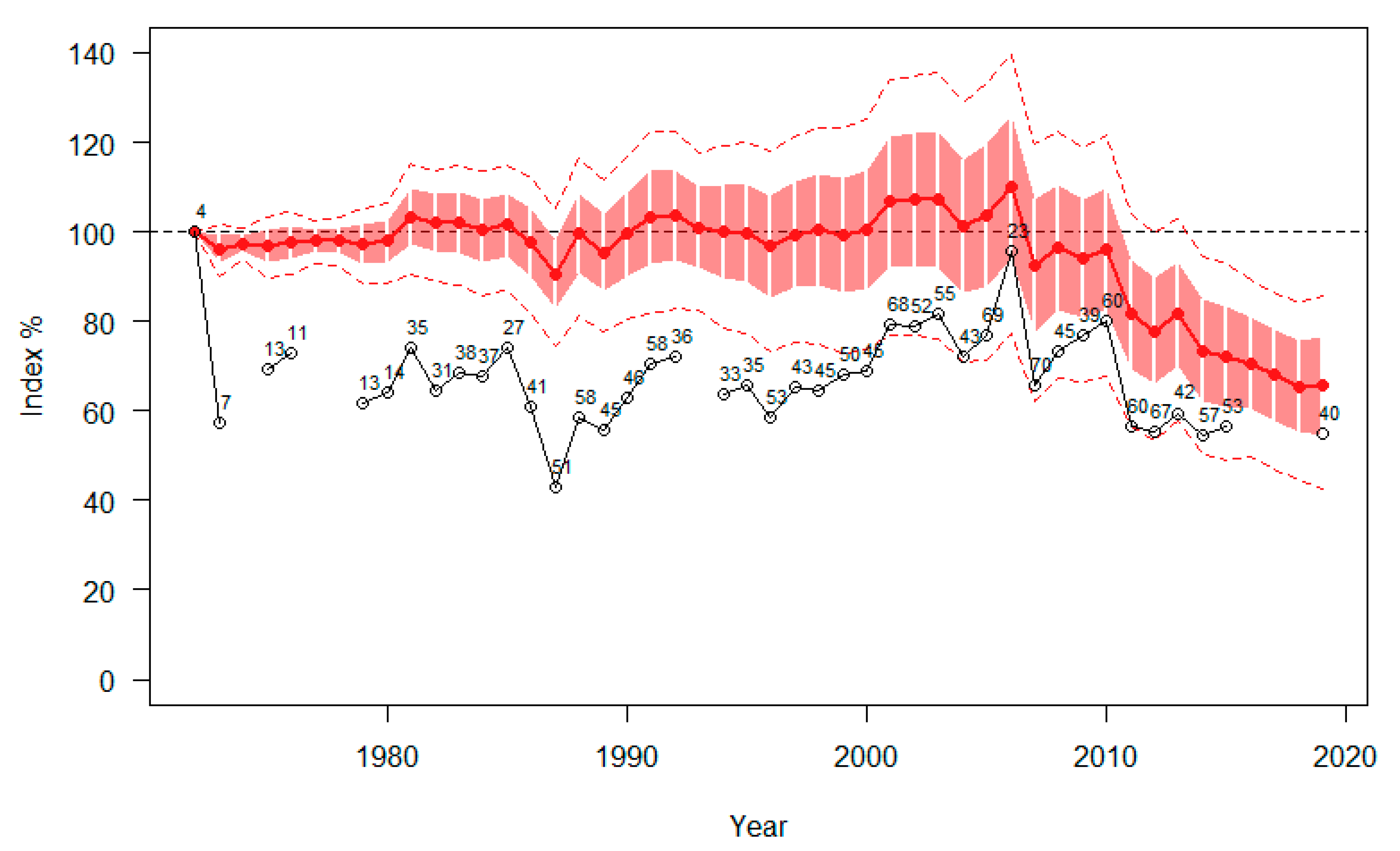
3.1. Population Trend
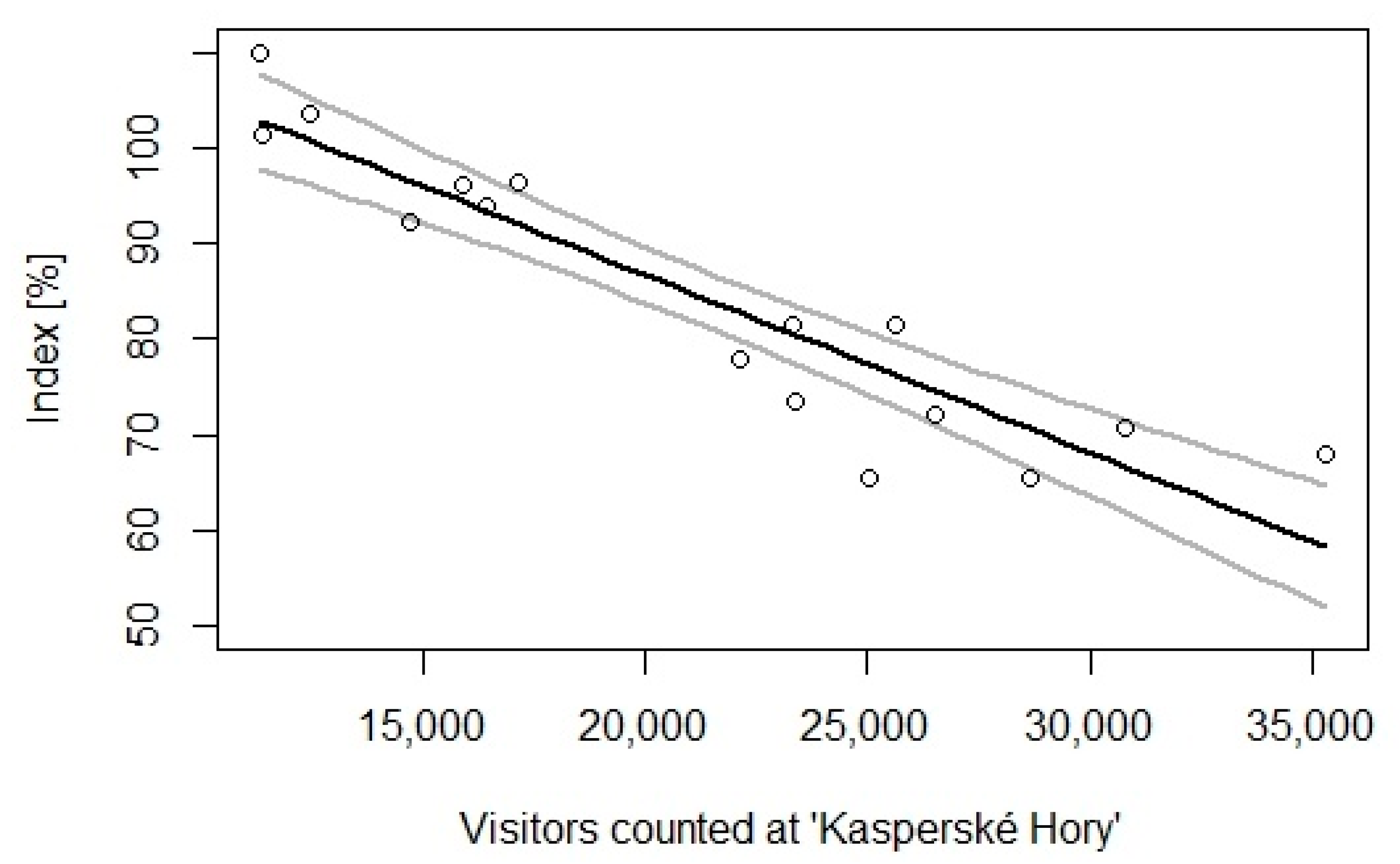
3.2. Negative Impact of Forestry and Disturbance by Tourism on Hazel Grouse
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scherzinger, W. Rauhfuß-Hühner. In Nationalpark Bayerischer Wald; Bayer. Staatsministerium: München, Germany, 1976; Volume 2, p. 71. [Google Scholar]
- Bergmann, H.H.; Klaus, S.; Müller, F.; Scherzinger, W.; Swenson, J.E.; Wiesner, J. Die Haselhühner; Westarp Wissenschaften: Magdeburg, Germany, 1996; ISBN 3-89432-499-6. [Google Scholar]
- Pynnönen, A. Beitrage zur Kenntnis der Lebensweise des Haselhuhns, Tetrastes bonasia (L.). Riistatiet. Julk. Pap. Game Res. 1954, 12, 1–90. [Google Scholar]
- Montadert, M.; Leonard, P. Survival in an expanding hazel grouse Bonasa bonasia population in the southeastern French Alps. Wildl. Biol. 2003, 9, 357–364. [Google Scholar] [CrossRef]
- Swenson, J.E. The ecology of Hazel Grouse and management of its habitat. Naturschutzreport 1995, 10, 227–238. [Google Scholar]
- Klaus, S.; Bergmann, H.-H. Auerhühner & Co.—Heimliche Vögel in Wilder Natur; Aula Verlag: Wiebelsheim, German, 2020; ISBN 978-3-89104-835-1. [Google Scholar]
- BirdLife International Bonasa bonasia. IUCN Red List Threat. Species. Available online: https://www.iucn.org/resources/conservation-tools/iucn-red-list-threatened-species (accessed on 16 March 2021).
- Storch, I. Grouse: Status Survey and Conservation Action Plan 2006–2010; World Pheasant Association & IUCN and Fordingbridge, UK: Gland, Switzerland, 2007; ISBN 978-2-8317-1009-9. [Google Scholar]
- Šťastný, K.; Bejček, V.; Němec, M. Červený seznam ptáků České republiky. In Červený Seznam Ohrožených Druhů České Republiky. (Red list of Birds, in Czech.); Chobot, N.M., Ed.; Příroda Praha: Prague, Czech Republic, 2017; Volume 34, pp. 107–154. [Google Scholar]
- Grüneberg, C.; Bauer, H.-G.; Haupt, H.; Hüppop, O.; Ryslavy, T.; Südbeck, P. Rote Liste der Brutvögel Deutschlands. Ber. Vogelschutz 2015, 52, 19–67. [Google Scholar]
- Zbinden, N.; Klaus, S.; Keller, V. Bonasa bonasia-Hazel Grouse. In European Breeding Bird Atlas 2. Distribution, Abundance and Change; Keller, V., Herrando, S., Voříšek, P., Franch, M., Kipson, M., Milanesi, P., Martí, D., Anton, M., Klvaňová, A., Kalyakin, M.V., et al., Eds.; European Bird Census Council & Lynx Edicions: Barcelona, Spain, 2020; pp. 86–87. ISBN 978-84-16728-38-1. [Google Scholar]
- Klaus, S. Effects of forestry on grouse populations: Case studies from the Thuringian and Bohemian Forests, Central Europe. Ornis Scand. 1991, 22, 218–223. [Google Scholar] [CrossRef]
- Klaus, S. Hazel grouse in the Bohemian Forest: Results of a 24-year-long study. Silva Gabreta 1996, 1, 209–220. [Google Scholar]
- Klaus, S.; Martens, J.; Andreev, A.; Sun, Y.H. Bonasa bonasia (Linnaeus, 1758) Haselhuhn. In Atlas der Verbreitung Palaearktischer Vögel, 20. Jg., Nr. 6; Martens, J., Eck, S., Sun, Y.-H., Eds.; Erwin-Stresemann-Gesellschaft für Paläarktische Faunistik e.V.: Berlin, Germany, 2003; p. 15. [Google Scholar]
- Kučera, L. Verbreitung und Populationsdichte von Auerhuhn (Tetrao urogallus), Birkhuhn (Lyrurus tetrix) und Haselhuhn (Tetrastes bonasia) im westlichen Teil von Šumava (CSSR). Ornithol. Mitt. 1975, 27, 160–169. [Google Scholar]
- Klaus, S. A 33-year Study of Hazel Grouse Bonasa bonasia in the Bohemian Forest, Šumava, Czech Republic: Effects of Weather on Density in Autumn. Wildl. Biol. 2007, 13, 105–108. [Google Scholar] [CrossRef]
- Klaus, S.; Ludwig, T. Ökologie, Verhalten und Schutz des Haselhuhns Bonasa bonasia im Böhmerwald (Šumava, Tschechien). In Proceedings of the Symposium Raufußhühner des Landesjagdverbandes Bayern—Bayerischer Jagdverband e.V. und der Bayerischen Akademie für Jagd und Natur; Schriftenreihe des Landesjagdverbandes Bayern e.V., Band 22: Freyung, Germany, 2016; pp. 45–54. [Google Scholar]
- Ter Braak, C.J.F.; van Strien, A.J.; Meijer, R.; Verstrael, T.J. Analysis of monitoring data with many missing values: Which method? In Proceedings 12th International Conference of IBCC and EOAC; Statistics Netherlands: Noordwijkerhout, The Netherlands, 1994; pp. 663–673. [Google Scholar]
- Klaus, S. Forest grouse and wilderness—Survival without management impacts. In Proceedings of the Europe’s wild Heart; Conference Report; Bavarian Forest National Park & Šumava National Park: Vimperk, Czech Republic; Grafenau, Germany, 2009; pp. 35–37. [Google Scholar]
- Klaus, S. Situation of the hazel grouse Tetrastes bonasia in the National Park Šumava and in the Šumava Landscape Reserve—Activities of the Galliforme Specialist Group of IUCN. Grouse News Newsl. Grouse Spec. Gr. 2014, 48, 7–8. [Google Scholar]
- Ludwig, T.; Klaus, S. Habitat selection in the post-breeding period by Hazel Grouse Tetrastes bonasia in the Bohemian Forest. J. Ornithol. 2017, 158, 101–112. [Google Scholar] [CrossRef]
- Klaus, S. Haselhühnerforschung im Böhmerwald. Nationalpark 1998, 3, 51–55. [Google Scholar]
- “Biosphärenreservat Šumava”. Available online: https://de.wikipedia.org/w/index.php?title=Biosphärenreservat_Šumava&oldid=207209923 (accessed on 8 February 2021).
- Klaus, S.; Berger, D.; Huhn, J. Capercaillie Tetrao urogallus decline and emissions from the iron industry. Wildl. Biol. 1997, 3, 131–136. [Google Scholar] [CrossRef]
- Cukor, J.; Linda, R.; Andersen, O.; Eriksen, L.F.; Vacek, Z.; Riegert, J.; Šálek, M. Evaluation of spatio-temporal patterns of predation risk to forest grouse nests in the central European mountain regions. Animals 2021, 11, 316. [Google Scholar] [CrossRef]
- Klaus, S. Predation among capercaillie in a reserve in Thuringia. In Proceedings of the 3rd International Grouse Symposium; Lovel, T., Hudson, P., Eds.; World Pheasant Association: New York, NY, USA, 1984; pp. 334–346. [Google Scholar]
- Kämmerle, J.-L.; Storch, I. Predation, predator control and grouse populations: A review. Wildl. Biol. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Wiesner, J.; Bergmann, H.H.; Klaus, S.; Müller, F. Siedlungsdichte und Habitatstruktur des Haselhuhns (Bonasa bonasia) im Waldgebiet von Bialowieza (Polen). J. Ornithol. 1977, 118, 1–20. [Google Scholar] [CrossRef]
- Swenson, J.E. Evaluation of a density index for territorial male Hazel Grouse Bonasa bonasia in spring and autumn. Ornis Fenn. 1991, 68, 57–65. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bogaart, A.P.; Van Der Loo, M.; Pannekoek, J. Rtrim: Trends and Indices for Monitoring Data. R Packag. Version 2016, 1.0.1. Available online: https://cran.r-project.org/package=rtrim (accessed on 16 March 2021).
- Pannekoek, J.; Van Strien, A. TRIM 3 Manual (TRends and Indices for Monitoring Data); CBS Statistics Netherlands: Dutch, The Netherlands, 2005. [Google Scholar]
- Montadert, M.; Leonhard, P. Natal dispersal affects population dynamics of Hazel Grouse in heterogenous landscapes. In Ecology, Conservation, and Management of Grouse; Sandercock, B.K., Martin, K., Segelbacher, G., Eds.; University of California: London, UK, 2011; pp. 89–103. [Google Scholar]
- Montadert, M.; Klaus, S. Hazel grouse in open landscapes. Grouse News 2011, 41, 13–22. [Google Scholar]
- Montadert, M. Fonctionnement Démographique et Sélection de L’habitat D’une Population en Phase D’expansion Géographique. Cas de la Gélinotte des Bois Dans les Alpes du Sud, France. Ph.D. Thesis, Université de Franche-Comté, Besançon, France, 2005. [Google Scholar]
- Sahlsten, J.; Wickström, F.; Höglund, J. Hazel grouse Bonasa bonasia population dynamics in a fragmented landscape: A metapopulation approach. Wildl. Biol. 2010, 16, 35–46. [Google Scholar] [CrossRef]
- Siivonen, L. The Problem of the Short-Term Fluctuations in Numbers of Tetraonids in Europe. Pap. Game Res. 1957, 19, 1–44. [Google Scholar]
- Semenov-Tjan-Shanskij, O.I. Ökologie der Birkhuhnvögel. Tr. Laplandskogo Gosudarvstveno Zapov. 1960, 5, 1–318, (In Russian, Translated into German). [Google Scholar]
- Lindström, J.; Ranta, E.; Lindén, H.; Lindstrom, J.; Linden, H. Large-Scale Synchrony in the Dynamics of Capercaillie, Black Grouse and Hazel Grouse Populations in Finland. Oikos 1996, 76, 221. [Google Scholar] [CrossRef]
- Lindstrom, J.; Ranta, E.; Linden, M.; Linden, H. Reproductive Output, Population Structure and Cyclic Dynamics in Capercaillie, Black Grouse and Hazel Grouse. J. Avian Biol. 1997, 28, 1. [Google Scholar] [CrossRef]
- Helle, P.; Lindén, H. Changes in Finnish grouse populations during the past half-century. Suom. Riista 2015, 56–66. [Google Scholar]
- Helle, P.; Belkin, V.; Bljudnik, L.; Danilov, P.; Jakimov, A. Changes in grouse populations in Finland and Russian Karelia during recent decades. Suom. Riista 2003, 49, 32–43. [Google Scholar]
- Ranta, E.; Helle, P.; Lindén, H. Forty years of grouse monitoring in Finland. Suom. Riista 2004, 50, 128–136. [Google Scholar]
- Baines, D.; Moss, R.; Dugan, D. Capercaillie breeding success in relation to forest habitat and predator abundance. J. Appl. Ecol. 2004, 41, 59–71. [Google Scholar] [CrossRef]
- Sim, I.M.W.; Eaton, M.A.; Setchfield, R.P.; Warren, P.K.; Lindley, P. Abundance of male Black Grouse Tetrao tetrix in Britain in 2005, and change since 1995–1996. Bird Study 2008, 55, 304–313. [Google Scholar] [CrossRef]
- Huhta, E.; Helle, P.; Nivala, V.; Nikula, A. The effect of human-modified landscape structure on forest grouse broods in two landscape types. Ecosphere 2017, 8, e01950. [Google Scholar] [CrossRef]
- Müller, D.; Schröder, B.; Müller, J. Modelling habitat selection of the cryptic Hazel Grouse Bonasa bonasia in a montane forest. J. Ornithol. 2009, 150, 717–732. [Google Scholar] [CrossRef]
- Kajtoch, Ł.; Żmihorski, M.; Bonczar, Z. Hazel Grouse occurrence in fragmented forests: Habitat quantity and configuration is more important than quality. Eur. J. For. Res. 2012, 131, 1783–1795. [Google Scholar] [CrossRef]
- Schäublin, S.; Bollmann, K. Winter habitat selection and conservation of Hazel Grouse (Bonasa bonasia) in mountain forests. J. Ornithol. 2011, 152, 179–192. [Google Scholar] [CrossRef]
- Swenson, J.E. Is the hazel grouse a poor disperser? In Proceedings of the 20th Congress of the International Union of Game Biologists, Gödöllö, Hungary, 21–26 August 1991; pp. 347–352. [Google Scholar]
- Åberg, J.; Swenson, J.E.; Angelstam, P. The habitat requirements of hazel grouse (Bonasa bonasia) in managed boreal forest and applicability of forest stand descriptions as a tool to identify suitable patches. For. Ecol. Manag. 2003, 175, 437–444. [Google Scholar] [CrossRef]
- Klaus, S.; Sewitz, A. Ecology and conservation of Hazel grouse Bonasa bonasia in the Bohemian Forest (Šumava, Czech Republic). In Proceedings of the International Conference Tetraonids at the Break of the Millenium (Tetřevovití—Tetraonidae na Přelomu Tisíciletí), Ceske Budejovice, Czech Republic, 24–26 March 2000; pp. 138–146. [Google Scholar]
- Rolstad, J.; Wegge, P. Effects of logging on capercaillie (Tetrao urogallus) leks. Scand. J. For. Res. 1989, 4, 129–135. [Google Scholar] [CrossRef]
- Haakana, H.; Huhta, E.; Hirvelä, H.; Packalen, T. Trade-offs between wood production and forest grouse habitats in two regions with distinctive landscapes. For. Ecosyst. 2020, 7, 21. [Google Scholar] [CrossRef]
- Lindén, H.; Wikman, M. Goshawk predation on hazel grouse. Suom. Riista 1987, 34, 96–106. [Google Scholar]
- Tornberg, R. Pattern of goshawk Accipiter gentilis predation on four forest grouse species in northern Finland. Wildl. Biol. 2001, 7, 245–256. [Google Scholar] [CrossRef]
- Tornberg, R.; Lindén, A.; Byholm, P.; Ranta, E.; Valkama, J.; Helle, P.; Lindén, H. Coupling in goshawk and grouse population dynamics in Finland. Oecologia 2013, 171, 863–872. [Google Scholar] [CrossRef]
- Marcstrom, V.; Kenward, R.; Engren, E. The impact of predation on boreal tetraonids during vole cycles: An experimental study. J. Anim. Ecol. 1988, 57, 859–872. [Google Scholar] [CrossRef]
- Lindström, E.R.; Andrén, H.; Angelstam, P.; Cederlund, G.; Hörnfeldt, B.; Jäderberg, L.; Lemnell, P.-A.; Martinsson, B.; Sköld, K.; Swenson, J.E. Disease Reveals the Predator: Sarcoptic Mange, Red Fox Predation, and Prey Populations. Ecology 1994, 75, 1042–1049. [Google Scholar] [CrossRef]
- Šťastný, K.; Bejček, V.; Hudec, K. Atlas Hnizdniho Rozšiřeni Ptaku v Česke Republice 1985–1989 (Atlas of Breeding Birds, in Czech); Jinočany H & H: Jihlava, Czech Republic, 1996. [Google Scholar]
- Kauhala, K.; Helle, P. The impact of predator abundance on grouse populations in Finland—A study based on wildlife monitoring counts. Ornis Fenn. 2002, 79, 14–25. [Google Scholar]
- Ukkola, M.; Helle, P.; Huhta, E.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.L. The impacts of ski resorts on wildlife in northern Finland. In Arctic Centre Reports: Environment, Local Society and Sustainable Tourism; Jokimäki, J., Kaisanlahti-Jokimäki, M.L., Tuulentie, S., Laine, K., Uusitalo, M., Eds.; Painatuskeskus: Rovaniemi, Finland, 2007; Volume 50, pp. 31–41. [Google Scholar]
- Storch, I. Human disturbance of grouse—Why and when? Wildl. Biol. 2013, 19, 390–403. [Google Scholar] [CrossRef]
- Le Corre, N.; Gélinaud, G.; Brigand, L. Bird disturbance on conservation sites in Brittany (France): The standpoint of geographers. J. Coast. Conserv. 2009, 13, 109–118. [Google Scholar] [CrossRef]
- Moss, R.; Leckie, F.; Biggins, A.; Poole, T.; Baines, D.; Kortland, K. Impacts of Human Disturbance on Capercaillie Tetrao urogallus Distribution and Demography in Scottish Woodland. Wildl. Biol. 2014, 20, 1–18. [Google Scholar] [CrossRef]
- Kortmann, M.; Heurich, M.; Latifi, H.; Rösner, S.; Seidl, R.; Müller, J.; Thorn, S. Forest structure following natural disturbances and early succession provides habitat for two avian flagship species, capercaillie (Tetrao urogallus) and hazel grouse (Tetrastes bonasia). Biol. Conserv. 2018, 226, 81–91. [Google Scholar] [CrossRef]
| From | Upto | Add | Se Add | Mul | Se Mul | p | Meaning |
|---|---|---|---|---|---|---|---|
| 1972 | 2019 | −0.0061 | 0.0032 | 0.9940 | 0.0032 | 0.0622 | Stable |
| 1972 | 2006 | −0.0008 | 0.0049 | 0.9992 | 0.0049 | 0.8782 | Stable |
| 2006 | 2019 | −0.0376 | 0.0126 | 0.9631 | 0.0121 | 0.0113 | Decrease (p < 0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaus, S.; Ludwig, T. Long-Term Trends of Hazel Grouse (Tetrastes bonasia) in the Bohemian Forest (Šumava), Czech Republic, 1972–2019. Birds 2021, 2, 127-137. https://doi.org/10.3390/birds2010009
Klaus S, Ludwig T. Long-Term Trends of Hazel Grouse (Tetrastes bonasia) in the Bohemian Forest (Šumava), Czech Republic, 1972–2019. Birds. 2021; 2(1):127-137. https://doi.org/10.3390/birds2010009
Chicago/Turabian StyleKlaus, Siegfried, and Tobias Ludwig. 2021. "Long-Term Trends of Hazel Grouse (Tetrastes bonasia) in the Bohemian Forest (Šumava), Czech Republic, 1972–2019" Birds 2, no. 1: 127-137. https://doi.org/10.3390/birds2010009
APA StyleKlaus, S., & Ludwig, T. (2021). Long-Term Trends of Hazel Grouse (Tetrastes bonasia) in the Bohemian Forest (Šumava), Czech Republic, 1972–2019. Birds, 2(1), 127-137. https://doi.org/10.3390/birds2010009






