Bioacoustics Reveal Species-Rich Avian Communities Exposed to Organophosphate Insecticides in Macadamia Orchards
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Section
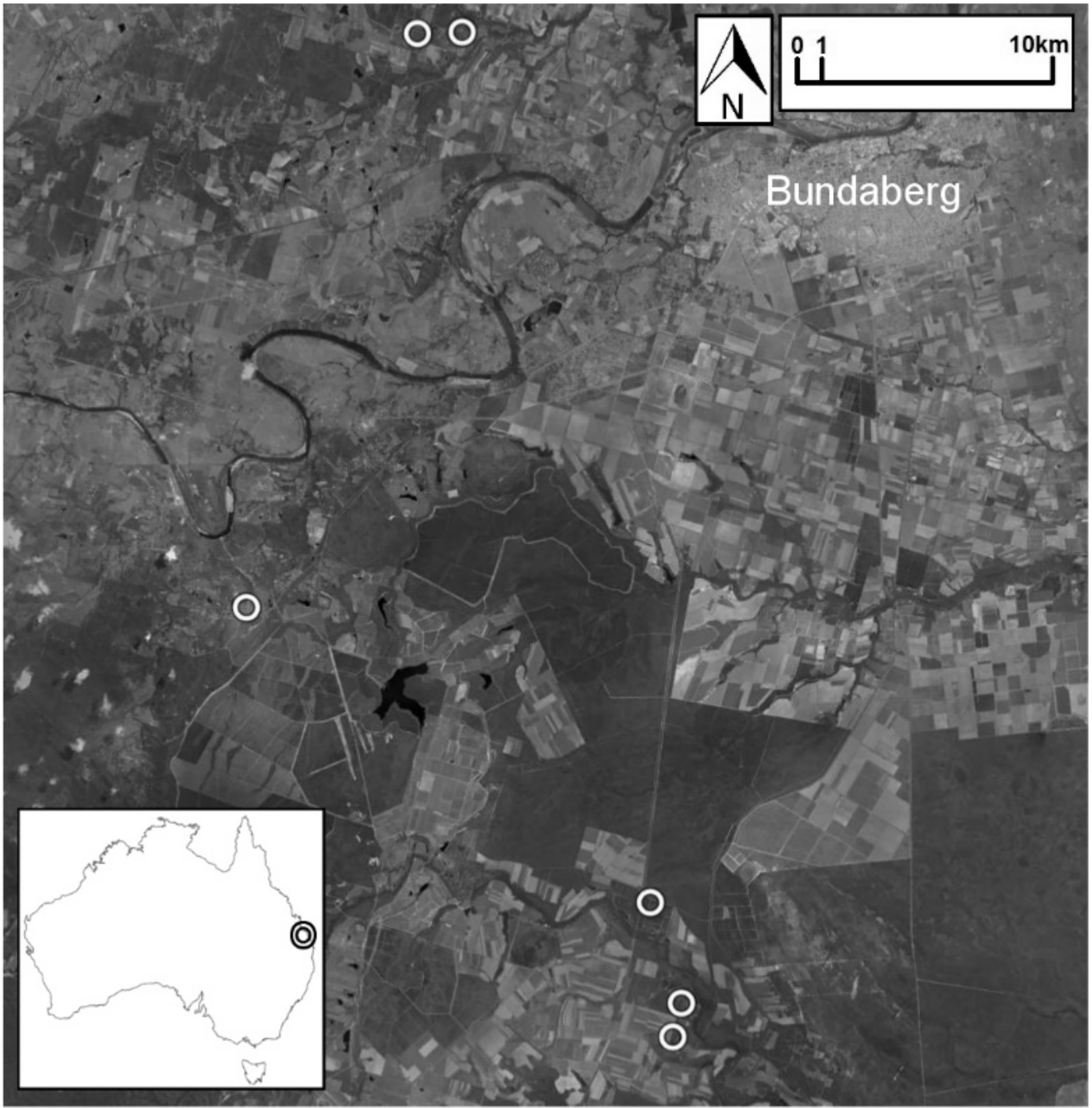
2.1. Study Area
2.2. Experimental Design
2.3. Acoustic Surveys
2.4. Arthropod Surveys
2.5. Statistical Analyses
3. Results
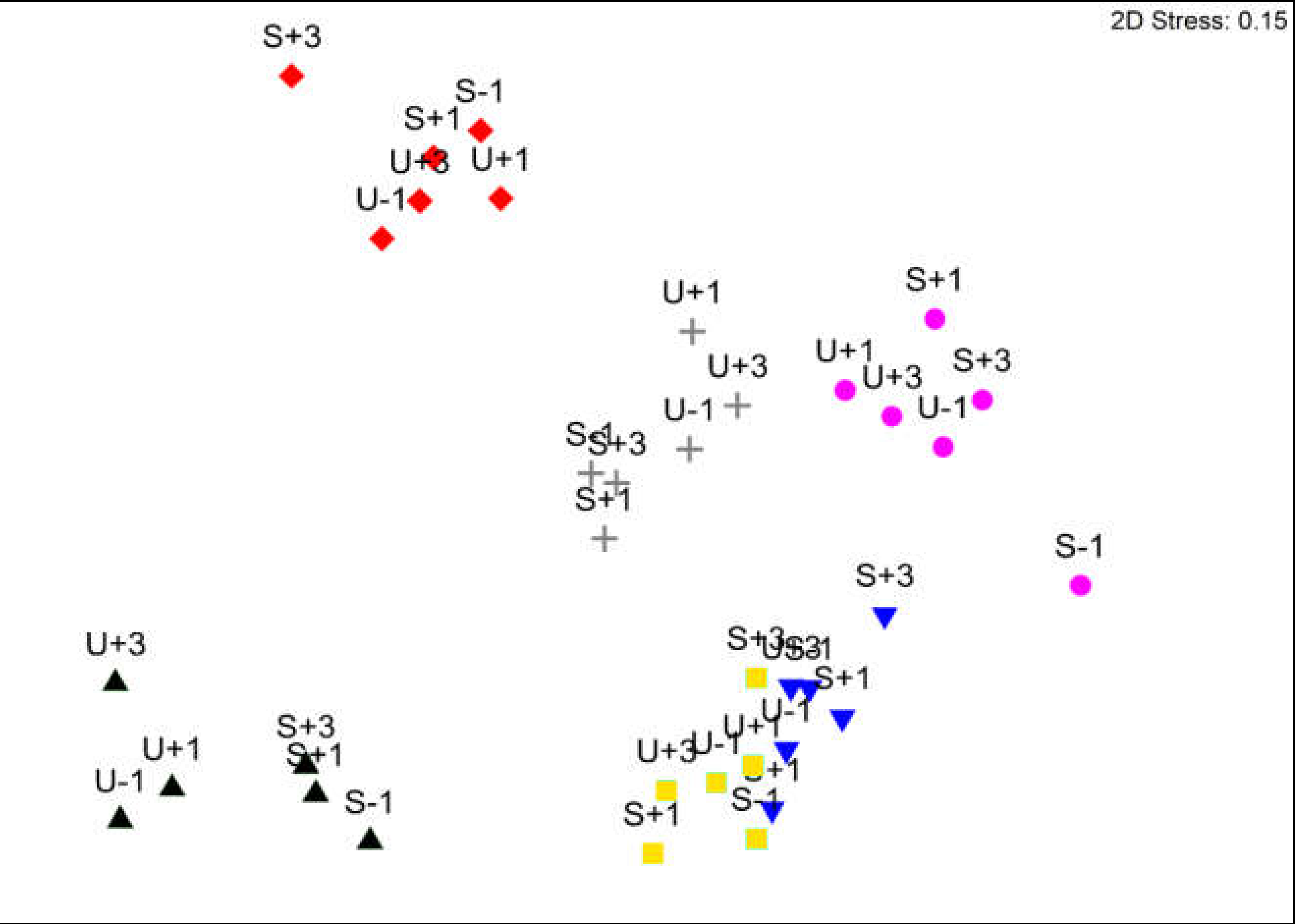
3.1. Avian Acoustic Activity
3.2. Arthropods
4. Discussion
4.1. The Importance of Macadamia Landscapes as Habitat for Birds and Arthropods
4.2. Bird Exposure to Organophosphates
4.3. Impacts of Organophosphates on Arthropods
4.4. Relation between Arthropods and Bird Acoustic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Bondarenko, S.; Gan, J.; Haver, D.L.; Kabashima, J.N. Persistence of selected organophosphate and carbamate insecticides in waters from a coastal watershed. Environ. Toxicol. Chem. 2004, 23, 2649–2654. [Google Scholar] [CrossRef]
- Zamy, C.; Mazellier, P.; Legube, B. Phototransformation of selected organophosphorus pesticides in dilute aqueous solutions. Water Res. 2004, 38, 2305–2314. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Trichlorfon Environmental Health Criteria. 1992. Available online: http://www.inchem.org/documents/ehc/ehc/ehc132.htm (accessed on 12 October 2020).
- Frank, R.; Mineau, P.; Braun, H.; Barker, I.; Kennedy, S.; Trudeau, S. Deaths of Canada geese following spraying of turf with diazinon. Bull. Environ. Contam. Toxicol. 1991, 46, 852–858. [Google Scholar] [CrossRef]
- Fleischli, M.A.; Franson, J.; Thomas, N.; Finley, D.; Riley, W., Jr. Avian mortality events in the United States caused by anticholinesterase pesticides: A retrospective summary of National Wildlife Health Center records from 1980 to 2000. Arch. Environ. Contam. Toxicol. 2004, 46, 542–550. [Google Scholar] [CrossRef]
- Millot, F.; Berny, P.; Decors, A.; Bro, E. Little field evidence of direct acute and short-term effects of current pesticides on the grey partridge. Ecotoxicol. Environ. Safe 2015, 117, 41–61. [Google Scholar] [CrossRef]
- Peakall, D.; Burger, J. Methodologies for assessing exposure to metals: Speciation, bioavailability of metals, and ecological host factors. Ecotoxicol. Environ. Safe 2003, 56, 110–121. [Google Scholar] [CrossRef]
- Golden, N.H.; Rattner, B.A. Ranking terrestrial vertebrate species for utility in biomonitoring and vulnerability to environmental contaminants. Rev. Environ. Contam. Toxicol. 2003, 176, 67–136. [Google Scholar] [PubMed]
- Goldstein, M.; Lacher, T.E.; Woodbridge, B.; Bechard, M.J.; Canavelli, S.B.; Zaccagnini, M.E.; Cobb, G.P.; Scollon, E.J.; Tribolet, R.; Hopper, M.J. Monocrotophos-Induced mass mortality of Swainson’s Hawks in Argentina, 1995–1996. Ecotoxicology 1999, 8, 201–214. [Google Scholar] [CrossRef]
- Goldstein, M.I.; Woodbridge, B.; Zaccagnini, M.E.; Canavelli, S.B. An assessment of mortality of Swainson’s Hawks on wintering grounds in Argentina. J. Raptor Res. 1996, 30, 106–107. [Google Scholar]
- Grue, C.E.; Shipley, B.K. Sensitivity of nestling and adult starlings to dicrotophos, an organophosphate pesticide. Environ. Res. 1984, 35, 454–465. [Google Scholar] [CrossRef]
- Busby, D.; White, L.; Pearce, P. Effects of aerial spraying of fenitrothion on breeding white-throated sparrows. J. Appl. Ecol. 1990, 27, 743–755. [Google Scholar] [CrossRef]
- Bennett, R.S.; Etterson, M.A. Estimating pesticide effects on fecundity rates of wild birds using current laboratory reproduction tests. Hum. Ecol. Risk Assess. 2006, 12, 762–781. [Google Scholar] [CrossRef]
- Rands, M. Pesticide use on cereals and the survival of grey partridge chicks: A field experiment. J. Appl. Ecol. 1985, 22, 49–54. [Google Scholar] [CrossRef]
- Morris, A.J.; Wilson, J.D.; Whittingham, M.J.; Bradbury, R.B. Indirect effects of pesticides on breeding yellowhammer (Emberiza citrinella). Agric. Ecosyst. Environ. 2005, 106, 1–16. [Google Scholar] [CrossRef]
- Hart, J.; Milsom, T.; Fisher, G.; Wilkins, V.; Moreby, S.; Murray, A.; Robertson, P. The relationship between yellowhammer breeding performance, arthropod abundance and insecticide applications on arable farmland. J. Appl. Ecol. 2006, 43, 81–91. [Google Scholar] [CrossRef]
- Campbell, L.; Cooke, A. The Indirect Effects of Pesticides on Birds, 1st ed.; Joint Nature Conservation Committee: London, UK, 1997. [Google Scholar]
- Boatman, N.D.; Brickle, N.W.; Hart, J.D.; Milsom, T.P.; Morris, A.J.; Murray, A.W.; Murray, K.A.; Robertson, P.A. Evidence for the indirect effects of pesticides on farmland birds. IBIS 2004, 146, 131–143. [Google Scholar] [CrossRef]
- Newton, I. Bird conservation problems resulting from agricultural intensification in Europe. In Avian Conservation, Research and Management; Marzluff, J., Sallabanks, R., Eds.; Island Press: Washington, DC, USA, 1998; pp. 307–322. [Google Scholar]
- Moulding, J.D. Effects of a low-persistence insecticide on forest bird populations. AUK 1976, 93, 692–708. [Google Scholar]
- Hunter, M.L., Jr.; Witham, J.W. Effects of a carbaryl-induced depression of arthropod abundance on the behavior of Parulinae warblers. Can. J. Zool. 1985, 63, 2612–2616. [Google Scholar] [CrossRef]
- Gard, N.W.; Hooper, M.J.; Bennett, R. An assessment of potential hazards of pesticides and environmental contaminants. In Ecology and Management of Neotropical Migratory Birds: A Synthesis and Review of Critical Issues; Martin, T.E., Finch, D.M., Eds.; Oxford University Press: New York, NY, USA, 1995; pp. 294–310. [Google Scholar]
- Dawson, D.K.; Efford, M.G. Bird population density estimated from acoustic signals. J. Appl. Ecol. 2009, 46, 1201–1209. [Google Scholar] [CrossRef]
- Haselmayer, J.; Quinn, J.S. A comparison of point counts and sound recording as bird survey methods in Amazonian southeast Peru. Condor 2000, 102, 887–893. [Google Scholar] [CrossRef]
- Celis-Murillo, A.; Deppe, J.L.; Allen, M.F. Using soundscape recordings to estimate bird species abundance, richness, and composition. J. F. Ornithol. 2009, 80, 64–78. [Google Scholar] [CrossRef]
- Borker, A.L.; McKown, M.W.; Ackerman, J.T.; Eagles-Smith, C.A.; Tershy, B.R.; Croll, D.A. Vocal activity as a low cost and scalable index of seabird colony size. Conserv. Biol. 2014, 28, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Australian Nut Industry Council. 2020. Available online: http://www.nutindustry.org.au/about-macadamia-nuts.html (accessed on 12 February 2020).
- Crisol-Martínez, E.; Moreno-Moyano, L.T.; Wormington, K.R.; Brown, P.H.; Stanley, D. Using next-generation sequencing to contrast the diet and explore pest-reduction services of sympatric bird species in macadamia orchards in Australia. PLoS ONE 2016, 11, e0150159. [Google Scholar] [CrossRef] [PubMed]
- Crisol-Martínez, E.; Ford, G.; Horgan, F.G.; Brown, P.H.; Wormington, K.R. Ecology and conservation of insectivorous bats in fragmented areas of macadamia production in eastern Australia. Austral Ecol. 2017, 42, 597. [Google Scholar] [CrossRef]
- Danne, A.W.; Llewellyn, R.; Huwer, R.K.; Furlong, M.J. Fruitspotting bugs, Amblypelta nitida Stål and A. lutescens lutescens Distant (Hemiptera: Coreidae): A review of the potential for integrated management practices. Austral Entomol. 2014, 53, 112–123. [Google Scholar] [CrossRef]
- Cuperus, G.; Radcliffe, E. Effect of trichlorfon sprays and alfalfa (Medicago sativa L.) cultivars on pea aphid, Acyrthosiphon pisum (Harris). Crop Prot. 1984, 3, 199–208. [Google Scholar] [CrossRef]
- Hill, E.F.; Heath, R.G.; Spawn, J.W.; Williams, J.D. Lethal Dietary Toxicities of Environmental Pollutants to Birds, 1st ed.; US Fish and Wildlife Service: Washington, DC, USA, 1975. [Google Scholar]
- De Oliveira, G.H.; Moreira, V.; Goes, S.P.R. Organophosphate induced delayed neuropathy in genetically dissimilar chickens: Studies with tri-ortho-cresyl phosphate (TOCP) and trichlorfon. Toxicol. Lett. 2002, 136, 143–150. [Google Scholar] [CrossRef]
- Zinkl, J.G.; Henny, C.J.; DeWeese, L.R. Brain cholinesterase activities of birds from forests sprayed with trichlorfon (Dylox) and carbaryl (Sevin-4-oil). Bull. Environ. Contam. Toxicol. 1977, 17, 379–386. [Google Scholar] [CrossRef]
- Crisol-Martínez, E.; Moreno-Moyano, L.T.; Wilkinson, N.; Prasai, T.; Brown, P.H.; Moore, R.J.; Stanley, D. A low dose of an organophosphate insecticide causes dysbiosis and sex-dependent responses in the intestinal microbiota of the Japanese quail (Coturnix japonica). PeerJ 2016, 4, e2002. [Google Scholar] [CrossRef] [Green Version]
- BO. Bureau of Meteorology, Australian Government. 2020. Available online: http://www.bom.gov.au/ (accessed on 10 September 2020).
- Wimmer, J.; Towsey, M.; Roe, P.; Williamson, I. Sampling environmental acoustic recordings to determine bird species richness. Ecol. Appl. 2013, 23, 1419–1428. [Google Scholar] [CrossRef] [Green Version]
- Higgins, P.; Peter, J.; Steele, W. Handbook of Australian, New Zealand and Antarctic Birds Vol. 5: Tyrant-Flycatchers to Chats, 1st ed.; Oxford University Press: Melbourne, VIC, Australia, 2001. [Google Scholar]
- Higgins, P.; Peter, J. Handbook of Australian, New Zealand and Antarctic Birds Vol. 6: Pardalotes to Shrike-Thrushes, 1st ed.; Oxford University Press: Melbourne, VIC, Australia, 2002. [Google Scholar]
- Higgins, P.; Peter, J.; Cowling, S. Handbook of Australian, New Zealand and Antarctic Birds Vol. 7: Boatbills to Starlings, 1st ed.; Oxford University Press: Melbourne, VIC, Australia, 2006. [Google Scholar]
- Zborowski, P.; Storey, R. Field Guide to Insects in Australia, 3rd ed.; New Holland Publishers: Wahroonga, NSW, Australia, 2010. [Google Scholar]
- Biaggini, M.; Consorti, R.; Dapporto, L.; Dellacasa, M.; Paggetti, E.; Corti, C. The taxonomic level order as a possible tool for rapid assessment of arthropod diversity in agricultural landscapes. Agric. Ecosyst. Environ. 2007, 122, 183–191. [Google Scholar] [CrossRef]
- Anderson, M.; Gorley, R.N.; Clarke, R.K. Permanova+ for Primer: Guide to Software and Statistical Methods, 1st ed.; Primer-e: Auckland, New Zealand, 2008. [Google Scholar]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Birdlife. The State of Australia’s Birds 2015. Headline Trends for Terrestrial Birds; Electronic Edition; Birdlife and, the Department of the Environment: Melbourne, VIC, Australia, 2015; Available online: http://birdlife.org.au/education-publications/publications/state-of-australias-birds (accessed on 17 July 2020).
- Lyon, R.; McNabb, E.; Cheers, G. Embedded Remnant Forest Patches as Habitat for Birds in the Green Triangle of South-Western Victoria and South-eastern South Australia, 1st ed.; Victorian Government: Melbourne, VIC, Australia, 2010. [Google Scholar]
- Muñoz, J.C.; Aerts, R.; Thijs, K.W.; Stevenson, P.R.; Muys, B.; Sekercioglu, C.H. Contribution of woody habitat islands to the conservation of birds and their potential ecosystem services in an extensive Colombian rangeland. Agric. Ecosyst. Environ. 2013, 173, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Linden, V.M.; Grass, I.; Joubert, E.; Tscharntke, T.; Weier, S.M.; Taylor, P.J. Ecosystem services and disservices by birds, bats and monkeys change with macadamia landscape heterogeneity. J. Appl. Ecol. 2019, 56, 2069. [Google Scholar] [CrossRef]
- Bouvier, J.C.; Ricci, B.; Agerberg, J.; Lavigne, C. Apple orchard pest control strategies affect bird communities in southeastern France. Environ. Toxicol. Chem. 2011, 30, 212–219. [Google Scholar] [CrossRef] [PubMed]
- George, T.L.; McEwen, L.C.; Petersen, B.E. Effects of grasshopper control programs on rangeland breeding bird populations. J. Range Manag. 1995, 48, 336–342. [Google Scholar] [CrossRef]
- Norelius, E.; Lockwood, J. The effects of reduced agent-area insecticide treatments for rangeland grasshopper (Orthoptera: Acrididae) control on bird densities. Arch. Environ. Contam. Toxicol. 1999, 37, 519–528. [Google Scholar] [CrossRef]
- Wolf, C.; Riffel, M.; Weyman, G.; Douglas, M.; Norman, S. Telemetry-based field studies for assessment of acute and short-term risk to birds from spray applications of chlorpyrifos. Environ. Toxicol. Chem. 2010, 29, 1795–1803. [Google Scholar] [CrossRef]
- Mineau, P. Barking up the wrong perch: Why we should stop ignoring non dietary routes of pesticide exposure in birds. Integr. Environ. Assess. 2011, 7, 297–299. [Google Scholar] [CrossRef]
- Hooper, M.J.; Detrich, P.J.; Weisskopf, C.P.; Wilson, B.W. Organophosphorus insecticide exposure in hawks inhabiting orchards during winter dormant-spraying. Bull. Environ. Contam. Toxicol. 1989, 42, 651–659. [Google Scholar] [CrossRef]
- Wilson, B.W.; Hooper, M.J.; Littrell, E.E.; Detrich, P.J.; Hansen, M.E.; Weisskopf, C.P.; Seiber, J.N. Orchard dormant sprays and exposure of red-tailed hawks to organophosphates. Bull. Environ. Contam. Toxicol. 1991, 47, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Vyas, N.B.; Spann, J.W.; Hulse, C.S.; Gentry, S.; Borges, S.L. Dermal insecticide residues from birds inhabiting an orchard. Environ. Monit. Assess. 2007, 133, 209. [Google Scholar] [CrossRef] [PubMed]
- Driver, C.J.; Drown, D.B.; Ligotke, M.W.; Van Voris, P.; McVeety, B.D.; Greenspan, B.J. Routes of uptake and their relative contribution to the toxicologic response of northern bobwhite (Colinus virginianus) to an organophosphate pesticide. Environ. Toxicol. Chem. 1991, 10, 21–33. [Google Scholar] [CrossRef]
- Mineau, P.; Sundaram, K.M.; Sundaram, A.; Feng, C.; Busby, D.G.; Pearce, P.A. An improved method to study the impact of pesticide sprays on small song birds. J. Environ. Sci. Health Part B 1990, 25, 105–135. [Google Scholar] [CrossRef]
- Mineau, P. Estimating the probability of bird mortality from pesticide sprays on the basis of the field study record. Environ. Toxicol. Chem. 2002, 21, 1497–1506. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Torchio, P.F. The effects of field applications of naled and trichlorfon on the alfalfa leafcutting bee, Megachile rotundata (Fabricius). J. Kans. Entomol. Soc. 1983, 56, 62–68. [Google Scholar]
- Epstein, D.; Zack, R.; Brunner, J.; Gut, L.; Brown, J. Effects of broad-spectrum insecticides on epigeal arthropod biodiversity in Pacific Northwest apple orchards. Environ. Entomol. 2000, 29, 340–348. [Google Scholar] [CrossRef]
- Pekár, S. Effect of IPM practices and conventional spraying on spider population dynamics in an apple orchard. Agric. Ecosyst. Environ. 1999, 73, 155–166. [Google Scholar] [CrossRef]
- Wisniewska, J.; Prokopy, R.J. Pesticide effect on faunal composition, abundance, and body length of spiders (Araneae) in apple orchards. Environ. Entomol. 1997, 26, 763–776. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Tscharntke, T. The role of perennial habitats for Central European farmland spiders. Agric. Ecosyst. Environ. 2005, 105, 235–242. [Google Scholar] [CrossRef]
- Frith, H.; Wolfe, T.; Barker, R. Food of eight species of Columbidae, in the genera Geopelia, Phaps, Geophaps and Petrophassa. Wildl. Res. 1976, 3, 159–171. [Google Scholar] [CrossRef]
- Hart, A.; Thompson, H.; Fletcher, M.; Greig-Smith, P.; Hardy, A.; Langton, S.; Frampton, G.; Hardy, T. Effects of summer aphicides on tree sparrows. In Pesticides, Cereal Farming and the Environment; Greig-Smith, P.W., Frampton, G.K., Hardy, A.R., Eds.; HMSO: London, UK, 1992; pp. 175–193. [Google Scholar]
- Nicolaus, L.K.; Lee, H. Low acute exposure to organophosphate produces long-term changes in bird feeding behavior. Ecol. Appl. 1999, 9, 1039–1049. [Google Scholar] [CrossRef]
- White, D.H.; King, K.A.; Mitchell, C.A.; Hill, E.F.; Lamont, T.G. Parathion causes secondary poisoning in a laughing gull breeding colony. Bull. Environ. Contam. Toxicol. 1979, 23, 281–284. [Google Scholar] [CrossRef] [PubMed]
- DeWeese, L.R.; McEwen, L.C.; Settimi, L.A.; Deblinger, R.D. Effects on birds of fenthion aerial application for mosquito control. J. Econ. Entomol. 1983, 76, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Rainwater, T.R.; Leopold, V.A.; Hooper, M.J.; Kendall, R.J. Avian exposure to organophosphorus and carbamate pesticides on a coastal South Carolina golf course. Environ. Toxicol. Chem. 1995, 14, 2155–2161. [Google Scholar] [CrossRef]
- Simon, S.; Bouvier, J.C.; Debras, J.F.; Sauphanor, B. Biodiversity and pest management in orchard systems. In Sustainable Agriculture Volume 2; Lichtfouse, E., Hamelin, M., Navarrete, M., Debaeke, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 693–709. [Google Scholar]
- Bengtsson, J.; Ahnström, J.; Weibull, A.C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Genghini, M.; Gellini, S.; Gustin, M. Organic and integrated agriculture: The effects on bird communities in orchard farms in northern Italy. Biodivers. Conserv. 2006, 15, 3077–3094. [Google Scholar] [CrossRef]

| Common Name | Scientific Name | Guild 1 | Loc 2 | % 3 Calls 3 | Acoustic Activity Ratios (Average ± SE) 4 | ||
|---|---|---|---|---|---|---|---|
| Pre 5 | Post 1 | Post 3 | |||||
| Silvereye 7 | Zosterops lateralis | Sh-omni | 6 | 38.04 | 0.50 ± 0.12 | 0.44 ± 0.20 | 0.44 ± 0.15 |
| Bar-shouldered Dove 7 | Geopelia humeralis | Gr-grani | 6 | 6.94 | 0.33 ± 0.12 | 0.60 ± 0.20 | 0.35 ± 0.23 |
| Rufous Whistler 7 | Pachycephala rufiventris | Sh-insect | 6 | 6.88 | 0.46 ± 0.23 | 0.45 ± 0.18 | 0.57 ± 0.25 |
| Little Friarbird 7 | Philemon citreogularis | Ca-nectinsect | 6 | 6.68 | 0.41 ± 0.10 | 0.43 ± 0.12 | 0.47 ± 0.25 |
| Noisy Friarbird 7 | Philemon corniculatus | Ca-omni | 6 | 4.60 | 0.46 ± 0.45 | 0.52 ± 0.43 | 0.42 ± 0.37 |
| Torresian Crow 7 | Corvus orru | Gr-omni | 6 | 4.48 | 0.44 ± 0.32 | 0.66 ± 0.22 | 0.53 ± 0.25 |
| Little Shrike-thrush 7 | Colluricincla megarhyncha | Ca-insect | 6 | 2.73 | 0.55 ± 0.12 | 0.43 ± 0.15 | 0.50 ± 0.16 |
| Olive-backed Oriole 7 | Oriolus sagittatus | Ca-nectinsect | 6 | 1.94 | 0.65 ± 0.22 | 0.51 ± 0.17 | 0.46 ± 0.33 |
| Pied Butcherbird 7 | Cracticus nigrogularis | Ge-omni | 6 | 1.82 | 0.54 ± 0.19 | 0.45 ± 0.14 | 0.57 ± 0.14 |
| White-browed Scrubwren | Sericornis frontalis | Sh-insect | 6 | 1.76 | 0.54 ± 0.35 | 0.56 ± 0.38 | 0.58 ± 0.33 |
| Brown Honeyeater 6,7 | Lichmera indistincta | Sh-nectinsect | 6 | 1.30 | 0.20 ± 0.21 a | 0.47 ± 0.24 ab | 0.66 ± 0.27 b |
| Willie Wagtail 7 | Rhipidura leucophrys | Sh-insect | 6 | 1.27 | 0.35 ± 0.39 | 0.69 ± 0.37 | 0.30 ± 0.31 |
| Dusky Honeyeater | Myzomela obscura | Ca-nectinsect | 6 | 1.26 | 0.52 ± 0.45 | 0.22 ± 0.38 | 0.25 ± 0.34 |
| Eastern Yellow Robin 7 | Eopsaltria australis | Gr-insect | 6 | 1.16 | 0.68 ± 0.33 | 0.53 ± 0.29 | 0.67 ± 0.29 |
| Magpie-lark 7 | Grallina cyanoleuca | Gr-insect | 6 | 1.11 | 0.47 ± 0.26 | 0.46 ± 0.28 | 0.58 ± 0.25 |
| Grey Butcherbird 7 | Cracticus torquatus | Ca-omni | 6 | 0.94 | 0.51 ± 0.26 | 0.44 ± 0.26 | 0.73 ± 0.22 |
| Australian Magpie 7 | Gymnorhina tibicen | Gr-insect | 6 | 0.60 | 0.37 ± 0.34 | 0.59 ± 0.24 | 0.37 ± 0.20 |
| Lewin’s Honeyeater 7 | Meliphaga lewinii | Ca-nectinsect | 6 | 0.31 | 0.45 ± 0.38 | 0.24 ± 0.28 | 0.62 ± 0.34 |
| Grey Shrike-thrush 7 | Colluricincla harmonica | Ge-insect | 6 | 0.30 | 0.64 ± 0.37 | 0.55 ± 0.34 | 0.57 ± 0.39 |
| Eastern Koel 7 | Eudynamys orientalis | Ca-frugi | 5 | 2.16 | 0.58 ± 0.30 | 0.35 ± 0.22 | 0.64 ± 0.21 |
| Fairy Gerygone 7 | Gerygone palpebrosa | Ca-insect | 5 | 0.70 | 1.00 ± 0.00 | 0.42 ± 0.42 | 0.43 ± 0.47 |
| White-winged Chough 7 | Corcorax melanorhamphos | Gr-omni | 5 | 0.48 | 0.55 ± 0.41 | 0.86 ± 0.21 | 0.51 ± 0.16 |
| Black-faced Cuckoo-shrike 6,7 | Coracina novaehollandiae | Ca-insect | 5 | 0.25 | 0.25 ± 0.20 | 0.39 ± 0.37 | 0.61 ± 0.29 |
| Eastern Rosella 7 | Platycercus eximius | Gr-grani | 5 | 0.19 | 0.67 ± 0.00 | 0.25 ± 0.43 | 0.90 ± 0.15 |
| Brush Cuckoo | Cacomantis variolosus | Sh-insect | 5 | 0.17 | 1.00 ± 0.00 | 0.74 ± 0.24 | 0.69 ± 0.41 |
| Little Wattlebird | Anthochaera chrysoptera | Sh-nectinsect | 5 | 0.17 | 1.00 ± 0.00 | 0.33 ± 0.47 | 0.96 ± 0.04 |
| Laughing Kookaburra 6,7 | Dacelo novaeguineae | Gr-carni | 5 | 0.08 | 0.59 ± 0.07 | 0.16 ± 0.20 | 0.65 ± 0.15 |
| Purple Swamphen 7 | Porphyrio porphyrio | Aq-omni | 5 | 0.06 | 0.49 ± 0.33 | 0.50 ± 0.50 | 0.33 ± 0.47 |
| Noisy Miner 7 | Manorina melanocephala | Ca-nectinsect | 4 | 1.79 | 0.88 ± 0.13 | 0.66 ± 0.47 | 0.47 ± 0.47 |
| Pheasant Coucal 7 | Centropus phasianinus | Gr-carni | 4 | 0.24 | 0.66 ± 0.36 | 0.21 ± 0.21 | 0.60 ± 0.12 |
| Rainbow Lorikeet 7 | Trichoglossus moluccanus | Ca-nectinsect | 4 | 0.23 | 0.64 ± 0.35 | 0.45 ± 0.32 | 0.11 ± 0.11 |
| White-throated Treecreeper 7 | Cormobates leucophaea | Ca-insect | 4 | 0.22 | 1.00 ± 0.00 | 0.42 ± 0.00 | 0.65 ± 0.46 |
| Sulphur-crested Cockatoo 7 | Cacatua galerita | Ge-grani | 4 | 0.20 | 0.88 ± 0.00 | 0.50 ± 0.50 | 0.39 ± 0.39 |
| Double-barred Finch 6,7 | Taeniopygia bichenovii | Sh-grani | 4 | 0.09 | 0.00 ± 0.00 | - | 0.50 ± 0.50 |
| Black Cockatoo 7 | Calyptorhynchus banksii | Ca-frugi | 3 | 1.65 | 0.66 ± 0.00 | 0.99 ± 0.01 | 0.87 ± 0.19 |
| Peaceful Dove 6,7 | Geopelia placida | Gr-grani | 3 | 0.89 | 0.00 ± 0.00 | 0.28 ± 0.33 | 0.18 ± 0.00 |
| Channel-billed Cuckoo 7 | Scythrops novaehollandiae | Ca-frugi | 3 | 0.60 | - | 0.00 ± 0.00 | 0.57 ± 0.10 |
| Blue-faced Honeyeater | Entomyzon cyanotis | Ca-nectinsect | 3 | 0.37 | 0.48 ± 0.41 | 0.11 ± 0.04 | 0.32 ± 0.27 |
| White-throated Gerygone 7 | Gerygone olivacea | Ca-insect | 3 | 0.13 | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.94 ± 0.00 |
| Cicadabird | Coracina tenuirostris | Ca-insect | 3 | 0.12 | 0.00 ± 0.00 | 0.50 ± 0.50 | 0.50 ± 0.50 |
| Rainbow Bee-eater 6, 7 | Merops ornatus | Ae-insect | 3 | 0.11 | 0.78 ± 0.22 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Fan-tailed Cuckoo 7 | Cacomantis flabelliformis | Sh-insect | 3 | 0.04 | 0.50 ± 0.50 | 1.00 ± 0.00 | 0.73 ± 0.03 |
| Pied Currawong 7 | Strepera graculina | Sh-omni | 3 | 0.03 | 1.00 ± 0.00 | - | 1.00 ± 0.00 |
| Sacred Kingfisher | Todiramphus sanctus | Gr-carni | 3 | 0.02 | 0.00 ± 0.00 | 1.00 ± 0.00 | 0.00 ± 0.00 |
| Leaden Flycatcher 7 | Myiagra rubecula | Sh-insect | 2 | 0.64 | 0.39 ± 0.00 | 0.54 ± 0.00 | 0.72 ± 0.28 |
| Dusky Moorhen 7 | Gallinula tenebrosa | Aq-omni | 2 | 0.40 | 0.00 ± 0.00 | 0.42 ± 0.25 | 0.27 ± 0.27 |
| Masked Lapwing 7 | Vanellus miles | Gr-insect | 2 | 0.19 | 0.50 ± 0.50 | 0.15 ± 0.15 | - |
| Wonga Pigeon | Leucosarcia melanoleuca | Gr-grani | 2 | 0.08 | 0.50 ± 0.50 | 1.00 ± 0.00 | - |
| Spangled Drongo 6,7 | Dicrurus bracteatus | Ca-omni | 2 | 0.07 | - | 0.00 ± 0.00 | 0.36 ± 0.00 |
| Red-browed Finch | Neochmia temporalis | Sh-grani | 2 | 0.06 | 1.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Dollarbird 7 | Eurystomus orientalis | Ae-insect | 2 | 0.04 | - | 1.00 ± 0.00 | 0.00 ± 0.00 |
| Varied Triller 7 | Lalage leucomela | Ca-omni | 2 | 0.03 | 0.50 ± 0.50 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Galah 7 | Eolophus roseicapilla | Gr-grani | 2 | 0.03 | - | 0.69 ± 0.00 | 1.00 ± 0.00 |
| White-breasted Woodswallow | Artamus leucorynchus | Ae-insect | 2 | 0.01 | 0.00 ± 0.00 | 0.30 ± 0.30 | - |
| Mistletoe | Dicaeum hirundinaceum | Ca-frugi | 2 | 0.01 | 1.00 ± 0.00 | - | 1.00 ± 0.00 |
| Source of Variation | df | Arthropod Abundance by Sampling Method | Avian Acoustic Activity | |||
|---|---|---|---|---|---|---|
| Light-Traps | Pit-Fall Traps | Sweep-Nets | Log-Transformed Data | Presence/abs. Data | ||
| Time | 2 | 1.970 | 2.413 * | 1.346 | 1.038 | 1.087 |
| Spray | 1 | 0.721 | 1.597 | 1.125 | 0.704 | 0.805 |
| Location (Loc) | 5 | 4.636 *** | 5.695 *** | 14.319 *** | 22.680 *** | 15.503 *** |
| Time × Spray | 2 | 1.079 | 2.087 * | 2.167 * | 1.492 | 1.651 |
| Time × Loc | 10 | 1.879 * | 1.260 | 1.594 | 2.802 *** | 2.074 *** |
| Spray × Loc | 5 | 1.930 * | 2.686 *** | 3.101 ** | 3.423 *** | 2.346 *** |
| Trap | Taxon 1 | Abundance | Abundance Ratios (Average ± SE) 2 | ||
|---|---|---|---|---|---|
| Pre 3 | Post 1 | Post 3 | |||
| Sweep-net | Hemiptera | 0.68 ± 0.08 | 0.42 ± 0.14 | 0.56 ± 0.10 | |
| Sweep-net | Coleoptera | 7123 | 0.57 ± 0.08 | 0.52 ± 0.12 | 0.54 ± 0.11 |
| Sweep-net | Diptera | 3205 | 0.54 ± 0.05 | 0.43 ± 0.09 | 0.52 ± 0.04 |
| Sweep-net | Hymenoptera | 1921 | 0.58 ± 0.06 | 0.43 ± 0.07 | 0.50 ± 0.05 |
| Sweep-net | Araneae | 1488 | 0.53 ± 0.05 a | 0.40 ± 0.06 b | 0.35 ± 0.03 b |
| Sweep-net | Psocoptera | 993 | 0.57 ± 0.08 | 0.51 ± 0.05 | 0.48 ± 0.09 |
| Sweep-net | Formicidae | 694 | 0.58 ± 0.06 | 0.45 ± 0.11 | 0.38 ± 0.05 |
| Sweep-net | Lepidoptera | 503 | 0.69 ± 0.14 | 0.39 ± 0.15 | 0.29 ± 0.14 |
| Sweep-net | Neuroptera | 413 | 0.52 ± 0.07 | 0.54 ± 0.09 | 0.45 ± 0.05 |
| Sweep-net | Thysanoptera | 205 | 0.69 ± 0.11 | 0.39 ± 0.13 | 0.30 ± 0.12 |
| Sweep-net | Orthoptera | 50 | 0.70 ± 0.20 | 0.75 ± 0.16 | 0.53 ± 0.22 |
| Pit-fall | Formicidae | 1932 | 0.60 ± 0.08 | 0.35 ± 0.15 | 0.52 ± 0.12 |
| Pit-fall | Collembola | 923 | 0.40 ± 0.13 | 0.20 ± 0.12 | 0.25 ± 0.13 |
| Pit-fall | Diptera | 86 | 0.47 ± 0.16 | 0.49 ± 0.15 | 0.23 ± 0.15 |
| Pit-fall | Coleoptera | 83 | 0.63 ± 0.14 | 0.78 ± 0.14 | 0.50 ± 0.29 |
| Pit-fall | Araneae | 61 | 1.00 ± 0.35 | 0.47 ± 0.23 | 0.43 ± 0.16 |
| Light-trap | Lepidoptera | 1198 | 0.46 ± 0.16 | 0.43 ± 0.12 | 0.53 ± 0.11 |
| Light-trap | Coleoptera | 337 | 0.57 ± 0.10 | 0.45 ± 0.15 | 0.45 ± 0.19 |
| Light-trap | Diptera | 315 | 0.55 ± 0.18 | 0.60 ± 0.11 | 0.50 ± 0.07 |
| Light-trap | Hymenoptera | 57 | 0.53 ± 0.08 | 0.50 ± 0.16 | 0.29 ± 0.20 |
| Light-trap | Hemiptera | 51 | 0.50 ± 0.50 | 0.58 ± 0.30 | 0.60 ± 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crisol-Martínez, E.; Moreno-Moyano, L.T.; Horgan, F.G. Bioacoustics Reveal Species-Rich Avian Communities Exposed to Organophosphate Insecticides in Macadamia Orchards. Birds 2020, 1, 35-52. https://doi.org/10.3390/birds1010005
Crisol-Martínez E, Moreno-Moyano LT, Horgan FG. Bioacoustics Reveal Species-Rich Avian Communities Exposed to Organophosphate Insecticides in Macadamia Orchards. Birds. 2020; 1(1):35-52. https://doi.org/10.3390/birds1010005
Chicago/Turabian StyleCrisol-Martínez, Eduardo, Laura T. Moreno-Moyano, and Finbarr G. Horgan. 2020. "Bioacoustics Reveal Species-Rich Avian Communities Exposed to Organophosphate Insecticides in Macadamia Orchards" Birds 1, no. 1: 35-52. https://doi.org/10.3390/birds1010005
APA StyleCrisol-Martínez, E., Moreno-Moyano, L. T., & Horgan, F. G. (2020). Bioacoustics Reveal Species-Rich Avian Communities Exposed to Organophosphate Insecticides in Macadamia Orchards. Birds, 1(1), 35-52. https://doi.org/10.3390/birds1010005





