Repeat Sequence Mapping Shows Different W Chromosome Evolutionary Pathways in Two Caprimulgiformes Families
Abstract
Simple Summary
Abstract
1. Introduction
2. Experimental Section
3. Results
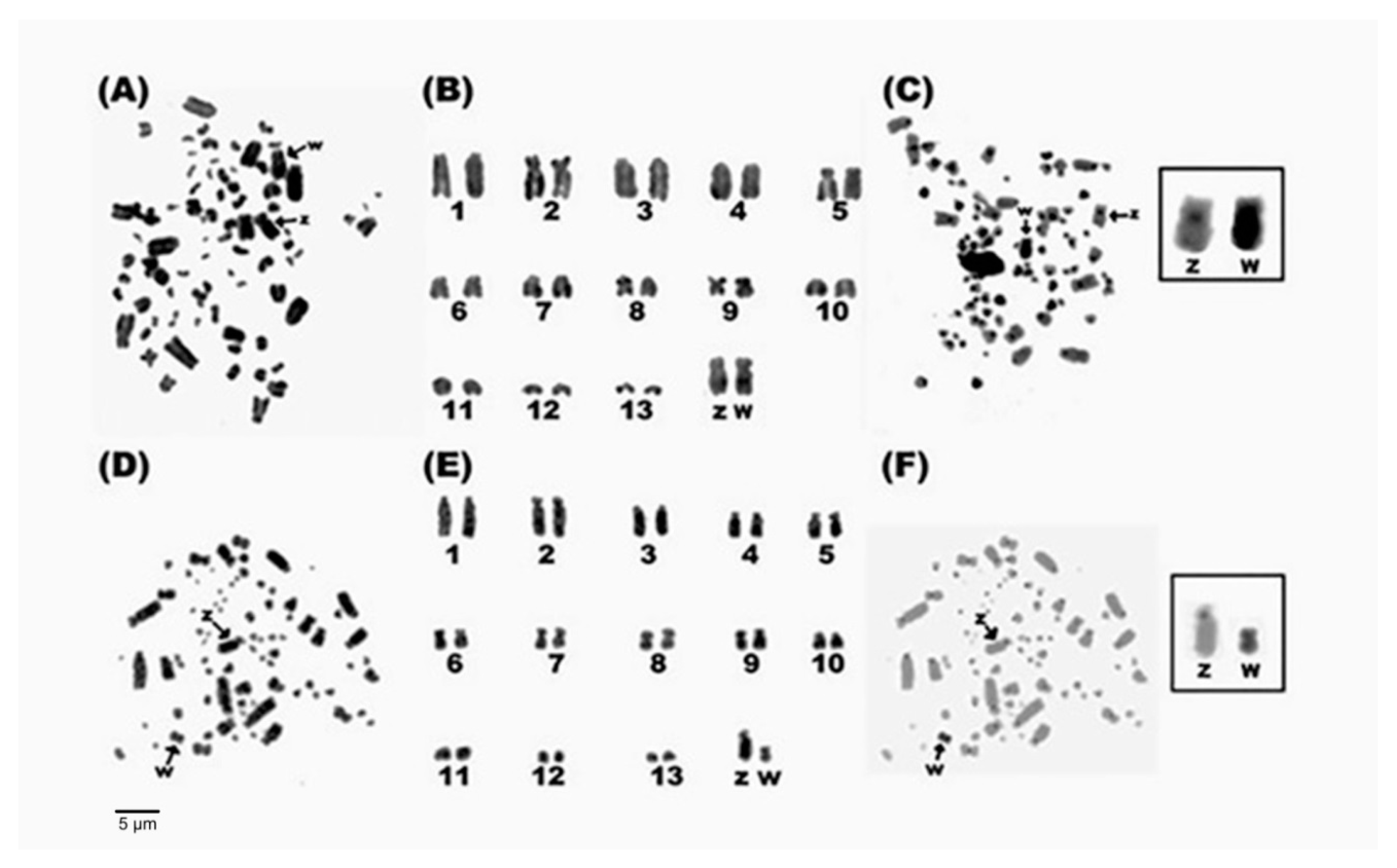
3.1. Karyotype Description
3.2. C-Banding
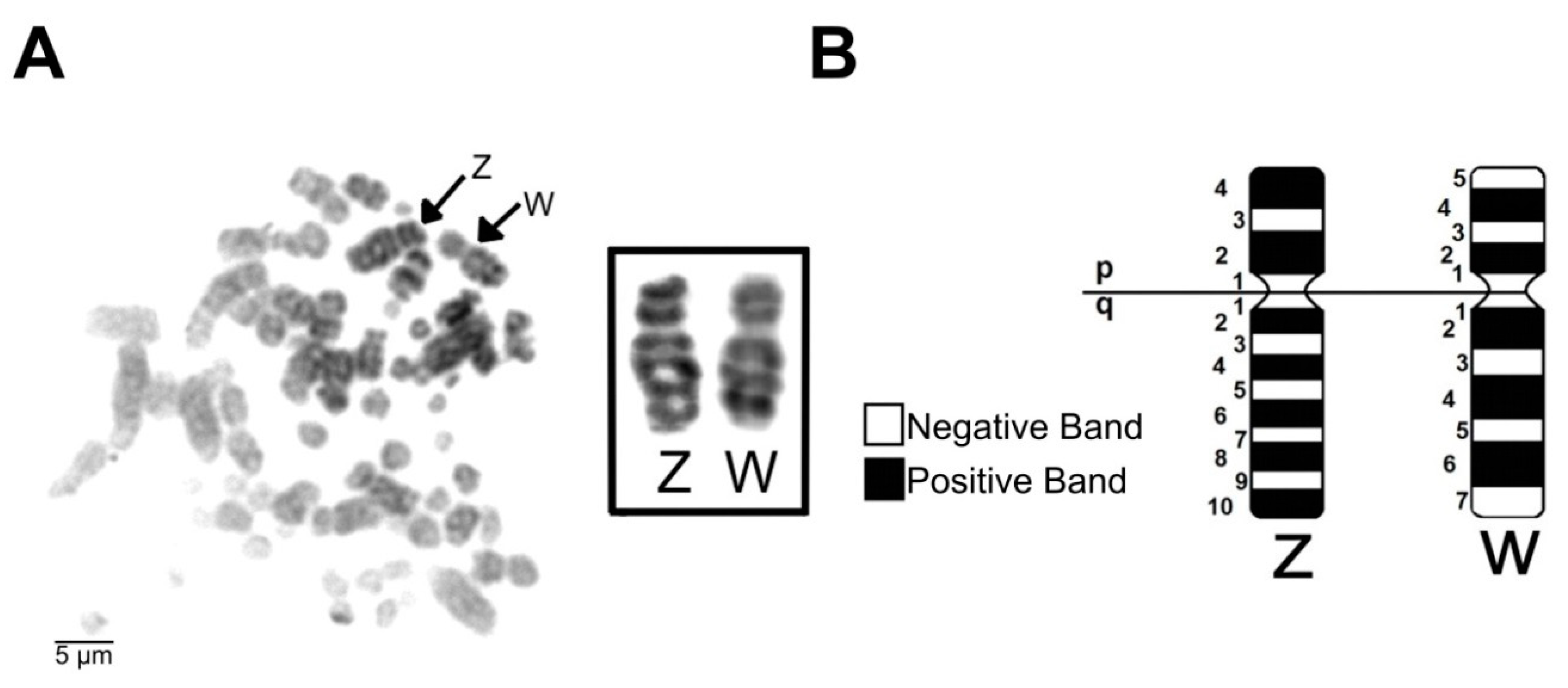
3.3. G-Banding in the ZW Sex Chromosomes of the Common Potoo
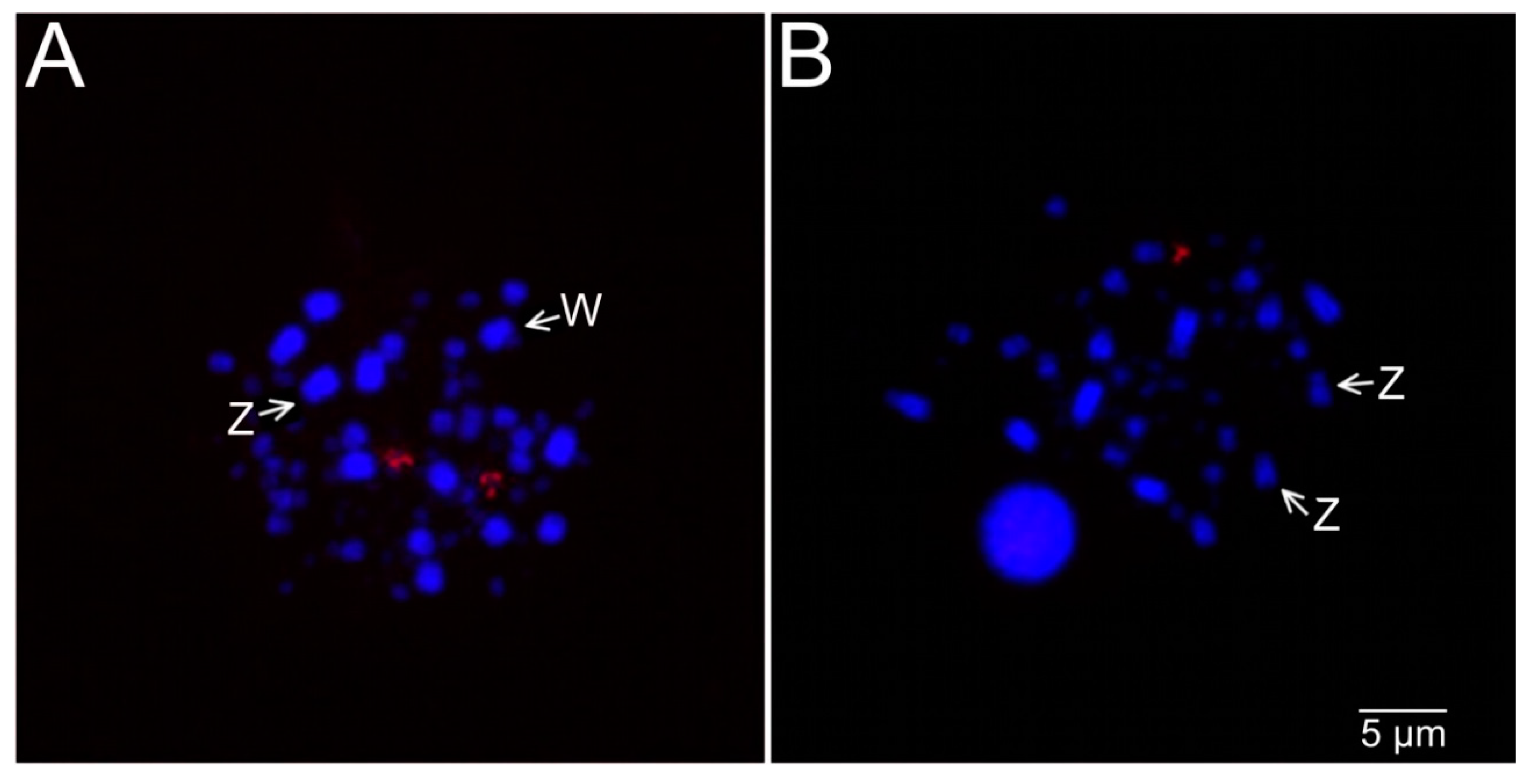
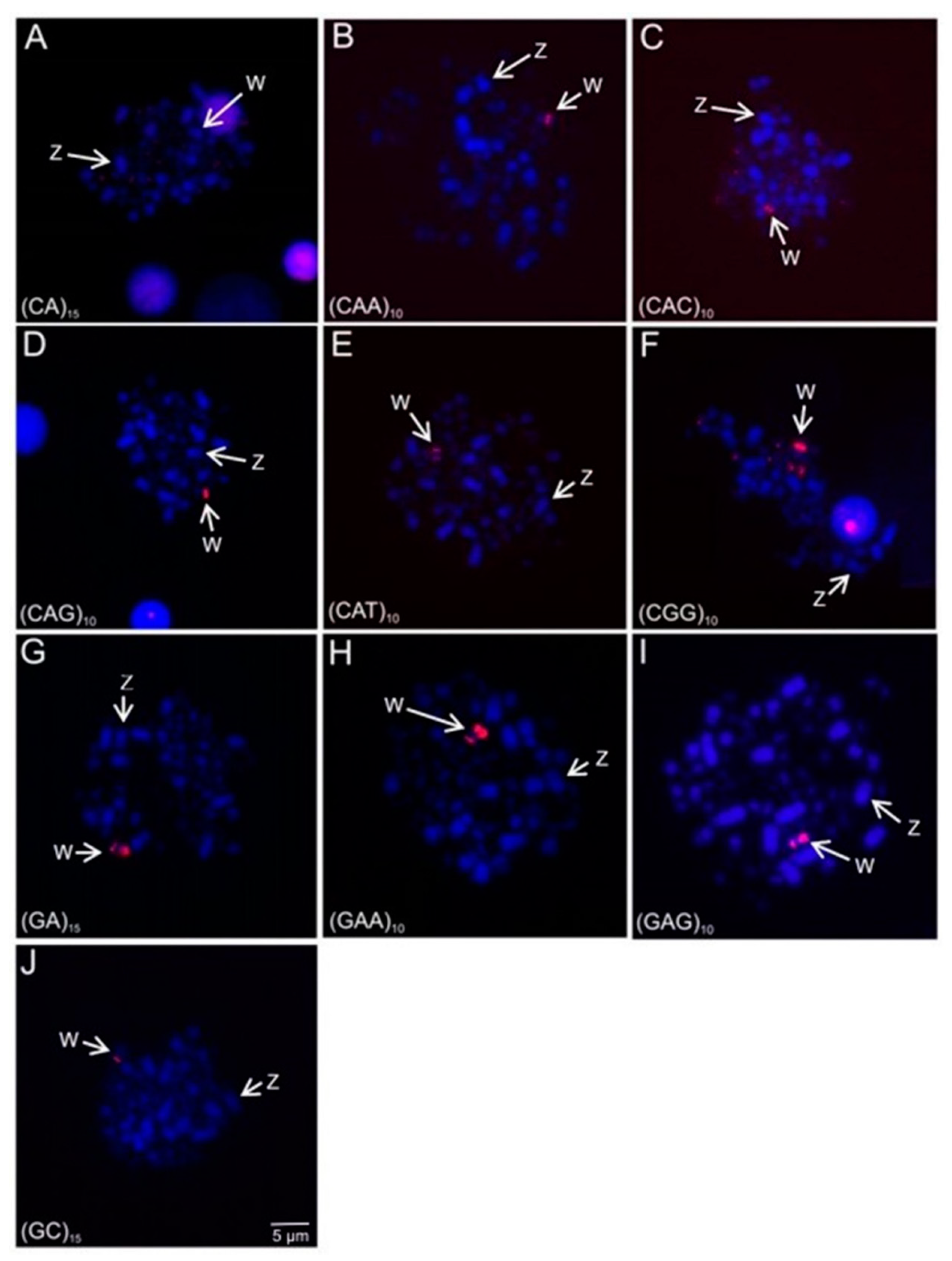
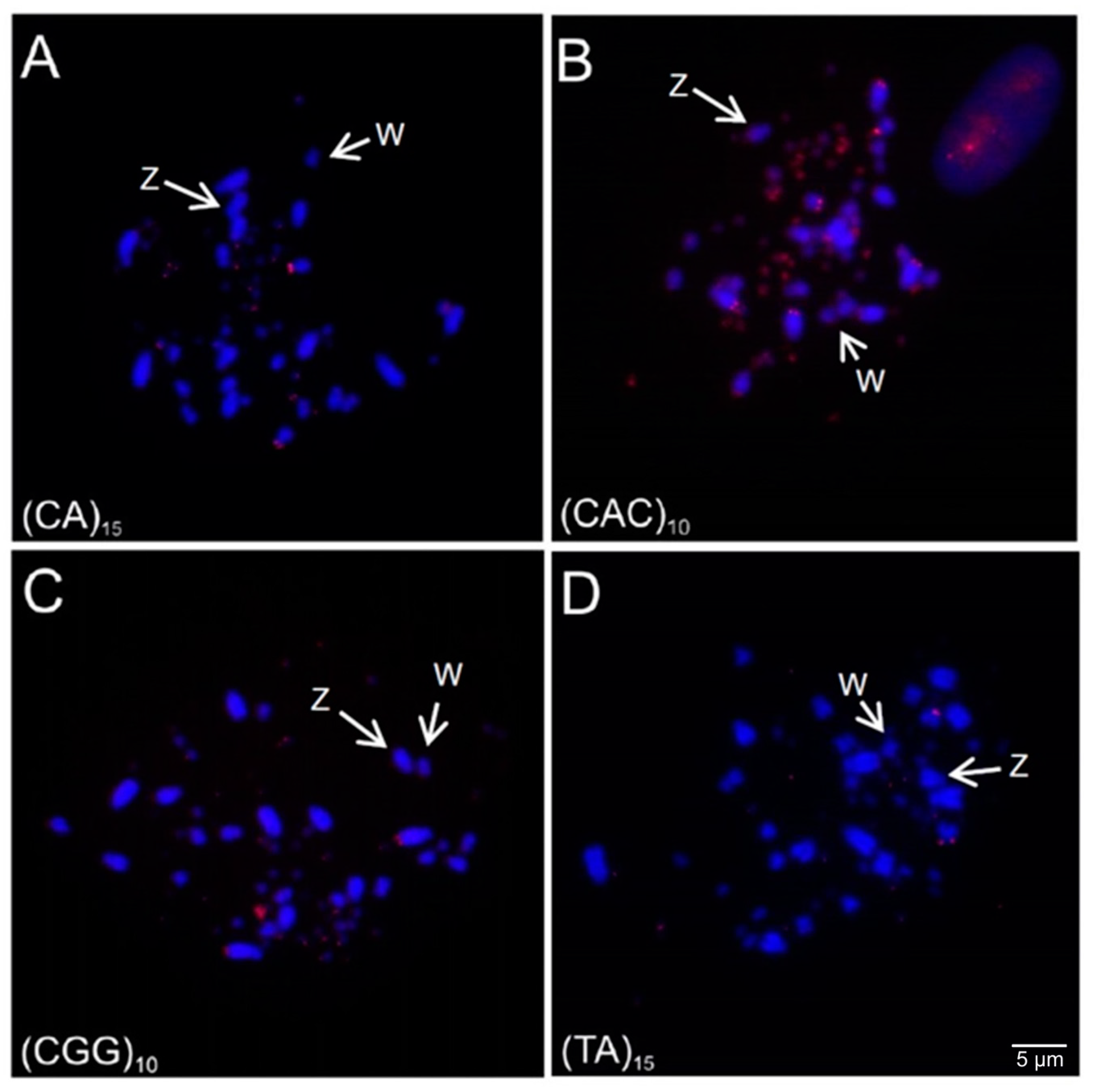
3.4. Microsatellite and 18S rDNA Hybridization
3.5. Common Potoo W Sex Chromosome
4. Discussion
4.1. Chromosomal Divergences between Common Potoo and Scissor-Tailed Nightjar
4.2. The Sex Chromosomes of Common Potoo and Scissor-Tailed Nightjar: Same Origin, Different Evolutionary Pathways
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mank, J.E.; Ellegren, H. Parallel divergence and degradation of the avian W sex chromosome. Trends Ecol. Evol. 2007, 22, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.K.; Nordén, A.K.; Hansson, B. Sex chromosome evolution: Historical insights and future perspectives. Proc. R. Soc. B 2017, 284. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; O’Meally, D.; Azad, B.; Georges, A.; Sarre, S.D.; Graves, J.A.M.; Matsuda, Y.; Ezaz, T. Amplification of microsatellite repeat motifs is associated with the evolutionary differentiation and heterochromatinization of sex chromosomes in Sauropsida. Chromosoma 2016, 125, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, D.; Charlesworth, B.; Marais, G. Steps in the evolution of heteromorphic sex chromosomes. Heredity 2005, 95, 118. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Camacho, J.P.; Bertollo, L.A. Repetitive DNAs and differentiation of sex chromosomes in Neotropical fishes. Cytogenet. Genome Res. 2011, 132, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Nanda, I.; Shan, Z.; Schartl, M.; Burt, D.W.; Koehler, M.; Nothwang, H.; Grützner, F.; Paton, I.R.; Windsor, D.; Dunn, I.; et al. 300 million years of conserved synteny between chicken Z and human chromosome 9. Nat. Genet. 1999, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.A. Sex chromosome specialization and degeneration in mammals. Cell 2006, 124, 901–914. [Google Scholar] [CrossRef]
- Yazdi, H.P.; Silva, W.T.; Suh, A. Why do some sex chromosomes degenerate more slowly than others? The odd case of ratite sex chromosomes. Genes 2020, 11, 1153. [Google Scholar] [CrossRef]
- Nanda, I.; Schmid, M. Conservation of avian Z chromosomes as revealed by comparative mapping of the Z-linked aldolase B gene. Cytogenet. Genome Res. 2002, 96, 176–178. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Q. The Female-Specific W Chromosomes of Birds Have Conserved Gene Contents but Are Not Feminized. Genes 2020, 11, 1126. [Google Scholar] [CrossRef]
- Matsubara, K.; Tarui, H.; Toriba, M.; Yamada, K.; Nishida-Umehara, C.; Agata, K.; Matsuda, Y. Evidence for different origin of sex chromosomes in snakes, birds, and mammals and step-wise differentiation of snake sex chromosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 18190–18195. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Murata, K.; Mizuno, S. The location of Z-and W-linked marker genes and sequence on the homomorphic sex chromosomes of the ostrich and the emu. Proc. Natl. Acad. Sci. USA 1998, 95, 4415–4418. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Griffin, D.K.; Graves, J.A.M. Comparative painting reveals strong chromosome homology over 80 million years of bird evolution. Chromosome Res. 1999, 7, 289–295. [Google Scholar] [CrossRef]
- Takagi, N.; Itoh, M.; Sasaki, M. Chromosome studies in four species of Ratitae (Aves). Chromosoma 1972, 36, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Gunski, R.J.; Giannoni, M.L. Nucleolar organizer regions and a new chromosome number for Rhea americana (Aves: Rheiformes). Genet. Mol. Biol. 1998, 21, 207–210. [Google Scholar] [CrossRef]
- Sumner, A.T. Euchromatin and the Longitudinal Differentiation of Chromosomes. Chromosomes-Organization and Function, 1st ed.; Blackwell: Oxford, UK, 2003; pp. 117–132. [Google Scholar]
- Nishida-Umehara, C.; Tsuda, Y.; Ishijima, J.; Ando, J.; Fujiwara, A.; Matsuda, Y.; Griffin, D.K. The molecular basis of chromosome orthologies and sex chromosomal differentiation in palaeognathous birds. Chromosome Res. 2007, 15, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Pigozzi, M.I. Diverse stages of sex-chromosome differentiation in tinamid birds: Evidence from crossover analysis in Eudromia elegans and Crypturellus tataupa. Genetica 2011, 139, 771. [Google Scholar] [CrossRef]
- Fridolfsson, A.K.; Cheng, H.; Copeland, N.G.; Jenkins, N.A.; Liu, H.C.; Raudsepp, T.; Woodage, T.; Chowdhary, B.; Halverson, J.; Ellegren, H. Evolution of the avian sex chromosomes from an ancestral pair of autosomes. Proc. Nat. Acad. Sci. USA 1998, 95, 8147–8152. [Google Scholar] [CrossRef]
- Barcellos, S.A.; Kretschmer, R.; de Souza, M.S.; Costa, A.L.; Degrande, T.M.; dos Santos, M.S.; de Oliveira, E.H.; Cioffi, M.B.; Gunski, R.J.; Garnero, A.D.V. Karyotype evolution and distinct evolutionary history of the W chromosomes in swallows (Aves, Passeriformes). Cytogenet. Genome Res. 2019, 158, 98–105. [Google Scholar] [CrossRef]
- Schartl, M.; Schmid, M.; Nanda, I. Dynamics of vertebrate sex chromosome evolution: From equal size to giants and dwarfs. Chromosoma 2016, 125, 553–571. [Google Scholar] [CrossRef]
- Degrandi, T.M.; del Valle Garnero, A.; O’Brien, P.C.; Ferguson-Smith, M.A.; Kretschmer, R.; de Oliveira, E.H.; Gunski, R.J. Chromosome painting in Trogon surrucura (Aves, Trogoniformes) reveals a karyotype derived by chromosomal fissions, fusions, and inversions. Cytogenet. Genome Res. 2017, 151, 208–215. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Furo, I.; Kretschmer, R.; dos Santos, M.S.; de Lima Carvalho, C.A.; Gunski, R.J.; O’Brien, P.C.; Ferguson-Smith, M.A.; Cioffi, M.B.; de Oliveira, E.H. Chromosomal mapping of repetitive DNAs in Myiopsitta monachus and Amazona aestiva (Psittaciformes, Psittacidae) with emphasis on the sex chromosomes. Cytogenet. Genome Res. 2017, 151, 151–160. [Google Scholar] [CrossRef]
- Gunski, R.J.; Kretschmer, R.; de Souza, M.S.; de Oliveira Furo, I.; Barcellos, S.A.; Costa, A.L.; Cioffi, M.B.; de Oliveira, E.H.C.; del Valle Garnero, A. Evolution of bird sex chromosomes narrated by repetitive sequences: Unusual W chromosome enlargement in Gallinula melanops (Aves: Gruiformes: Rallidae). Cytogenet. Genome Res. 2019, 158, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, C.; Li, Q.; Li, B.; Xu, L.; Pan, H.; Wang, Z.; Jin, L.; Zhang, P.; Larkin, D.M.; et al. Comparative genomics reveals insights into avian genome evolution and adaptation. Science 2014, 346, 1311–1320. [Google Scholar] [CrossRef]
- Hughes, A.L.; Hughes, M.K. Small genomes for better flyers. Nature 1995, 377, 391. [Google Scholar] [CrossRef]
- Kapusta, A.; Suh, A.; Feschotte, C. Dynamics of genome size evolution in birds and mammals. Proc. Natl. Acad. Sci. USA 2017, 114, E1460–E1469. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Nanda, I.; Guttenbach, M.; Steinlein, C.; Hoehn, M.; Schartl, M.; Haaf, T.; Weigend, S.; Fries, R.; Buerstedde, J.M.; et al. First report on chicken genes and chromosomes 2000. Cytogenet. Cell Genet. 2000, 90, 169–218. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Piontkivska, H. DNA repeat arrays in chicken and human genomes and the adaptive evolution of avian genome size. BMC Evol. Biol. 2005, 5, 12. [Google Scholar] [CrossRef]
- Stevens, L. Sex chromosomes and sex determining mechanisms in birds. Sci. Progress. 1997, 80, 197–216. [Google Scholar]
- Hughes, A.L.; Friedman, R. Genome size reduction in the chicken has involved massive loss of ancestral protein-coding genes. Mol. Biol. Evol. 2008, 25, 2681–2688. [Google Scholar] [CrossRef]
- Ellegren, H. Microsatellites: Simple sequences with complex evolution. Nat. Rev. Genet. 2004, 5, 435. [Google Scholar] [CrossRef]
- Pokorná, M.; Kratochvíl, L.; Kejnovský, E. Microsatellite distribution on sex chromosomes at different stages of heteromorphism and heterochromatinization in two lizard species (Squamata: Eublepharidae: Coleonyx elegans and Lacertidae: Eremias velox). BMC Genet. 2011, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.D.; Kretschmer, R.; Bertocchi, N.A.; Degrandi, T.M.; de Oliveira, E.H.C.; de Bello Cioffi, M.; del Valle Garnero, A.; Gunski, R.J. Genomic organization of repetitive DNA in woodpeckers (Aves, Piciformes): Implications for karyotype and ZW sex chromosome differentiation. PLoS ONE 2017, 12, e0169987. [Google Scholar] [CrossRef]
- Mayr, G. Osteological evidence for paraphyly of the avian order Caprimulgiformes (nightjars and allies). J. Ornithol. 2002, 143, 82–97. [Google Scholar] [CrossRef]
- Belterman, R.H.R.; De Boer, L.E.M. A karyological study of 55 species of birds, including karyotypes of 39 species new to cytology. Genetica 1984, 65, 39–82. [Google Scholar] [CrossRef]
- Nieto, L.M.; Gunski, R.J. Estudios cromossomicos em Atajacaminos (Aves, Caprimulgidae). Boll. Soc. Biol. Concepc. 1998, 69, 161–169. [Google Scholar]
- Francisco, M.R.; Lunardi, O.; Garcia, C.; Junior, P.M. Karyotype description of the Semicollared Nighthawk, Lurocalis semitorquatus (Caprimulgidae). Revist. Bras. Ornit. 2006, 14, 63–65. [Google Scholar]
- Nieto, L.M.; Kretschmer, R.; Ledesma, M.A.; Garnero, A.D.V.; Gunski, R.J. Karyotype morphology suggests that the Nyctibius griseus (Gmelin, 1789) carries an ancestral ZW-chromosome pair to the order Caprimulgiformes (Aves). Comp. Cytogenet. 2012, 6, 379. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef]
- Kretschmer, R.; de Lima, V.L.C.; de Souza, M.S.; Costa, A.L.; O’Brien, P.C.; Ferguson-Smith, M.A.; de Oliveira, E.H.C.; Gunski, R.J.; Garnero, A.D.V. Multidirectional chromosome painting in Synallaxis frontalis (Passeriformes, Furnariidae) reveals high chromosomal reorganization, involving fissions and inversions. Comp. Cytogenet. 2018, 12, 97. [Google Scholar] [CrossRef]
- Kretschmer, R.; Ferguson-Smith, M.A.; de Oliveira, E.H.C. Karyotype evolution in birds: From conventional staining to chromosome painting. Genes 2018, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Garnero, A.V.; Gunski, R.J. Comparative analysis of the karyotypes of Nothura maculosa and Rynchotus rufescens (Aves: Tinamidae): A case of chromosomal polymorphism. Nucleus 2000, 43, 64–70. [Google Scholar]
- Sasaki, M.; Ikeuchi, T.; Makino, S. A feather pulp culture for avian chromosomes with notes on the chromosomes of the peafowl and the ostrich. Experientia 1968, 24, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef]
- Ledesma, M.A.; Garnero, A.D.V.; Gunski, R.J. Análise do Cariótipo de duas espécies da Família Formicariidae (Aves: Passeriformes). Ararajuba 2002, 10, 15–19. [Google Scholar]
- Howe, B.; Umrigar, A.; Tsien, F. Chromosome preparation from cultured cells. JoVE J. Vis. Exp. 2014, 83. [Google Scholar] [CrossRef]
- Guerra, M. FISH: Conceitos e Aplicações na Citogenética, 1st ed.; Sociedade Brasileira de Genética: Ribeirão Preto, Brazil, 2004; pp. 88–89. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.J.; Taylor, J.L. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Daniels, L.M.; Delany, M.E. Molecular and cytogenetic organization of the 5S ribosomal DNA array in chicken (Gallus gallus). Chromosome Res. 2003, 11, 305–317. [Google Scholar] [CrossRef]
- Kubat, Z.; Hobza, R.; Vyskot, B.; Kejnovsky, E. Microsatellite accumulation on the Y chromosome in Silene latifolia. Genome 2008, 51, 350–356. [Google Scholar] [CrossRef]
- Burt, D.W. Origin and evolution of avian microchromosomes. Cytogenet. Genome Res. 2002, 96, 97–112. [Google Scholar] [CrossRef]
- Skinner, B.M.; Griffin, D.K. Intrachromosomal rearrangements in avian genome evolution: Evidence for regions prone to breakpoints. Heredity 2012, 108, 37–41. [Google Scholar] [CrossRef][Green Version]
- Degrandi, T.M.; Gunski, R.J.; Garnero, A.D.V.; de Oliveira, E.H.C.; Kretschmer, R.; de Souza, M.S.; Barcellos, S.A.; Hass, I. The distribution of 45S rDNA sites in bird chromosomes suggests multiple evolutionary histories. Genet. Mol. Biol. 2020, 43, e20180331. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, E.D.; Mirarab, S.; Aberer, A.J.; Li, B.; Houde, P.; Li, C.; Ho, S.Y.; Faircloth, B.C.; Nabholz, B.; Howard, J.T.; et al. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 2014, 346, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Dyomin, A.G.; Koshel, E.I.; Kiselev, A.M.; Saifitdinova, A.F.; Galkina, S.A.; Fukagawa, T.; Kostareva, A.A.; Gaginskaya, E.R. Chicken rRNA gene cluster structure. PLoS ONE 2016, 11, e0157464. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.S.; Kretschmer, R.; Gunski, R.J.; Garnero, A.D.V.; O’Brien, P.C.M.; Ferguson-Smith, M.A.; de Oliveira, E.H.C. Chromosome painting in tyrant flycatchers confirms a set of inversions shared by Oscines and Suboscines (Aves, Passeriformes). Cytogenet. Genome Res. 2018, 153, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, R.; de Oliveira, T.D.; Furo, I.O.; Silva, F.A.O.; Gunski, R.J.; Garnero, A.D.V.; Cioffi, M.B.; de Oliveira, E.H.C.; de Freitas, T.R.O. Repetitive DNAs and shrink genomes: A chromosomal analysis in nine Columbidae species (Aves, Columbiformes). Genet. Mol. Biol. 2018, 41, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Yano, C.F.; Poltronieri, J.; Bertollo, L.A.C.; Artoni, R.F.; Liehr, T.; de Bello Cioffi, M. Chromosomal mapping of repetitive DNAs in Triportheus trifurcatus (Characidae, Characiformes): Insights into the differentiation of the Z and W chromosomes. PLoS ONE 2014, 9, e90946. [Google Scholar] [CrossRef]
- Smith, C.A.; Roeszler, K.N.; Hudson, Q.J.; Sinclair, A.H. Avian sex determination: What, when and where? Cytogenet. Genome Res. 2007, 117, 165–173. [Google Scholar] [CrossRef]
- López-Flores, I.; Garrido-Ramos, M.A. The repetitive DNA content of eukaryotic genomes. Repetitive DNA 2012, 7, 1–28. [Google Scholar] [CrossRef]
- Kejnovsky, E.; Hobza, R.; Cermak, T.; Kubat, Z.; Vyskot, B. The role of repetitive DNA in structure and evolution of sex chromosomes in plants. Heredity 2009, 102, 533–541. [Google Scholar] [CrossRef]
- Cioffi, M.B.; Moreira-Filho, O.; Almeida-Toledo, L.F.; Bertollo, L.A.C. The contrasting role of heterochromatin in the differentiation of sex chromosomes: An overview from Neotropical fishes. J. Fish Biol. 2012, 80, 2125–2139. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, M.S.; Kretschmer, R.; Barcellos, S.A.; Costa, A.L.; Cioffi, M.d.B.; de Oliveira, E.H.C.; Garnero, A.D.V.; Gunski, R.J. Repeat Sequence Mapping Shows Different W Chromosome Evolutionary Pathways in Two Caprimulgiformes Families. Birds 2020, 1, 19-34. https://doi.org/10.3390/birds1010004
de Souza MS, Kretschmer R, Barcellos SA, Costa AL, Cioffi MdB, de Oliveira EHC, Garnero ADV, Gunski RJ. Repeat Sequence Mapping Shows Different W Chromosome Evolutionary Pathways in Two Caprimulgiformes Families. Birds. 2020; 1(1):19-34. https://doi.org/10.3390/birds1010004
Chicago/Turabian Stylede Souza, Marcelo Santos, Rafael Kretschmer, Suziane Alves Barcellos, Alice Lemos Costa, Marcelo de Bello Cioffi, Edivaldo Herculano Corrêa de Oliveira, Analía Del Valle Garnero, and Ricardo José Gunski. 2020. "Repeat Sequence Mapping Shows Different W Chromosome Evolutionary Pathways in Two Caprimulgiformes Families" Birds 1, no. 1: 19-34. https://doi.org/10.3390/birds1010004
APA Stylede Souza, M. S., Kretschmer, R., Barcellos, S. A., Costa, A. L., Cioffi, M. d. B., de Oliveira, E. H. C., Garnero, A. D. V., & Gunski, R. J. (2020). Repeat Sequence Mapping Shows Different W Chromosome Evolutionary Pathways in Two Caprimulgiformes Families. Birds, 1(1), 19-34. https://doi.org/10.3390/birds1010004










