Radionuclide-Dependent Stimulation of Antitumor Immunity in GD2-Targeted Radiopharmaceutical Therapy Combined with Immune Checkpoint Inhibitors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture
2.2. Murine Tumor Models
2.2.1. Imaging Studies
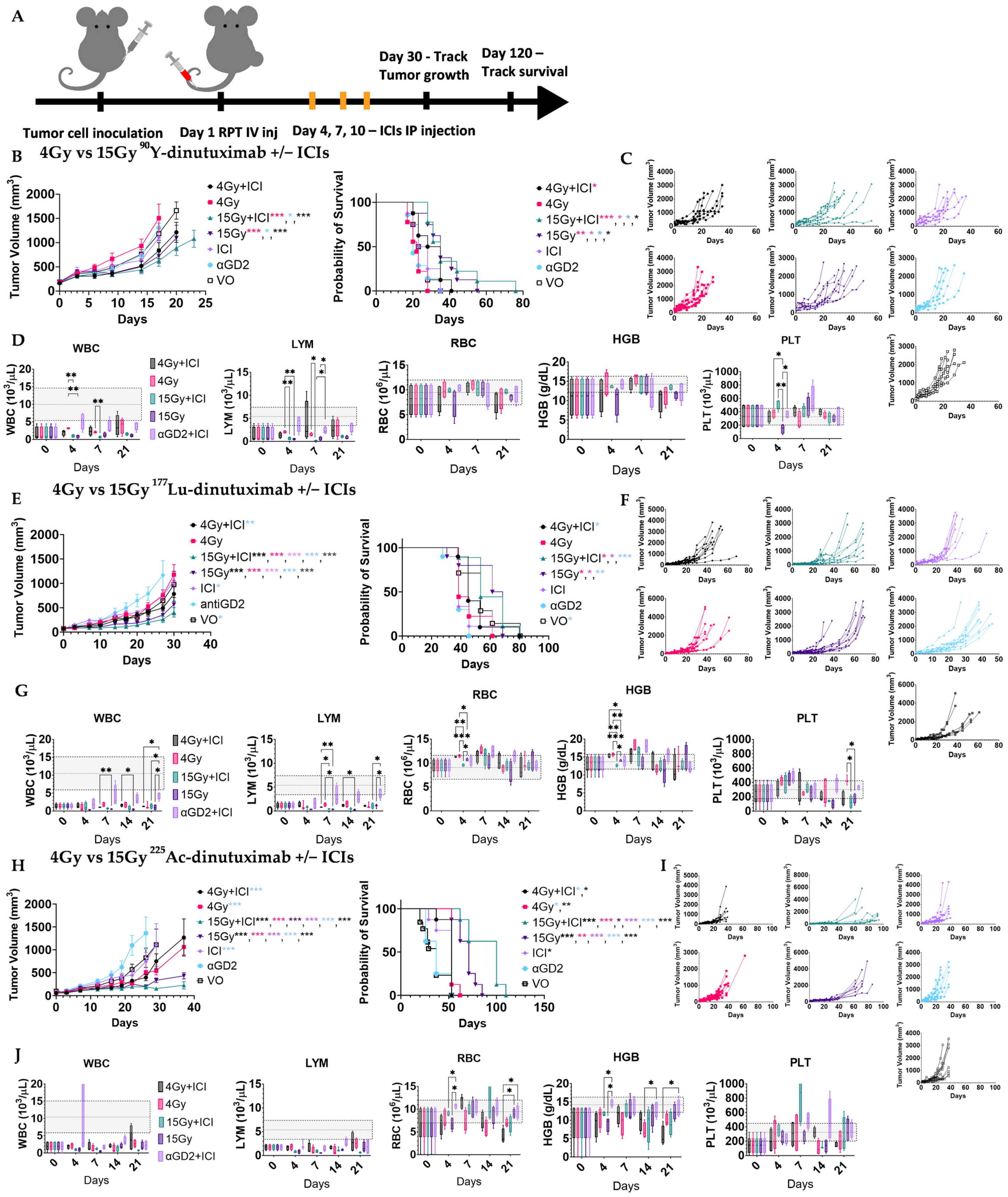
2.2.2. Therapy Studies
2.2.3. Toxicity and Immunoprofiling Studies
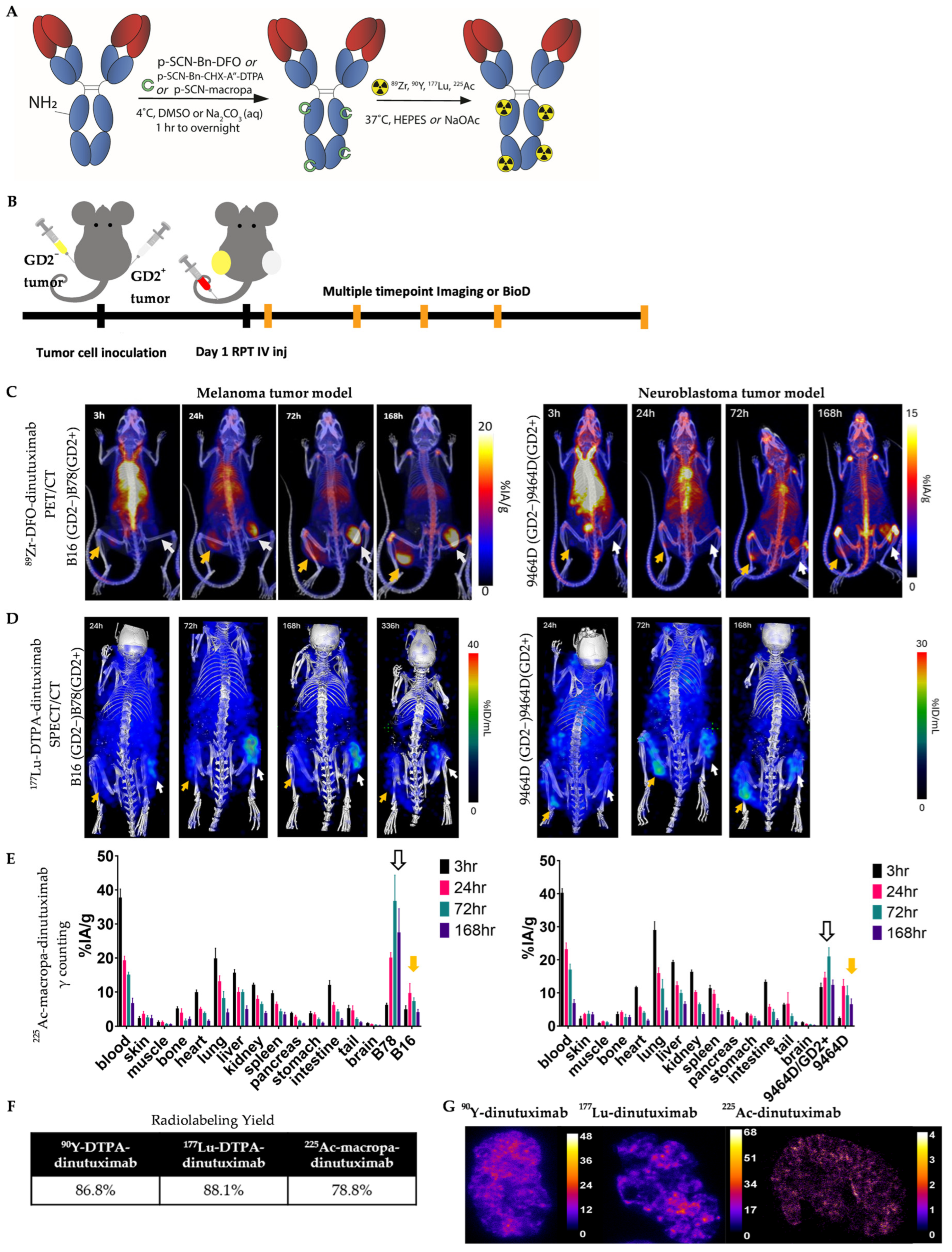
2.3. Radiopharmaceuticals
2.4. PET/CT Imaging
2.5. SPECT/CT Imaging
2.6. Alpha Camera Imaging
2.7. Ex Vivo Biodistribution
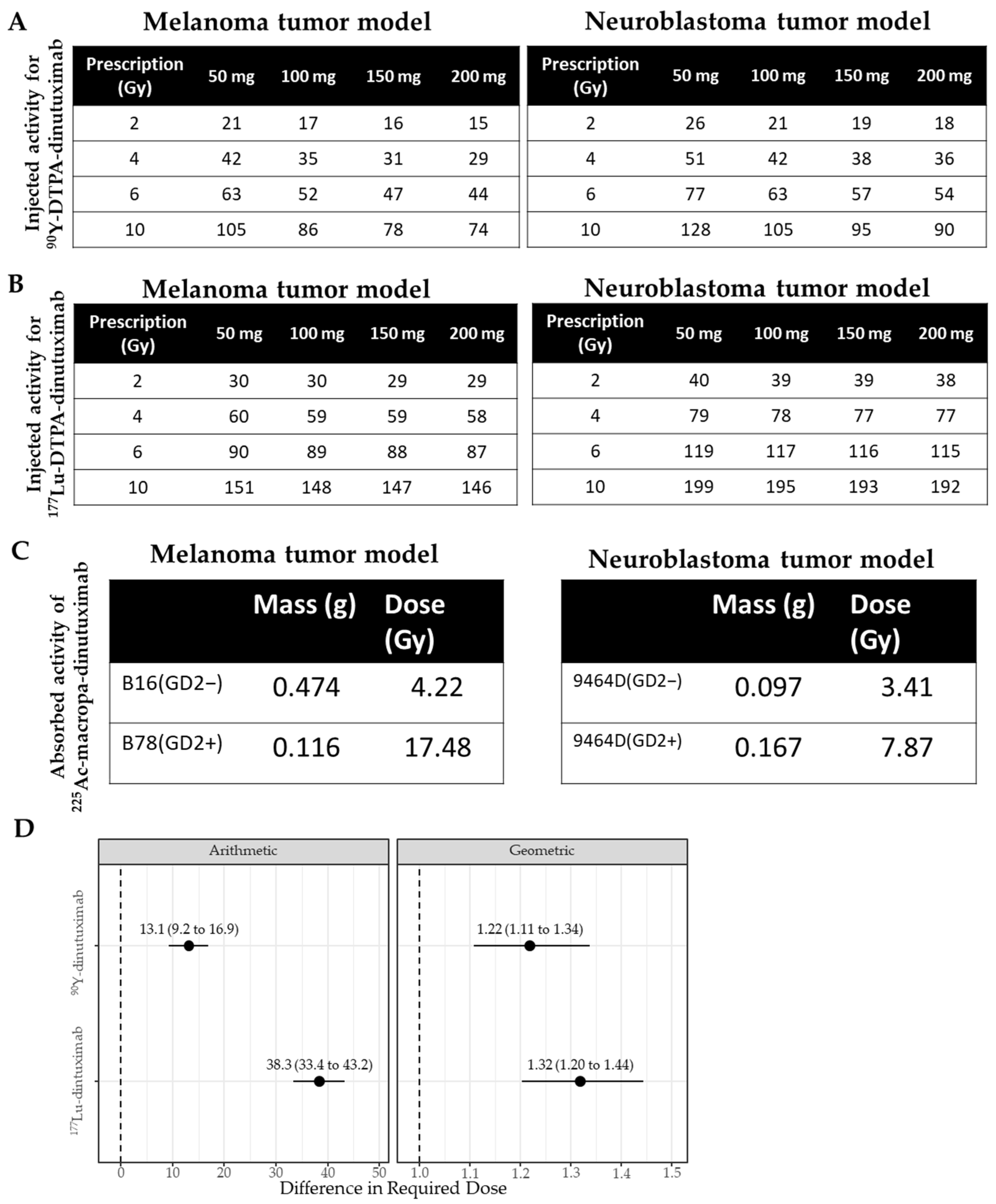
2.8. Dosimetry Calculations
2.9. Toxicity Assessments
2.10. Radiopharmaceutical Therapy (RPT)
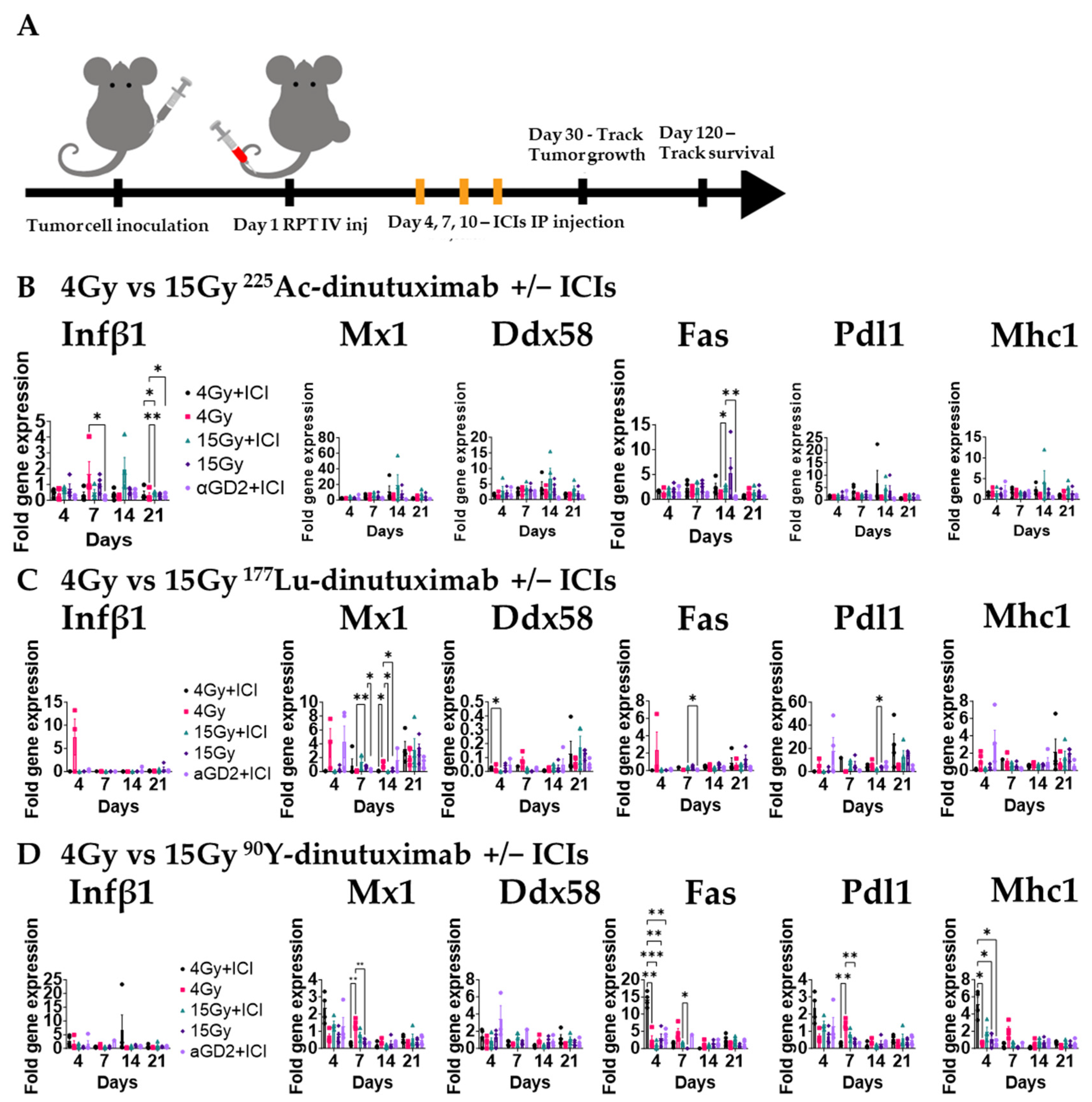
2.11. Gene Expression Analysis
2.12. Flow Cytometry
2.13. Statistical Analysis
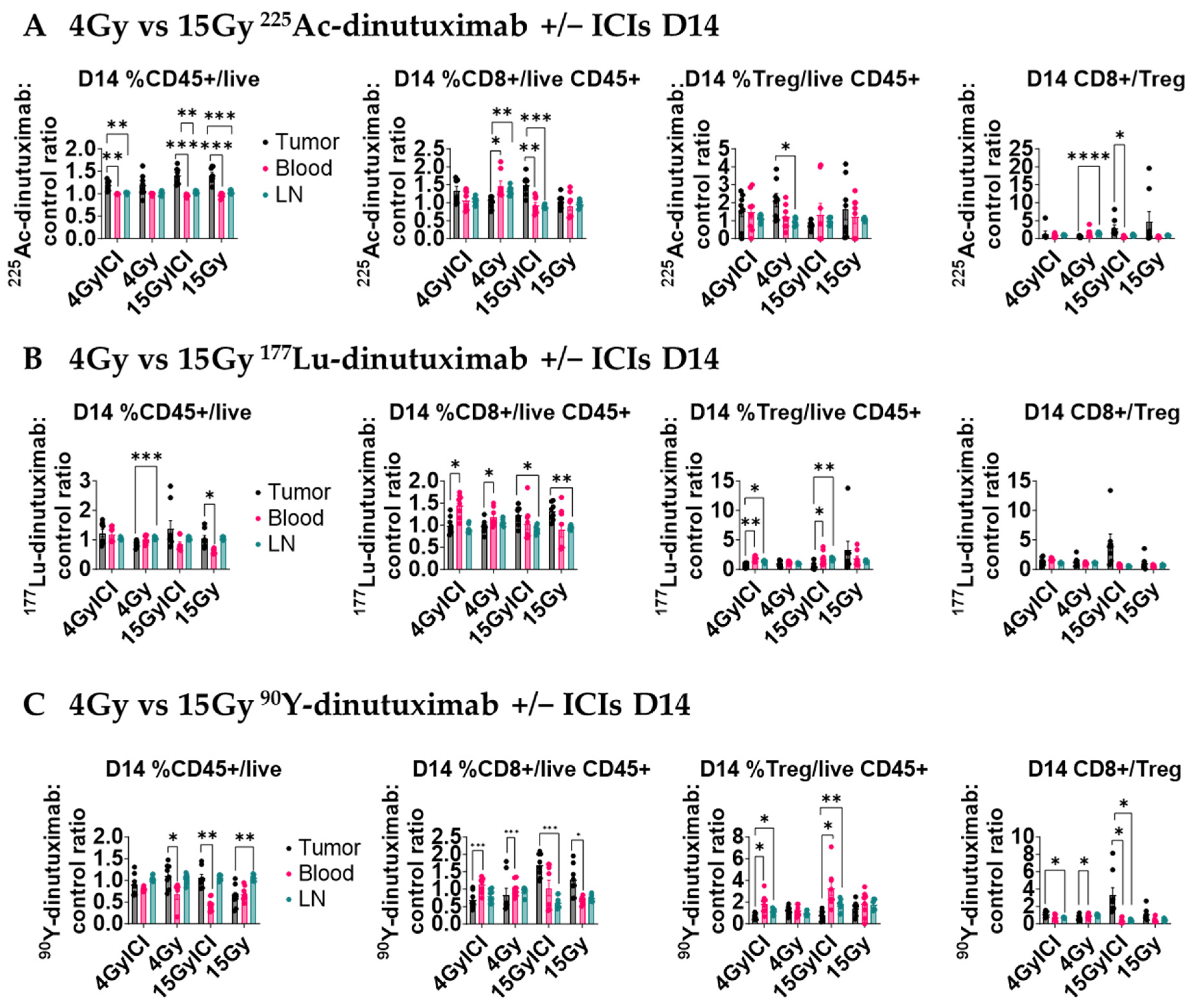
3. Results
3.1. Imaging, Uptake, and Distribution of 89Zr-DFO-, 177Lu-DTPA-, and 225Ac-Macropa-Dinutuximab
3.2. Dosimetry Highlights the Need for Absorbed Dose-Based RPT Evaluation
3.3. RPT and ICI Synergy Is Both Dose- and Radionuclide-Dependent
3.4. 225Ac-Dinutuximab Induces Sustained Type I Interferon Responses
3.5. 15 Gy 90Y- and 225Ac-Dinutuximab Enhance CD8+ T Cell Infiltration and CD8+/Treg Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 225Ac | Actinium-225 |
| 89Zr | Zirconium-89 |
| 90Y | Yttrium-90 |
| 177Lu | Lutetium-177 |
| Ab | Antibody |
| B78 | Murine melanoma cell line |
| CD4 | Cluster of Differentiation 4 (helper T cell marker) |
| CD8 | Cluster of Differentiation 8 (cytotoxic T cell marker) |
| CD11b | Cluster of Differentiation 11b (myeloid cell marker) |
| CT | Computed Tomography |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| DFO | Desferrioxamine |
| DTPA | Diethylenetriaminepentaacetic Acid |
| Fas | Tumor Necrosis Factor Receptor Superfamily Member 6 (apoptosis marker) |
| GD2 | Disialoganglioside 2 |
| Gy | Gray (unit of absorbed dose) |
| γH2AX | Phosphorylated Histone H2AX (marker of DNA damage) |
| ICI | Immune Checkpoint Inhibitor |
| IFN | Interferon |
| kBq | Kilobecquerel |
| MBq | Megabecquerel |
| MHC I | Major Histocompatibility Complex Class I |
| mAb | Monoclonal Antibody |
| MX1 | Myxovirus Resistance Protein 1 (Type I IFN response marker) |
| NK | Natural Killer (cell) |
| PBS | Phosphate-Buffered Saline |
| PD-L1 | Programmed Death-Ligand 1 |
| PET | Positron Emission Tomography |
| qPCR | Quantitative Polymerase Chain Reaction |
| RPT | Radiopharmaceutical Therapy |
| SPECT | Single Photon Emission Computed Tomography |
| TME | Tumor Microenvironment |
| Treg | Regulatory T Cell |
References
- Sgouros, G. Radiopharmaceutical Therapy. Health Phys. 2019, 116, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Decazes, P.; Bohn, P. Immunotherapy by Immune Checkpoint Inhibitors and Nuclear Medicine Imaging: Current and Future Applications. Cancers 2020, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Kleinendorst, S.C.; Oosterwijk, E.; Bussink, J.; Westdorp, H.; Konijnenberg, M.W.; Heskamp, S. Combining targeted radionuclide therapy and immune checkpoint inhibition for cancer treatment. Clin. Cancer Res. 2022, 28, 3652–3657. [Google Scholar] [CrossRef]
- Patel, R.B.; Hernandez, R.; Carlson, P.; Grudzinski, J.; Bates, A.M.; Jagodinsky, J.C.; Erbe, A.; Marsh, I.R.; Arthur, I.; Aluicio-Sarduy, E.; et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci. Transl. Med. 2021, 13, eabb3631. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Walker, K.L.; Grudzinski, J.J.; Aluicio-Sarduy, E.; Patel, R.; Zahm, C.D.; Pinchuk, A.N.; Massey, C.F.; Bitton, A.N.; Brown, R.J.; et al. Y-NM600 targeted radionuclide therapy induces immunologic memory in syngeneic models of T-cell Non-Hodgkin’s Lymphoma. Commun. Biol. 2019, 2, 79. [Google Scholar] [CrossRef]
- Hernandez, R.; Grudzinski, J.J.; Aluicio-Sarduy, E.; Massey, C.F.; Pinchuk, A.N.; Bitton, A.N.; Patel, R.; Zhang, R.; Rao, A.V.; Iyer, G.; et al. Lu-NM600 Targeted Radionuclide Therapy Extends Survival in Syngeneic Murine Models of Triple-Negative Breast Cancer. J. Nucl. Med. 2020, 61, 1187–1194. [Google Scholar] [CrossRef]
- Kerr, C.P.; Sheehan-Klenk, J.; Grudzinski, J.J.; Adam, D.P.; Nguyen, T.P.T.; Ferreira, C.A.; Bates, A.M.; Jin, W.J.; Kwon, O.; Olson, A.P.; et al. The effects of clinically relevant radionuclides on the activation of an ifn1 response correlate with radionuclide half-life and linear energy transfer and influence radiopharmaceutical antitumor efficacy. Cancer Immunol. Res. 2025, 13, 1190–1206. [Google Scholar] [CrossRef]
- Kerr, C.P.; Jin, W.J.; Liu, P.; Grudzinski, J.J.; Ferreira, C.A.; Rojas, H.C.; Oñate, A.J.; Kwon, O.; Hyun, M.; Idrissou, M.B.; et al. Priming versus propagating: Distinct immune effects of an alpha- versus beta-particle emitting radiopharmaceutical when combined with immune checkpoint inhibition. bioRxiv 2024. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sam, S.L.; Koshkin, V.; Small, E.; Feng, F.; Kouchkovsky, I.; Kwon, D.; Friedlander, T.; Borno, H.; Bose, R.; et al. Immunogenic priming with 177Lu-PSMA-617 plus pembrolizumab in metastatic castration resistant prostate cancer (mCRPC): A phase 1b study. J. Clin. Oncol. 2021, 39, 5053. [Google Scholar] [CrossRef]
- Sandhu, S.; Subramaniam, S.; Hofman, M.; Stockler, M.; Martin, A.; Pokorski, I.; Goh, J.; Pattison, D.; Dhiantravan, N.; Gedye, C.; et al. Evolution: Phase II study of radionuclide 177Lu-PSMA-617 therapy versus 177Lu-PSMA-617 in combination with ipilimumab and nivolumab for men with metastatic castration-resistant prostate cancer (mCRPC.; ANZUP 2001). J. Clin. Oncol. 2023, 41, TPS271. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Hetzheim, H.; Kratochwil, C.; Benesova, M.; Eder, M.; Neels, O.C.; Eisenhut, M.; Kübler, W.; Holland-Letz, T.; Giesel, F.L.; et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J. Nucl. Med. 2015, 56, 1697. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Theodorescu, D. Determinants of Resistance to Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 1594. [Google Scholar] [CrossRef] [PubMed]
- Taunk, N.K.; Escorcia, F.E.; Lewis, J.S.; Bodei, L. Radiopharmaceuticals for Cancer Diagnosis and Therapy: New Targets, New Therapies-Alpha-Emitters, Novel Targets. Cancer J. 2024, 30, 218–223. [Google Scholar] [CrossRef]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef]
- Machy, P.; Mortier, E.; Birklé, S. Biology of GD2 ganglioside: Implications for cancer immunotherapy. Front. Pharmacol. 2023, 14, 1249929. [Google Scholar] [CrossRef] [PubMed]
- Achbergerová, M.; Hederová, S.; Hrašková, A.; Kolenová, A. Dinutuximab beta in the treatment of high-risk neuroblastoma: A follow-up of a case series in Bratislava. Medicine 2022, 101, e28716. [Google Scholar] [CrossRef]
- Lee, C.M.; Tannock, I.F. The distribution of the therapeutic monoclonal antibodies cetuximab and trastuzumab within solid tumors. BMC Cancer 2010, 10, 255. [Google Scholar] [CrossRef]
- Kabiljo, J.; Harpain, F.; Carotta, S.; Bergmann, M. Radiotherapy as a Backbone for Novel Concepts in Cancer Immunotherapy. Cancers 2019, 12, 79. [Google Scholar] [CrossRef]
- Zhao, X.; Shao, C. Radiotherapy-Mediated Immunomodulation and Anti-Tumor Abscopal Effect Combining Immune Checkpoint Blockade. Cancers 2020, 12, 2762. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 104. [Google Scholar] [CrossRef]
- Kerr, C.P.; Grudzinski, J.J.; Nguyen, T.P.; Hernandez, R.; Weichert, J.P.; Morris, Z.S. Developments in Combining Targeted Radionuclide Therapies and Immunotherapies for Cancer Treatment. Pharmaceutics 2022, 15, 128. [Google Scholar] [CrossRef]
- Bellavia, M.C.; Patel, R.B.; Anderson, C.J. Combined Targeted Radiopharmaceutical Therapy and Immune Checkpoint Blockade: From Preclinical Advances to the Clinic. J. Nucl. Med. 2022, 63, 1636–1641. [Google Scholar] [CrossRef]
- Page, D.B.; Bear, H.; Prabhakaran, S.; Gatti-Mays, M.E.; Thomas, A.; Cobain, E.; McArthur, H.; Balko, J.M.; Gameiro, S.R.; Nanda, R.; et al. Two may be better than one: PD-1/PD-L1 blockade combination approaches in metastatic breast cancer. NPJ Breast Cancer 2019, 5, 34. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, L.; Xiao, Y.; Guo, X.; Hu, Y. Iodine-125 seed brachytherapy combined with pembrolizumab for advanced non-small-cell lung cancer after failure of first-line chemotherapy: A report of two cases and literature review. J. Contemp. Brachytherapy 2023, 15, 81–88. [Google Scholar] [CrossRef]
- Jagodinsky, J.C.; Vera, J.M.; Jin, W.J.; Shea, A.G.; Clark, P.A.; Sriramaneni, R.N.; Havighurst, T.C.; Chakravarthy, I.; Allawi, R.H.; Kim, K.; et al. Intratumoral radiation dose heterogeneity augments antitumor immunity in mice and primes responses to checkpoint blockade. Sci. Transl. Med. 2024, 16, eadk0642. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Barsoumian, H.; Verma, V.; Cortez, M.A.; Welsh, J.W. Low-Dose Radiation Decreases Cancer-Associated Fibroblasts and May Increase T-Cell Trafficking into Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e530–e531. [Google Scholar] [CrossRef]
- Haraguchi, M.; Yamashiro, S.; Yamamoto, A.; Furukawa, K.; Takamiya, K.; Lloyd, K.O.; Shiku, H. Isolation of GD3 synthase gene by expression cloning of GM3 alpha-2,8-sialyltransferase cDNA using anti-GD2 monoclonal antibody. Proc. Natl. Acad. Sci. USA 1994, 91, 10455–10459. [Google Scholar] [CrossRef]
- Rakhmilevich, A.L.; Imboden, M.; Hao, Z.; Macklin, M.D.; Roberts, T.; Wright, K.M.; Albertini, M.R.; Yang, N.S.; Sondel, P.M. Effective particle-mediated vaccination against mouse melanoma by coadministration of plasmid DNA encoding Gp100 and granulocyte-macrophage colony-stimulating factor. Clin. Cancer Res. 2001, 7, 952–961. [Google Scholar]
- Becker, J.C.; Varki, N.; Gillies, S.D.; Furukawa, K.; Reisfeld, R.A. An antibody-interleukin 2 fusion protein overcomes tumor heterogeneity by induction of a cellular immune response. Proc. Natl. Acad. Sci. USA 1996, 93, 7826–7831. [Google Scholar] [CrossRef]
- Aiken, T.J.; Komjathy, D.; Rodriguez, M.; Stuckwisch, A.; Feils, A.; Subbotin, V.; Birstler, J.; Gillies, S.D.; Rakhmilevich, A.L.; Erbe, A.K.; et al. Short-course neoadjuvant in situ vaccination for murine melanoma. J. Immunother. Cancer 2022, 10, e003586. [Google Scholar] [CrossRef] [PubMed]
- Voeller, J.; Erbe, A.K.; Slowinski, J.; Rasmussen, K.; Carlson, P.M.; Hoefges, A.; VandenHeuvel, S.; Stuckwisch, A.; Wang, X.; Gillies, S.D.; et al. Combined innate and adaptive immunotherapy overcomes resistance of immunologically cold syngeneic murine neuroblastoma to checkpoint inhibition. J. Immunother. Cancer 2019, 7, 344. [Google Scholar] [CrossRef] [PubMed]
- Norris, M.D.; Burkhart, C.A.; Marshall, G.M.; Weiss, W.A.; Haber, M. Expression of N-myc and MRP genes and their relationship to N-myc gene dosage and tumor formation in a murine neuroblastoma model. Med. Pediatr. Oncol. 2000, 35, 585–589. [Google Scholar] [CrossRef]
- Aiken, T.J.; Erbe, A.K.; Zebertavage, L.; Komjathy, D.; Feils, A.S.; Rodriguez, M.; Stuckwisch, A.; Gillies, S.D.; Morris, Z.S.; Birstler, J.; et al. Mechanism of effective combination radio-immunotherapy against 9464D-GD2, an immunologically cold murine neuroblastoma. J. Immunother. Cancer 2022, 10, e004834. [Google Scholar] [CrossRef]
- Hernandez, R.; Sun, H.; England, C.G.; Valdovinos, H.F.; Ehlerding, E.B.; Barnhart, T.E.; Yang, Y.; Cai, W. CD146-targeted immunoPET and NIRF Imaging of Hepatocellular Carcinoma with a Dual-Labeled Monoclonal Antibody. Theranostics 2016, 6, 1918–1933. [Google Scholar] [CrossRef]
- England, C.G.; Jiang, D.; Ehlerding, E.B.; Rekoske, B.T.; Ellison, P.A.; Hernandez, R.; Barnhart, T.E.; McNeel, D.G.; Huang, P.; Cai, W. 89Zr-labeled nivolumab for imaging of T-cell infiltration in a humanized murine model of lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 110–120. [Google Scholar] [CrossRef] [PubMed]
- England, C.G.; Ehlerding, E.B.; Hernandez, R.; Rekoske, B.T.; Graves, S.A.; Sun, H.; Liu, G.; McNeel, D.G.; Barnhart, T.E.; Cai, W. Preclinical Pharmacokinetics and Biodistribution Studies of 89Zr-Labeled Pembrolizumab. J. Nucl. Med. 2017, 58, 162–168. [Google Scholar] [CrossRef]
- Bobba, K.N.; Bidkar, A.P.; Meher, N.; Fong, C.; Wadhwa, A.; Dhrona, S.; Sorlin, A.; Bidlingmaier, S.; Shuere, B.; He, J.; et al. Evaluation of 134Ce/134La as a PET Imaging Theranostic Pair for 225Ac α-Radiotherapeutics. J. Nucl. Med. 2023, 64, 1076–1082. [Google Scholar] [CrossRef]
- Wichmann, C.W.; Morgan, K.A.; Cao, Z.; Osellame, L.D.; Guo, N.; Gan, H.; Reilly, E.; Burvenich, I.J.G.; O’Keefe, G.J.; Donnelly, P.S.; et al. Radiolabeling and Preclinical Evaluation of Therapeutic Efficacy of (225)Ac-ch806 in Glioblastoma and Colorectal Cancer Xenograft Models. J. Nucl. Med. 2024, 65, 1456–1462. [Google Scholar] [CrossRef]
- Schatz, C.A.; Zitzmann-Kolbe, S.; Moen, I.; Klotz, M.; Nair, S.; Stargard, S.; Bjerke, R.M.; Wickstrøm Biseth, K.; Feng, Y.Z.; Indrevoll, B.; et al. Preclinical Efficacy of a PSMA-Targeted Actinium-225 Conjugate (225Ac-Macropa-Pelgifatamab): A Targeted Alpha Therapy for Prostate Cancer. Clin. Cancer Res. 2024, 30, 2531–2544. [Google Scholar] [CrossRef]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 14712–14717. [Google Scholar] [CrossRef]
- Wurzer, A.; Sun, B.; Saleh, S.; Brosch-Lenz, J.; Fischer, S.; Kossatz, S.; Hürkamp, K.; Li, W.B.; Eiber, M.; Morgenstern, A.; et al. [225Ac]Ac-PSMA I&T: A Preclinical Investigation on the Fate of Decay Nuclides and Their Influence on Dosimetry of Salivary Glands and Kidneys. J. Nucl. Med. 2025, 66, 1964–1969. [Google Scholar]
- Merkx, R.I.J.; Rijpkema, M.; Franssen, G.M.; Kip, A.; Smeets, B.; Morgenstern, A.; Bruchertseifer, F.; Yan, E.; Wheatcroft, M.P.; Oosterwijk, E.; et al. Carbonic Anhydrase IX-Targeted α-Radionuclide Therapy with 225Ac Inhibits Tumor Growth in a Renal Cell Carcinoma Model. Pharmaceuticals 2022, 15, 570. [Google Scholar] [CrossRef]
- Besemer, A.E.; Yang, Y.M.; Grudzinski, J.J.; Hall, L.T.; Bednarz, B.P. Development and Validation of RAPID: A Patient-Specific Monte Carlo Three-Dimensional Internal Dosimetry Platform. Cancer Biother. Radiopharm. 2018, 33, 155–165. [Google Scholar] [CrossRef]
- Bednarz, B.; Grudzinski, J.; Marsh, I.; Besemer, A.; Baiu, D.; Weichert, J.; Otto, M. Murine-specific Internal Dosimetry for Preclinical Investigations of Imaging and Therapeutic Agents. Health Phys. 2018, 114, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.J.; Erbe, A.K.; Schwarz, C.N.; Jaquish, A.A.; Anderson, B.R.; Sriramaneni, R.N.; Jagodinsky, J.C.; Bates, A.M.; Clark, P.A.; Le, T.; et al. Tumor-Specific Antibody, Cetuximab, Enhances the In Situ Vaccine Effect of Radiation in Immunologically Cold Head and Neck Squamous Cell Carcinoma. Front. Immunol. 2020, 11, 591139. [Google Scholar] [CrossRef]
- Verel, I.; Visser, G.W.; Boellaard, R.; Boerman, O.C.; van Eerd, J.; Snow, G.B.; Lammertsma, A.A.; van Dongen, G.A. Quantitative 89Zr immuno-PET for in vivo scouting of 90Y-labeled monoclonal antibodies in xenograft-bearing nude mice. J. Nucl. Med. 2003, 44, 1663–1670. [Google Scholar]
- Zeglis, B.; Lewis, J. The bioconjugation and radiosynthesis of 89Zr-DFO-labeled antibodies. J. Vis. Exp. JoVE 2015, 96, 52521. [Google Scholar]
- Rösch, F.; Herzog, H.; Qaim, S. The Beginning and Development of the Theranostic Approach in Nuclear Medicine, as Exemplified by the Radionuclide Pair 86Y and 90Y. Pharmaceuticals 2017, 10, 56. [Google Scholar] [CrossRef]
- Sgouros, G. Dosimetry, Radiobiology and Synthetic Lethality: Radiopharmaceutical Therapy (RPT) With Alpha-Particle-Emitters. Semin. Nucl. Med. 2020, 50, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.A.; Hobbs, R.F. Dosimetry for Optimized, Personalized Radiopharmaceutical Therapy. Semin. Radiat. Oncol. 2021, 31, 37–44. [Google Scholar] [CrossRef]
- O’Donoghue, J.; Zanzonico, P.; Humm, J.; Kesner, A. Dosimetry in Radiopharmaceutical Therapy. J. Nucl. Med. 2022, 63, 1467–1474. [Google Scholar] [CrossRef]
- St James, S.; Bednarz, B.; Benedict, S.; Buchsbaum, J.C.; Dewaraja, Y.; Frey, E.; Hobbs, R.; Grudzinski, J.; Roncali, E.; Sgouros, G.; et al. Current Status of Radiopharmaceutical Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 891–901. [Google Scholar] [CrossRef]
- Lim, J.Y.; Gerber, S.A.; Murphy, S.P.; Lord, E.M. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol. Immunother. 2014, 63, 259–271. [Google Scholar] [CrossRef]
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.X.; Auh, S.L. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011, 71, 2488–2496. [Google Scholar] [CrossRef]
- Takashima, M.E.; Berg, T.J.; Morris, Z.S. The Effects of Radiation Dose Heterogeneity on the Tumor Microenvironment and Anti-Tumor Immunity. Semin. Radiat. Oncol. 2024, 34, 262–271. [Google Scholar] [CrossRef]
- Jagodinsky, J.C.; Jin, W.J.; Bates, A.M.; Hernandez, R.; Grudzinski, J.J.; Marsh, I.R.; Chakravarty, I.; Arthur, I.S.; Zangl, L.M.; Brown, R.J.; et al. Temporal analysis of type 1 interferon activation in tumor cells following external beam radiotherapy or targeted radionuclide therapy. Theranostics 2021, 11, 6120–6137. [Google Scholar] [CrossRef] [PubMed]
- Danforth, J.M.; Provencher, L.; Goodarzi, A.A. Chromatin and the Cellular Response to Particle Radiation-Induced Oxidative and Clustered DNA Damage. Front. Cell Dev. Biol. 2022, 10, 910440. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Jakobsson, V.; Greifenstein, L.; Khong, P.-L.; Chen, X.; Baum, R.P.; Zhang, J. Alpha-peptide receptor radionuclide therapy using actinium-225 labeled somatostatin receptor agonists and antagonists. Front. Med. 2022, 9, 1034315. [Google Scholar] [CrossRef]
- Uccelli, L.; Boschi, A.; Cittanti, C.; Martini, P.; Panareo, S.; Tonini, E.; Nieri, A.; Urso, L.; Caracciolo, M.; Lodi, L.; et al. 90Y/177Lu-DOTATOC: From Preclinical Studies to Application in Humans. Pharmaceutics 2021, 13, 1463. [Google Scholar] [CrossRef]
- Chew, V.; Lee, Y.H.; Pan, L.; Nasir, N.J.M.; Lim, C.J.; Chua, C.; Lai, L.; Hazirah, S.N.; Lim, T.K.H.; Goh, B.K.P.; et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019, 68, 335–346. [Google Scholar] [CrossRef]
- Hobbs, R.F.; Wahl, R.L.; Frey, E.C.; Kasamon, Y.; Song, H.; Huang, P.; Jones, R.J.; Sgouros, G. Radiobiologic optimization of combination radiopharmaceutical therapy applied to myeloablative treatment of non-Hodgkin lymphoma. J. Nucl. Med. 2013, 54, 1535–1542. [Google Scholar] [CrossRef]
- Ljungberg, M.; Celler, A.; Konijnenberg, M.W.; Eckerman, K.F.; Dewaraja, Y.K.; Sjögreen-Gleisner, K.; Bolch, W.E.; Brill, A.B.; Fahey, F.; Fisher, D.R.; et al. MIRD Pamphlet No. 26: Joint EANM/MIRD Guidelines for Quantitative 177Lu SPECT Applied for Dosimetry of Radiopharmaceutical Therapy. J. Nucl. Med. 2016, 57, 151–162. [Google Scholar]
- Wege, A.K. Humanized Mouse Models for the Preclinical Assessment of Cancer Immunotherapy. BioDrugs 2018, 32, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Shea, A.G.; Idrissou, M.B.; Torres, A.I.; Chen, T.; Hernandez, R.; Morris, Z.S.; Sodji, Q.H. Immunological effects of radiopharmaceutical therapy. Front. Nucl. Med. 2024, 4, 1331364. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Roncali, E.; Hobbs, R.; James, S.S.; Bednarz, B.; Benedict, S.; Dewaraja, Y.K.; Frey, E.; Grudzinski, J.; Sgouros, G.; et al. Toward Individualized Voxel-Level Dosimetry for Radiopharmaceutical Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 902–904. [Google Scholar] [CrossRef] [PubMed]
| RPT | Tumor Absorbed Dose Prescription (Gy) | Injected Activity |
|---|---|---|
| 90Y-dinutuximab | 4 | 1.369 MBq |
| 90Y-dinutuximab | 15 | 5.217 MBq |
| 177Lu-dinutuximab | 4 | 2.22 MBq |
| 177Lu-dinutuximab | 15 | 8.251 MBq |
| 225Ac-dinutuximab | 4 | 1.702 kBq |
| 225Ac-dinutuximab | 15 | 6.29 kBq |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lilieholm, C.; Zaborek, J.; Kwon, O.; Adeniyi, A.O.; Kerr, C.P.; Comas Rojas, H.; Bio Idrissou, M.; Ferreira, C.A.; Clark, P.A.; Jin, W.J.; et al. Radionuclide-Dependent Stimulation of Antitumor Immunity in GD2-Targeted Radiopharmaceutical Therapy Combined with Immune Checkpoint Inhibitors. Radiation 2025, 5, 39. https://doi.org/10.3390/radiation5040039
Lilieholm C, Zaborek J, Kwon O, Adeniyi AO, Kerr CP, Comas Rojas H, Bio Idrissou M, Ferreira CA, Clark PA, Jin WJ, et al. Radionuclide-Dependent Stimulation of Antitumor Immunity in GD2-Targeted Radiopharmaceutical Therapy Combined with Immune Checkpoint Inhibitors. Radiation. 2025; 5(4):39. https://doi.org/10.3390/radiation5040039
Chicago/Turabian StyleLilieholm, Cynthia, Jen Zaborek, Ohyun Kwon, Adedamola O. Adeniyi, Caroline P. Kerr, Hansel Comas Rojas, Malick Bio Idrissou, Carolina A. Ferreira, Paul A. Clark, Won Jong Jin, and et al. 2025. "Radionuclide-Dependent Stimulation of Antitumor Immunity in GD2-Targeted Radiopharmaceutical Therapy Combined with Immune Checkpoint Inhibitors" Radiation 5, no. 4: 39. https://doi.org/10.3390/radiation5040039
APA StyleLilieholm, C., Zaborek, J., Kwon, O., Adeniyi, A. O., Kerr, C. P., Comas Rojas, H., Bio Idrissou, M., Ferreira, C. A., Clark, P. A., Jin, W. J., Grudzinski, J. J., Erbe, A. K., Aluicio-Sarduy, E., Kanagasundaram, T., Wilson, J. J., Engle, J. W., Hernandez, R., Bednarz, B., Morris, Z. S., & Weichert, J. P. (2025). Radionuclide-Dependent Stimulation of Antitumor Immunity in GD2-Targeted Radiopharmaceutical Therapy Combined with Immune Checkpoint Inhibitors. Radiation, 5(4), 39. https://doi.org/10.3390/radiation5040039








