Whole Transcriptome Analysis of the Mouse Placenta Following Radiation-Induced Growth Restriction
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Animals and Irradiation Treatment
2.2. Genotyping
2.3. RNA Extraction and cDNA Synthesis
2.4. Transcriptomic Profiling and Secondary Analyses
2.5. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Statistical Analysis
3. Results
3.1. Conceptus and Placental Weight
3.2. Whole Transcriptome Analysis and Differentially Expressed Genes
3.3. RT-qPCR Validation
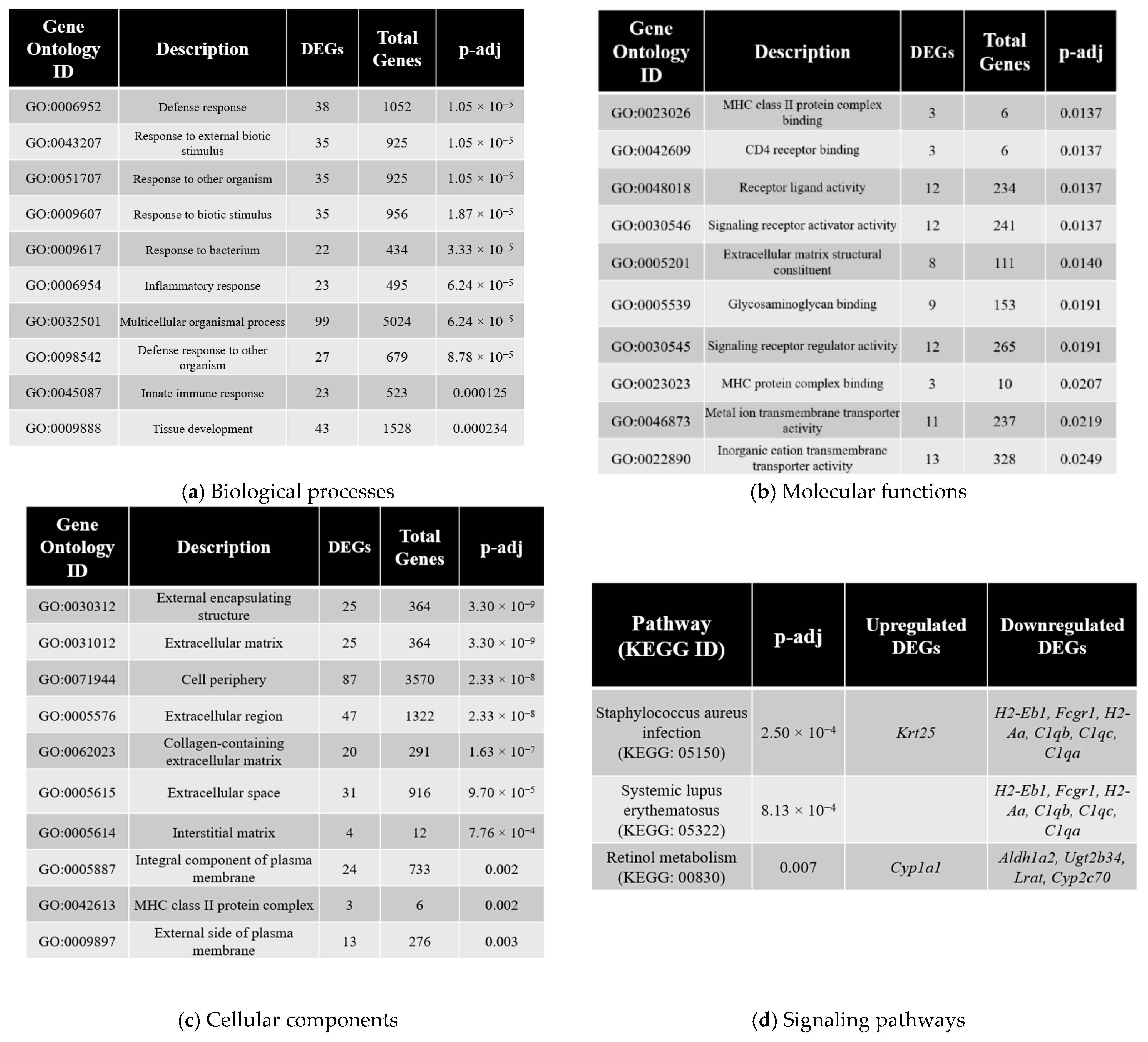
3.4. Gene Ontology Analysis
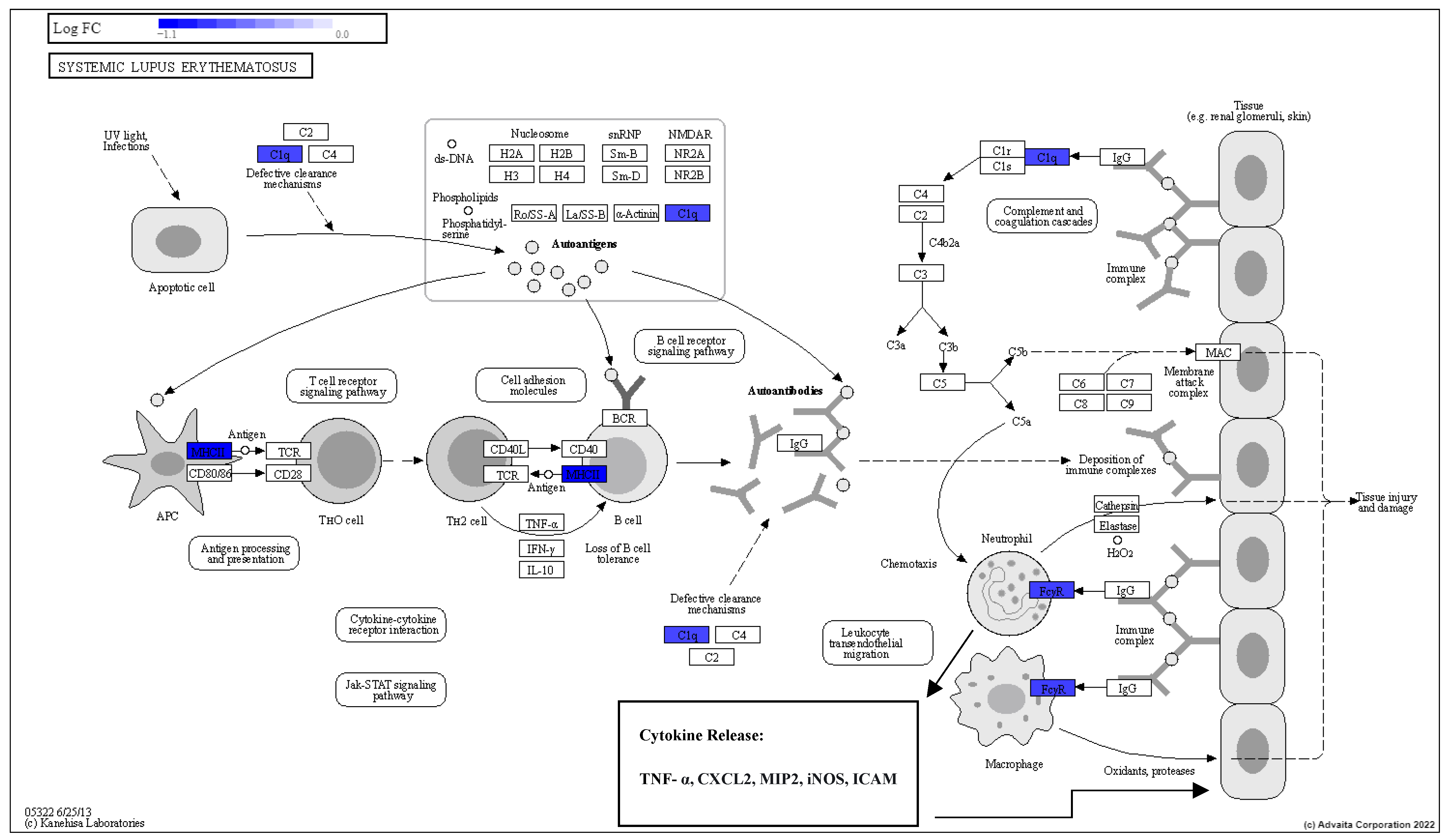
3.5. Pathway Enrichment Analysis
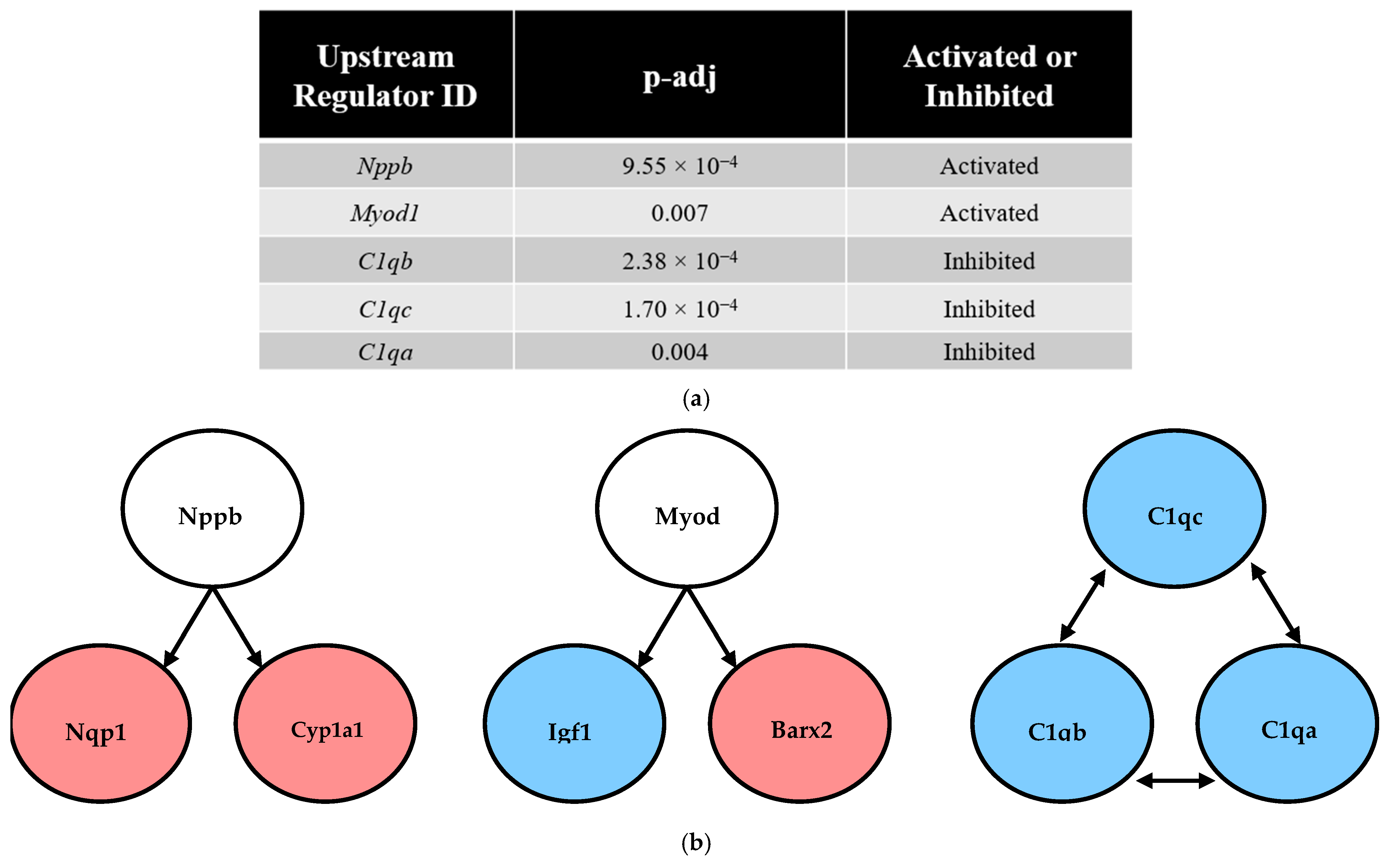
3.6. Predicted Upstream Regulator Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scifres, C.M.; Nelson, D.M. Intrauterine growth restriction, human placental development and trophoblast cell death: IGR, placental development and trophoblast cell death. J. Physiol. 2009, 587, 3453–3458. [Google Scholar] [CrossRef]
- Bernstein, I.M.; Horbar, J.D.; Badger, G.J.; Ohlsson, A.; Golan, A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. Am. J. Obstet. Gynecol. 2000, 182, 198–206. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal origins of adult hypertension. J. Hypertension. Suppl. Off. J. Int. Soc. Hypertens. 1992, 10, S39–S44. [Google Scholar]
- Barker, D.J.; Bagby, S.P.; Hanson, M.A. Mechanisms of Disease: In utero programming in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2006, 2, 700–707. [Google Scholar] [CrossRef]
- Lee, H.-S. Impact of Maternal Diet on the Epigenome during In Utero Life and the Developmental Programming of Diseases in Childhood and Adulthood. Nutrients 2015, 7, 9492–9507. [Google Scholar] [CrossRef]
- Seckl, J.R. Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Mol. Cell. Endocrinol. 2001, 185, 61–71. [Google Scholar] [CrossRef]
- Fajersztajn, L.; Veras, M.M. Hypoxia: From Placental Development to Fetal Programming: Influence of Hypoxia on Development. Birth Defects Res. 2017, 109, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Maritz, G.S.; Harding, R. Life-long Programming Implications of Exposure to Tobacco Smoking and Nicotine Before and Soon After Birth: Evidence for Altered Lung Development. Int. J. Environ. Res. Public Health 2011, 8, 875–898. [Google Scholar] [CrossRef] [PubMed]
- Frangione, B.; Hinton, P.; Villeneuve, P.J. Low-dose ionizing radiation and adverse birth outcomes: A systematic review and meta-analysis. Int. Arch. Occup. Environ. Health 2023, 96, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Misumi, M.; Sakata, R.; Brenner, A.V.; Utada, M.; Ozasa, K. Mortality among individuals exposed to atomic bomb radiation in utero: 1950–2012. Eur. J. Epidemiol. 2021, 36, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef]
- Watson, E.D.; Cross, J.C. Development of Structures and Transport Functions in the Mouse Placenta. Physiology 2005, 20, 180–193. [Google Scholar] [CrossRef]
- Chaddha, V.; Viero, S.; Huppertz, B.; Kingdom, J. Developmental biology of the placenta and the origins of placental insufficiency. Semin. Fetal Neonatal Med. 2004, 9, 357–369. [Google Scholar] [CrossRef]
- StarrLab. DNA Extraction from Tail Clips. Available online: https://sites.google.com/a/umn.edu/starrlab/protocols/dna/dna-extraction-from-tail-clips/ (accessed on 24 October 2022).
- The Jackson Laboratory. DNA Isolation Protocols. Available online: https://www.jax.org/jax-mice-and-services/customer-support/technical-support/genotyping-resources/dna-isolation-protocols/ (accessed on 24 October 2022).
- Clapcote, S.J.; Roder, J.C. Simplex PCR assay for sex determination in mice. BioTechniques 2005, 38, 702–706. [Google Scholar] [CrossRef]
- Pirkkanen, J.; Tharmalingam, S.; Morais, I.H.; Lam-Sidun, D.; Thome, C.; Zarnke, A.M.; Benjamin, L.V.; Losch, A.C.; Borgmann, A.J.; Sinex, H.C.; et al. Transcriptomic profiling of gamma ray induced mutants from the CGL1 human hybrid cell system reveals novel insights into the mechanisms of radiation-induced carcinogenesis. Free Radic. Biol. Med. 2019, 145, 300–311. [Google Scholar] [CrossRef]
- Tharmalingam, S.; Khurana, S.; Murray, A.; Lamothe, J.; Tai, T.C. Whole transcriptome analysis of adrenal glands from prenatal glucocorticoid programmed hypertensive rodents. Sci. Rep. 2020, 10, 18755. [Google Scholar] [CrossRef]
- Al-khayyat, W.; Pirkkanen, J.; Dougherty, J.; Laframboise, T.; Dickinson, N.; Khaper, N.; Lees, S.J.; Mendonca, M.S.; Boreham, D.R.; Tai, T.C.; et al. Overexpression of FRA1 (FOSL1) Leads to Global Transcriptional Perturbations, Reduced Cellular Adhesion and Altered Cell Cycle Progression. Cells 2023, 12, 2344. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Solano, M.E.; Thiele, K.; Kowal, M.K.; Arck, P.C. Identification of suitable reference genes in the mouse placenta. Placenta 2016, 39, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Draghici, S.; Khatri, P.; Tarca, A.L.; Amin, K.; Done, A.; Voichita, C.; Georgescu, C.; Romero, R. A systems biology approach for pathway level analysis. Genome Res. 2007, 17, 1537–1545. [Google Scholar] [CrossRef]
- Kristal, M.B.; DiPirro, J.M.; Thompson, A.C. Placentophagia in Humans and Nonhuman Mammals: Causes and Consequences. Ecol. Food Nutr. 2012, 51, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Kanter, D.J.; O’Brien, M.B.; Shi, X.-H.; Chu, T.; Mishima, T.; Beriwal, S.; Epperly, M.W.; Wipf, P.; Greenberger, J.S.; Sadovsky, Y. The impact of ionizing radiation on placental trophoblasts. Placenta 2014, 35, 85–91. [Google Scholar] [CrossRef]
- Rao, M.S.; Van Vleet, T.R.; Ciurlionis, R.; Buck, W.R.; Mittelstadt, S.W.; Blomme, E.A.G.; Liguori, M.J. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver From Short-Term Rat Toxicity Studies. Front. Genet. 2019, 9, 636. [Google Scholar] [CrossRef]
- Seyyed Mousavi, M.N.; Mehramuz, B.; Sadeghi, J.; Alizadeh, N.; Oskouee, M.A.; Kafil, H.S. The pathogenesis of Staphylococcus aureus in autoimmune diseases. Microb. Pathog. 2017, 111, 503–507. [Google Scholar] [CrossRef]
- Liu, Q.; Mazhar, M.; Miller, L.S. Immune and Inflammatory Reponses to Staphylococcus aureus Skin Infections. Curr. Dermatol. Rep. 2018, 7, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.M.; Lane, A.T.; Barnett, N.K.; Bias, W.B.; Arnett, F.C.; Provost, T.T. Neonatal lupus erythematosus. A clinical, serological and immunogenetic study with review of the literature. Medicine 1984, 63, 362–378. [Google Scholar] [CrossRef]
- Wei, S.; Lai, K.; Yang, Z.; Zeng, K. Systemic lupus erythematosus and risk of preterm birth: A systematic review and meta-analysis of observational studies. Lupus 2017, 26, 563–571. [Google Scholar] [CrossRef]
- Guillotin, V.; Bouhet, A.; Barnetche, T.; Richez, C.; Truchetet, M.-E.; Seneschal, J.; Duffau, P.; Lazaro, E. Hydroxychloroquine for the prevention of fetal growth restriction and prematurity in lupus pregnancy: A systematic review and meta-analysis. Jt. Bone Spine 2018, 85, 663–668. [Google Scholar] [CrossRef]
- Blomhoff, R.; Blomhoff, H.K. Overview of retinoid metabolism and function. J. Neurobiol. 2006, 66, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Wassef, L.; Hamberger, L.; Piantedosi, R.; Palczewski, K.; Blaner, W.S.; Quadro, L. Retinyl Ester Formation by Lecithin:Retinol Acyltransferase Is a Key Regulator of Retinoid Homeostasis in Mouse Embryogenesis. J. Biol. Chem. 2008, 283, 5611–5621. [Google Scholar] [CrossRef]
- Marceau, G.; Gallot, D.; Lemery, D.; Sapin, V. Metabolism of Retinol During Mammalian Placental and Embryonic Development. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2007; pp. 97–115. [Google Scholar]
- Balmer, J.E.; Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002, 43, 1773–1808. [Google Scholar] [CrossRef]
- Huang, L.-T.; Chou, H.-C.; Lin, C.-M.; Chen, C.-M. Uteroplacental Insufficiency Alters the Retinoid Pathway and Lung Development in Newborn Rats. Pediatr. Neonatol. 2016, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; Truscott, T. Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids—A Review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef]
- Fernandes-Silva, H.; Araújo-Silva, H.; Correia-Pinto, J.; Moura, R.S. Retinoic Acid: A Key Regulator of Lung Development. Biomolecules 2020, 10, 152. [Google Scholar] [CrossRef]
- Pasanen, M. The expression and regulation of drug metabolism in human placenta. Adv. Drug Deliv. Rev. 1999, 38, 81–97. [Google Scholar] [CrossRef]
- Suter, M.; Abramovici, A.; Showalter, L.; Hu, M.; Shope, C.D.; Varner, M.; Aagaard-Tillery, K. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism 2010, 59, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.G.; Gyselaers, W.; Byun, H.-M.; Roels, H.A.; Cuypers, A.; Baccarelli, A.A.; Nawrot, T.S. Placental mitochondrial DNA and CYP1A1 gene methylation as molecular signatures for tobacco smoke exposure in pregnant women and the relevance for birth weight. J. Transl. Med. 2017, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, E.; Kim, Y.-K.; Wassef, L.; Shete, V.; Quadro, L. Maternal–fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 88–98. [Google Scholar] [CrossRef]
- Laviola, L.; Perrini, S.; Belsanti, G.; Natalicchio, A.; Montrone, C.; Leonardini, A.; Vimercati, A.; Scioscia, M.; Selvaggi, L.; Giorgino, R.; et al. Intrauterine Growth Restriction in Humans Is Associated with Abnormalities in Placental Insulin-Like Growth Factor Signaling. Endocrinology 2005, 146, 1498–1505. [Google Scholar] [CrossRef]
- Setia, S.; Sridhar, M.G. Changes in GH/IGF-1 Axis in Intrauterine Growth Retardation: Consequences of Fetal Programming? Horm. Metab. Res. 2009, 41, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Nava, F.; Lanes, R. GH/IGF-1 Signaling and Current Knowledge of Epigenetics; a Review and Considerations on Possible Therapeutic Options. Int. J. Mol. Sci. 2017, 18, 1624. [Google Scholar] [CrossRef]
- Regal, J.F.; Burwick, R.M.; Fleming, S.D. The Complement System and Preeclampsia. Curr. Hypertens. Rep. 2017, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Pierik, E.; Prins, J.R.; van Goor, H.; Dekker, G.A.; Daha, M.R.; Seelen, M.A.J.; Scherjon, S.A. Dysregulation of Complement Activation and Placental Dysfunction: A Potential Target to Treat Preeclampsia? Front. Immunol. 2020, 10, 3098. [Google Scholar] [CrossRef]
- Agostinis, C.; Bulla, R.; Tripodo, C.; Gismondi, A.; Stabile, H.; Bossi, F.; Guarnotta, C.; Garlanda, C.; De Seta, F.; Spessotto, P.; et al. An Alternative Role of C1q in Cell Migration and Tissue Remodeling: Contribution to Trophoblast Invasion and Placental Development. J. Immunol. 2010, 185, 4420–4429. [Google Scholar] [CrossRef] [PubMed]
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef]
- Pennington, K.A.; Harper, J.L.; Sigafoos, A.N.; Beffa, L.M.; Carleton, S.M.; Phillips, C.L.; Schulz, L.C. Effect of Food Restriction and Leptin Supplementation on Fetal Programming in Mice. Endocrinology 2012, 153, 4556–4567. [Google Scholar] [CrossRef]
- Tarrade, A.; Panchenko, P.; Junien, C.; Gabory, A. Placental contribution to nutritional programming of health and diseases: Epigenetics and sexual dimorphism. J. Exp. Biol. 2015, 218, 50–58. [Google Scholar] [CrossRef]
- Kalisch-Smith, J.I.; Simmons, D.G.; Dickinson, H.; Moritz, K.M. Review: Sexual dimorphism in the formation, function and adaptation of the placenta. Placenta 2017, 54, 10–16. [Google Scholar] [CrossRef]
- Hoch, D.; Novakovic, B.; Cvitic, S.; Saffery, R.; Desoye, G.; Majali-Martinez, A. Sex matters: XIST and DDX3Y gene expression as a tool to determine fetal sex in human first trimester placenta. Placenta 2020, 97, 68–70. [Google Scholar] [CrossRef]
- Ontsouka, E.; Lüthi, M.; Zaugg, J.; Schroeder, M.; Albrecht, C. Establishment and validation of an approach allowing unequivocal fetal sex determination based on placental sex-specific genes. Placenta 2021, 112, 132–134. [Google Scholar] [CrossRef]
- Steinhauser, C.B.; Lambo, C.A.; Askelson, K.; Burns, G.W.; Behura, S.K.; Spencer, T.E.; Bazer, F.W.; Satterfield, M.C. Placental Transcriptome Adaptations to Maternal Nutrient Restriction in Sheep. Int. J. Mol. Sci. 2021, 22, 7654. [Google Scholar] [CrossRef]
- Gallou-Kabani, C.; Gabory, A.; Tost, J.; Karimi, M.; Mayeur, S.; Lesage, J.; Boudadi, E.; Gross, M.-S.; Taurelle, J.; Vigé, A.; et al. Sex- and Diet-Specific Changes of Imprinted Gene Expression and DNA Methylation in Mouse Placenta under a High-Fat Diet. PLoS ONE 2010, 5, e14398. [Google Scholar] [CrossRef]
- Gabory, A.; Ferry, L.; Fajardy, I.; Jouneau, L.; Gothié, J.-D.; Vigé, A.; Fleur, C.; Mayeur, S.; Gallou-Kabani, C.; Gross, M.-S.; et al. Maternal Diets Trigger Sex-Specific Divergent Trajectories of Gene Expression and Epigenetic Systems in Mouse Placenta. PLoS ONE 2012, 7, e47986. [Google Scholar] [CrossRef]
- de Barros Mucci, D.; Kusinski, L.C.; Wilsmore, P.; Loche, E.; Pantaleão, L.C.; Ashmore, T.J.; Blackmore, H.L.; Fernandez-Twinn, D.S.; Carmo, M.D.G.T.D.; Ozanne, S.E. Impact of maternal obesity on placental transcriptome and morphology associated with fetal growth restriction in mice. Int. J. Obes. 2020, 44, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Casero, D.; Thamotharan, S.; Wadehra, M.; Cosi, A.; Devaskar, S.U. The Placental Transcriptome in Late Gestational Hypoxia Resulting in Murine Intrauterine Growth Restriction Parallels Increased Risk of Adult Cardiometabolic Disease. Sci. Rep. 2019, 9, 1243. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Jain, A.; Denslow, N.D.; Nouri, M.-Z.; Chen, S.; Wang, T.; Zhu, N.; Koh, J.; Sarma, S.J.; Sumner, B.W.; et al. Bisphenol A and bisphenol S disruptions of the mouse placenta and potential effects on the placenta–brain axis. Proc. Natl. Acad. Sci. USA 2020, 117, 4642–4652. [Google Scholar] [CrossRef]
- Majewska, M.; Lipka, A.; Paukszto, L.; Jastrzebski, J.; Szeszko, K.; Gowkielewicz, M.; Lepiarczyk, E.; Jozwik, M.; Majewski, M. Placenta Transcriptome Profiling in Intrauterine Growth Restriction (IUGR). Int. J. Mol. Sci. 2019, 20, 1510. [Google Scholar] [CrossRef] [PubMed]
- Fryer, B.H.; Simon, M.C. Hypoxia, HIF and the Placenta. Cell Cycle 2006, 5, 495–498. [Google Scholar] [CrossRef]
- Bischoff, L.J.M.; Kuijper, I.A.; Schimming, J.P.; Wolters, L.; Braak, B.T.; Langenberg, J.P.; Noort, D.; Beltman, J.B.; Van De Water, B. A systematic analysis of Nrf2 pathway activation dynamics during repeated xenobiotic exposure. Arch. Toxicol. 2019, 93, 435–451. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Wolters Kluwer: Philadelphia, PA, USA, 2019; p. 597. [Google Scholar]
- Gundogan, F.; Gilligan, J.; Qi, W.; Chen, E.; Naram, R.; De La Monte, S.M. Dose effect of gestational ethanol exposure on placentation and fetal growth. Placenta 2015, 36, 523–530. [Google Scholar] [CrossRef]
- Simmers, M.D.; Hudson, K.M.; Baptissart, M.; Cowley, M. Epigenetic control of the imprinted growth regulator Cdkn1c in cadmium-induced placental dysfunction. Epigenetics 2022, 18, 2088173. [Google Scholar] [CrossRef]
- Gonzalez, P.N.; Gasperowicz, M.; Barbeito-Andrés, J.; Klenin, N.; Cross, J.C.; Hallgrímsson, B. Chronic Protein Restriction in Mice Impacts Placental Function and Maternal Body Weight before Fetal Growth. PLoS ONE 2016, 11, e0152227. [Google Scholar] [CrossRef]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef]
- Li, B.; Chang, S.; Liu, C.; Zhang, M.; Zhang, L.; Liang, L.; Li, R.; Wang, X.; Qin, C.; Zhang, T.; et al. Low Maternal Dietary Folate Alters Retrotranspose by Methylation Regulation in Intrauterine Growth Retardation (IUGR) Fetuses in a Mouse Model. Med. Sci. Monit. 2019, 25, 3354–3365. [Google Scholar] [CrossRef]
- Cao, C.; Prado, M.A.; Sun, L.; Rockowitz, S.; Sliz, P.; Paulo, J.A.; Finley, D.; Fleming, M.D. Maternal Iron Deficiency Modulates Placental Transcriptome and Proteome in Mid-Gestation of Mouse Pregnancy. J. Nutr. 2021, 151, 1073–1083. [Google Scholar] [CrossRef]
- Arias, A.; Schander, J.A.; Bariani, M.V.; Correa, F.; Domínguez Rubio, A.P.; Cella, M.; Cymeryng, C.B.; Wolfson, M.L.; Franchi, A.M.; Aisemberg, J. Dexamethasone-induced intrauterine growth restriction modulates expression of placental vascular growth factors and fetal and placental growth. Mol. Hum. Reprod. 2021, 27, gaab006. [Google Scholar] [CrossRef] [PubMed]
- Czamara, D.; Dieckmann, L.; Röh, S.; Kraemer, S.; Rancourt, R.C.; Sammallahti, S.; Kajantie, E.; Laivuori, H.; Eriksson, J.G.; Räikkönen, K.; et al. Betamethasone administration during pregnancy is associated with placental epigenetic changes with implications for inflammation. Clin. Epigenetics 2021, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- James, J.L.; Whitley, G.S.; Cartwright, J.E. Pre-eclampsia: Fitting together the placental, immune and cardiovascular pieces. J. Pathol. 2010, 221, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Magid, M.S.; Kaplan, C.; Sammaritano, L.R.; Peterson, M.; Druzin, M.L.; Lockshin, M.D. Placental pathology in systemic lupus erythematosus: A prospective study. Am. J. Obstet. Gynecol. 1998, 179, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Bordt, E.A.; Shook, L.L.; Atyeo, C.; Pullen, K.M.; De Guzman, R.M.; Meinsohn, M.-C.; Chauvin, M.; Fischinger, S.; Yockey, L.J.; James, K.; et al. Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses. Sci. Transl. Med. 2021, 13, eabi7428. [Google Scholar] [CrossRef] [PubMed]
- Yung, H.W.; Cox, M.; Patot, M.T.; Burton, G.J. Evidence of endoplasmic reticulum stress and protein synthesis inhibition in the placenta of non-native women at high altitude. FASEB J. 2012, 26, 1970–1981. [Google Scholar] [CrossRef] [PubMed]




| Gene | Sex | Fold Change (RNA-Seq) | p-adj | Fold Change (RT-qPCR) | p-adj |
|---|---|---|---|---|---|
| Rasl11b | Female | −1.00 | 0.994 | −1.02 | 0.972 |
| Male | +1.00 | 0.999 | −1.01 | 0.999 | |
| Hsd11b2 | Female | −1.04 | 0.947 | −1.11 | 0.273 |
| Male | −1.02 | 0.972 | −1.03 | 0.965 | |
| Sec61b | Female | +1.04 | 0.827 | +1.08 | 0.701 |
| Male | −1.03 | 0.878 | +1.08 | 0.643 | |
| Mtmr7 | Female | +1.02 | 0.951 | −1.00 | 0.999 |
| Male | −1.02 | 0.932 | −1.01 | 0.998 | |
| Slc5a5 | Female | +1.89 | 1.08 × 10−4 | +1.25 | 0.111 |
| Male | +1.92 | 3.26 × 10−4 | +1.48 | 1.30 × 10−3 | |
| Wsb1 | Female | −1.14 | 0.210 | −1.20 | 4.40 × 10−3 |
| Male | −1.16 | 0.152 | −1.07 | 0.575 | |
| Dio2 | Female | −2.21 | 0.0286 | −1.49 | 0.0469 |
| Male | −2.16 | 0.117 | −1.80 | 8.80 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreetharan, S.; Tharmalingam, S.; Hourtovenko, C.; Tubin, F.; McTiernan, C.D.; Thome, C.; Khaper, N.; Boreham, D.R.; Lees, S.J.; Tai, T.C. Whole Transcriptome Analysis of the Mouse Placenta Following Radiation-Induced Growth Restriction. Radiation 2025, 5, 35. https://doi.org/10.3390/radiation5040035
Sreetharan S, Tharmalingam S, Hourtovenko C, Tubin F, McTiernan CD, Thome C, Khaper N, Boreham DR, Lees SJ, Tai TC. Whole Transcriptome Analysis of the Mouse Placenta Following Radiation-Induced Growth Restriction. Radiation. 2025; 5(4):35. https://doi.org/10.3390/radiation5040035
Chicago/Turabian StyleSreetharan, Shayenthiran, Sujeenthar Tharmalingam, Cameron Hourtovenko, Felix Tubin, Christopher D. McTiernan, Christopher Thome, Neelam Khaper, Douglas R. Boreham, Simon J. Lees, and T.C. Tai. 2025. "Whole Transcriptome Analysis of the Mouse Placenta Following Radiation-Induced Growth Restriction" Radiation 5, no. 4: 35. https://doi.org/10.3390/radiation5040035
APA StyleSreetharan, S., Tharmalingam, S., Hourtovenko, C., Tubin, F., McTiernan, C. D., Thome, C., Khaper, N., Boreham, D. R., Lees, S. J., & Tai, T. C. (2025). Whole Transcriptome Analysis of the Mouse Placenta Following Radiation-Induced Growth Restriction. Radiation, 5(4), 35. https://doi.org/10.3390/radiation5040035






