Recalcitrant Pelvic Pain: Evaluating the Effectiveness of Radiofrequency Ablation for Pudendal, Genitofemoral, and Ilioinguinal Neuropathy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
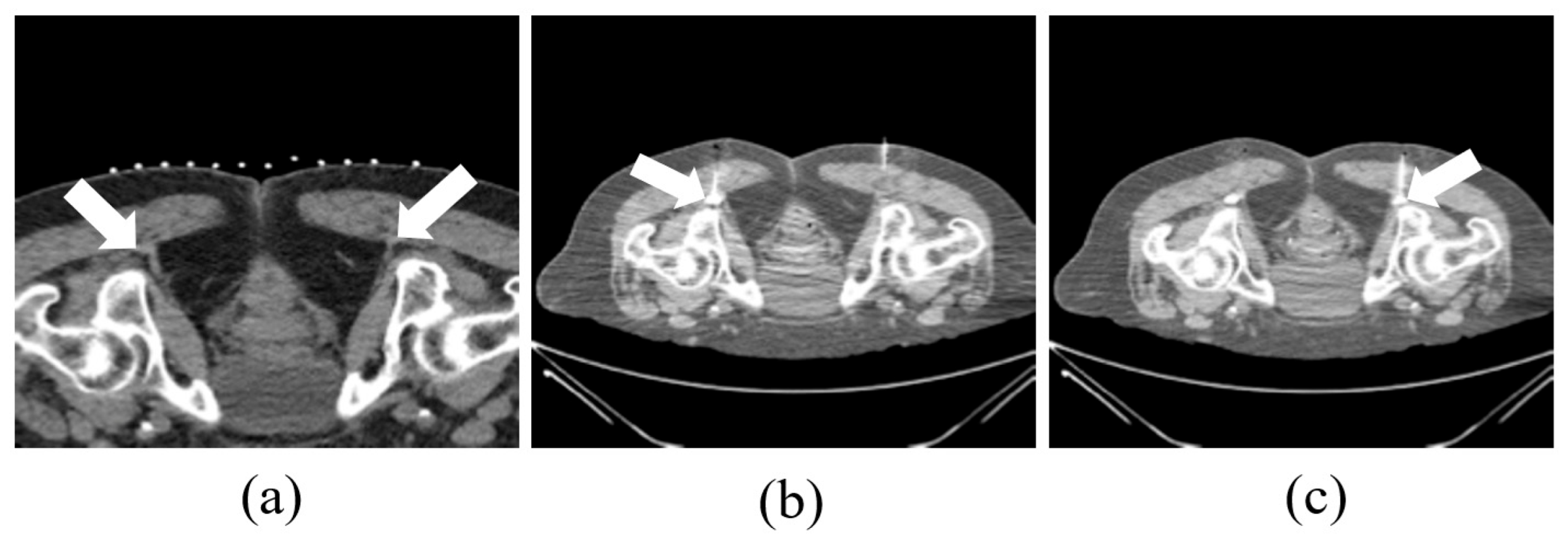
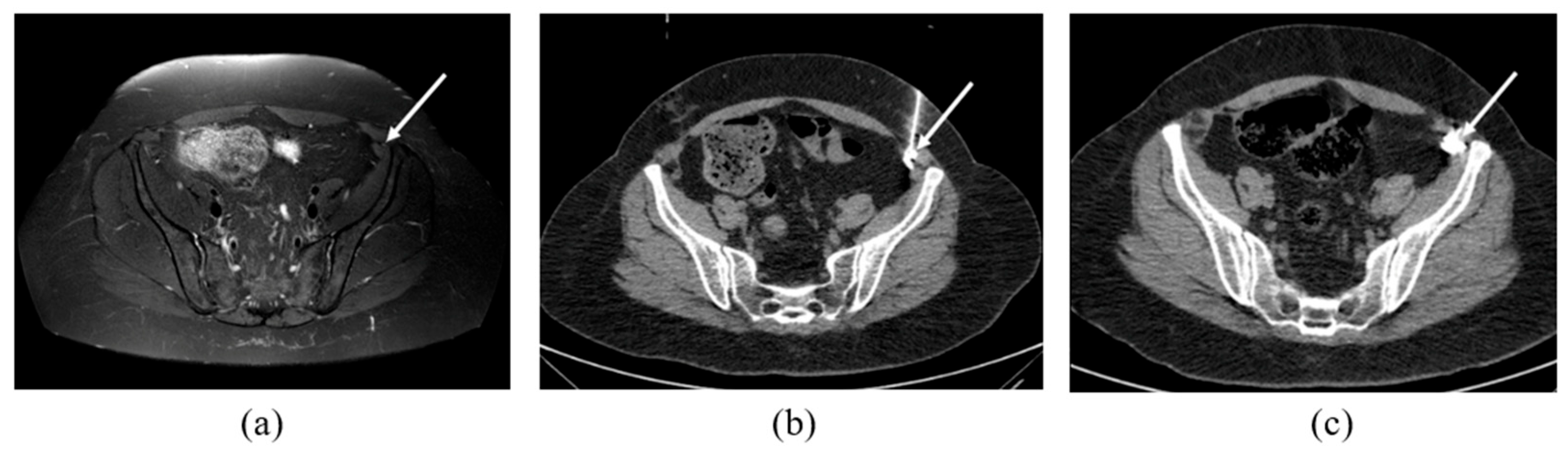
2.2. CT-Guided Procedures
2.3. Follow-Ups
2.4. Statistics
3. Results
3.1. Patient Characteristics and Demographics
3.2. MRN Findings
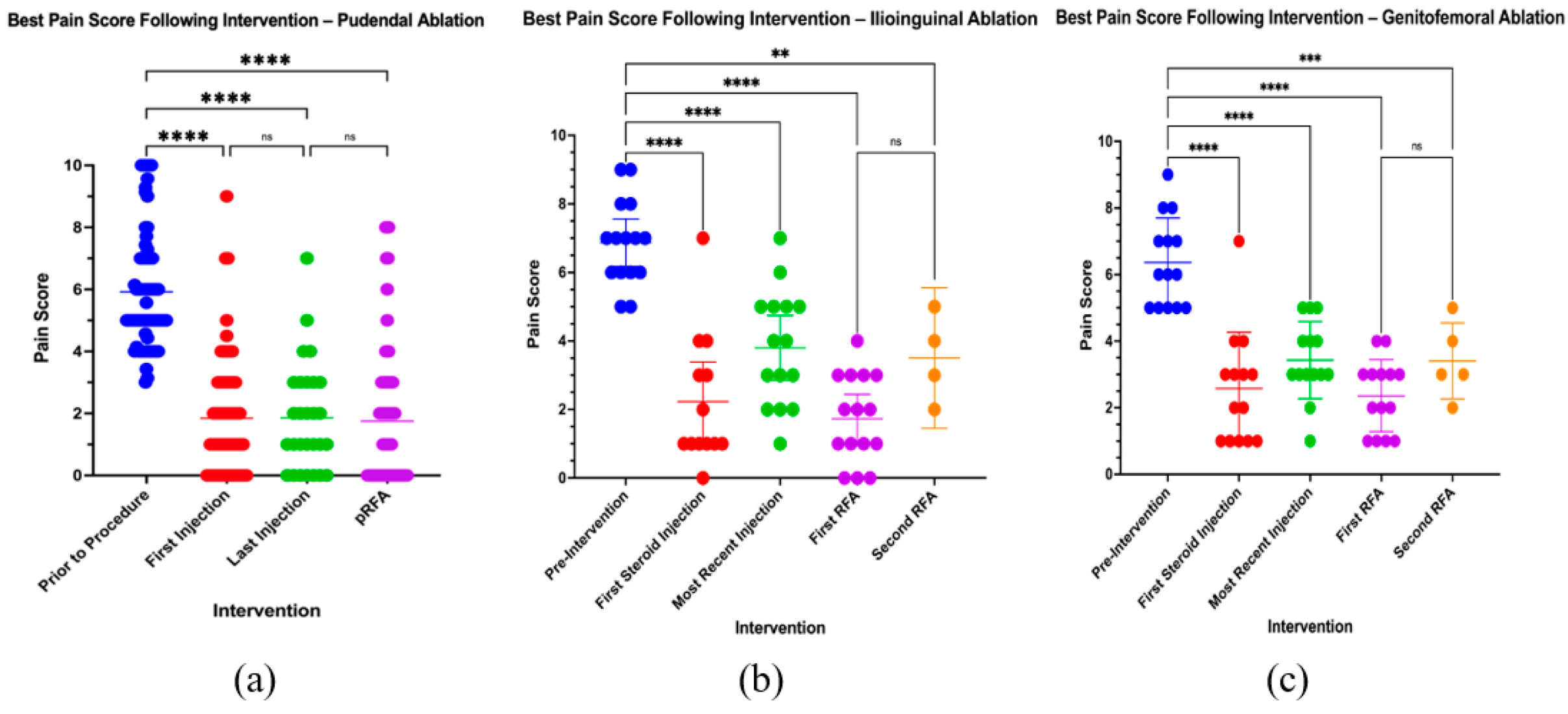
3.3. Pain Response
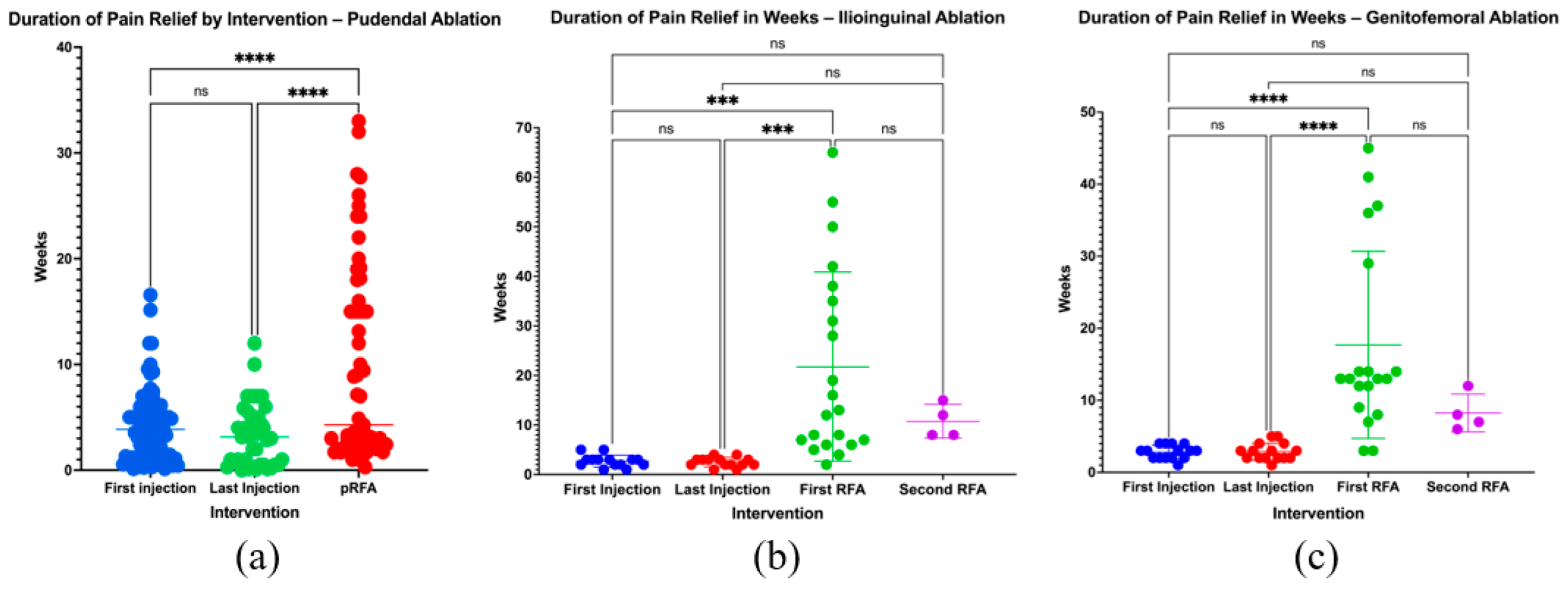
3.4. Duration of Pain Relief
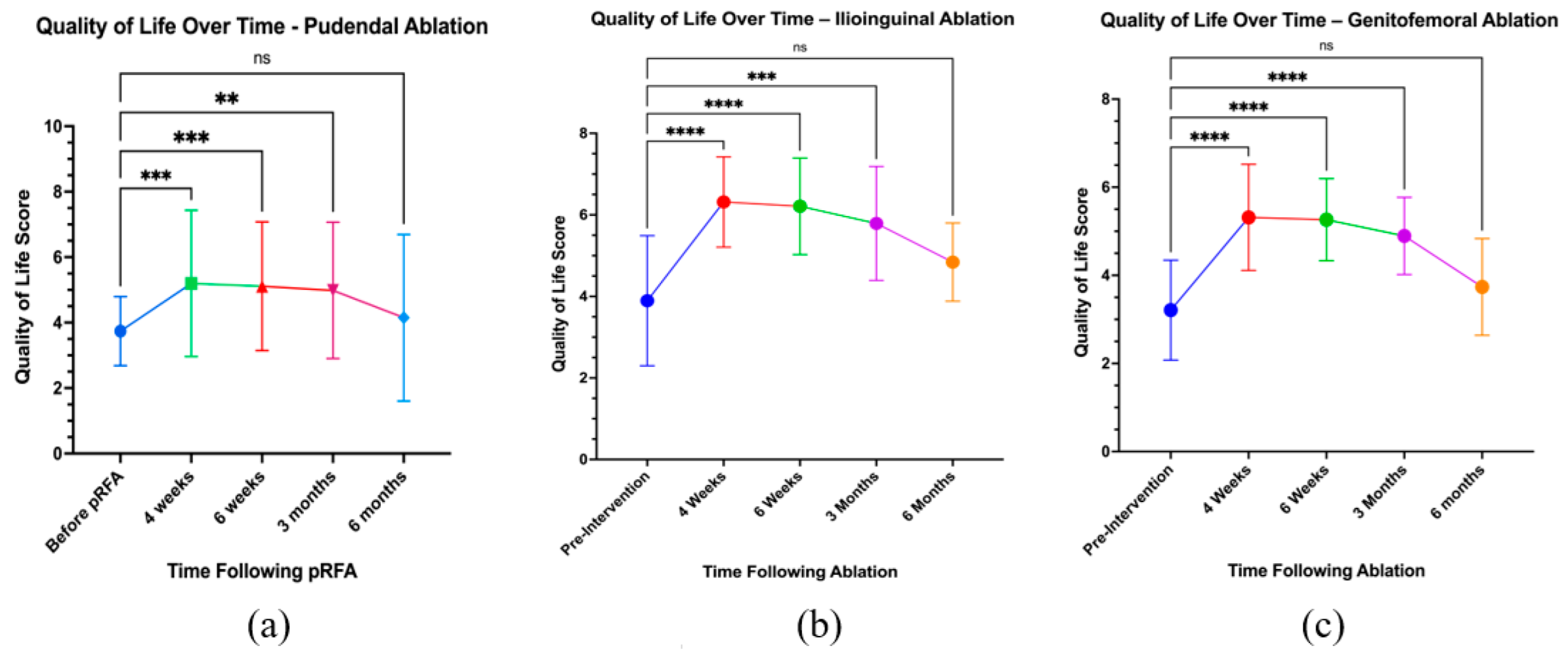
3.5. Quality of Life
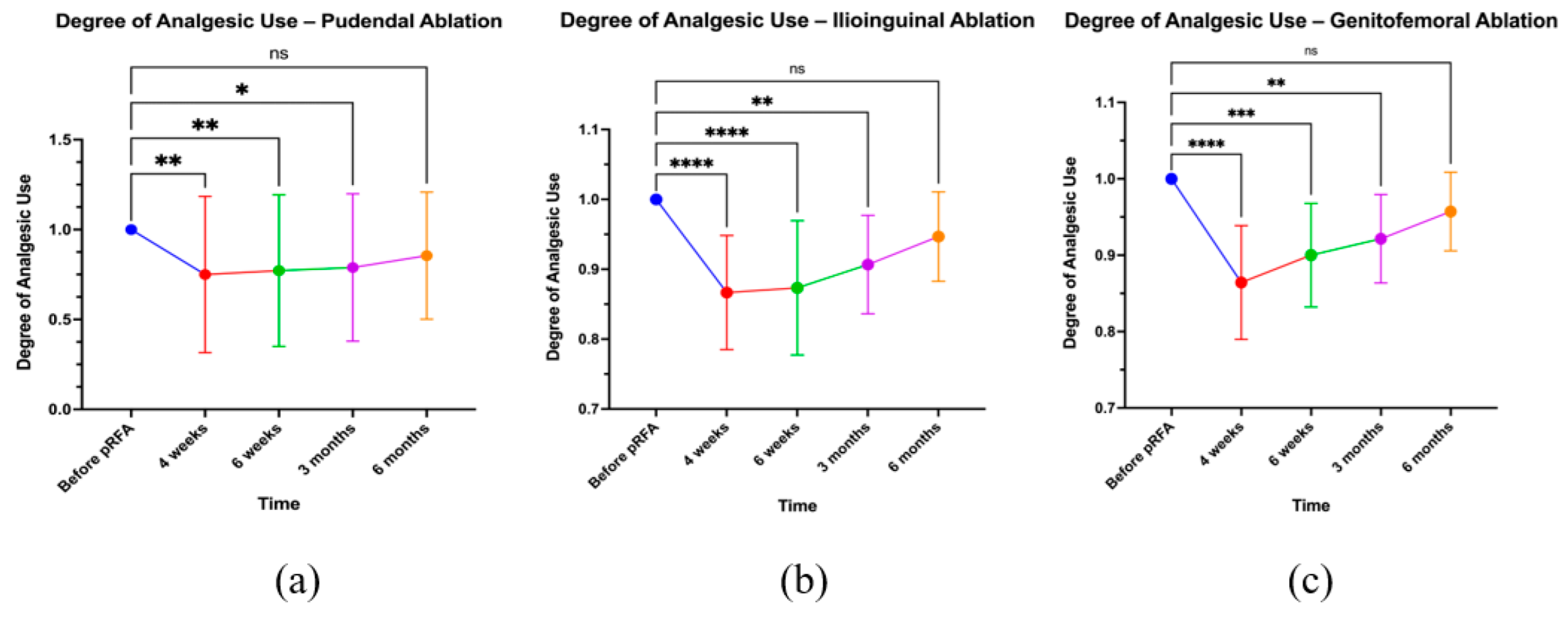
3.6. Analgesic Use
3.7. Correlations
3.8. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cRFA | Continuous Radiofrequency Ablation |
| pRF | Pulsed Radiofrequency |
| VAS | Visual Analog Scale |
| MRN | Magnetic Resonance Neurography |
| CT | Computed Tomography |
| RF | Radiofrequency |
| IRB | Institutional Review Board |
| HIPAA | Health Insurance Portability and Accountability Act |
| BMI | Body Mass Index |
| QoL | Quality of Life |
| ANOVA | Analysis of Variance |
| RFN | Radiofrequency Neurotomy |
References
- Lamvu, G.; Carrillo, J.; Ouyang, C.; Rapkin, A. Chronic Pelvic Pain in Women: A Review. JAMA 2021, 325, 2381–2391. [Google Scholar] [CrossRef]
- Doggweiler, R.; Whitmore, K.E.; Meijlink, J.M.; Drake, M.J.; Frawley, H.; Nordling, J.; Hanno, P.; Fraser, M.O.; Homma, Y.; Garrido, G.; et al. A Standard for Terminology in Chronic Pelvic Pain Syndromes: A Report from the Chronic Pelvic Pain Working Group of the International Continence Society. Neurourol Urodyn. 2017, 36, 984–1008. [Google Scholar] [CrossRef] [PubMed]
- Kothari, S. Neuromodulatory Approaches to Chronic Pelvic Pain and Coccygodynia. In Operative Neuromodulation; Sakas, D.E., Simpson, B.A., Krames, E.S., Eds.; Acta Neurochirurgica Supplements; Springer: Vienna, Austria, 2006; Volume 97, pp. 365–371. ISBN 978-3-211-33078-4. [Google Scholar]
- Latthe, P.; Latthe, M.; Say, L.; Gülmezoglu, M.; Khan, K.S. WHO Systematic Review of Prevalence of Chronic Pelvic Pain: A Neglected Reproductive Health Morbidity. BMC Public Health 2006, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, A. Prevalence of Chronic Pelvic Pain among Women: An Updated Review. Pain Physician 2014, 17, E141–E147. [Google Scholar] [CrossRef] [PubMed]
- Meisenheimer, E.S.; Carnevale, A.M. Chronic Pelvic Pain in Women: Evaluation and Treatment. Am. Fam. Physician. 2025, 111, 218–229. [Google Scholar]
- Hunter, C.W.; Stovall, B.; Chen, G.; Carlson, J.; Levy, R. Anatomy, Pathophysiology and Interventional Therapies for Chronic Pelvic Pain: A Review. Pain Physician 2018, 21, 147–167. [Google Scholar] [CrossRef]
- Shafik, A.; El-Sherif, M.; Youssef, A.; Olfat, E. Surgical Anatomy of the Pudendal Nerve and Its Clinical Implications. Clin. Anat. 1995, 8, 110–115. [Google Scholar] [CrossRef]
- Kaur, J.; Leslie, S.W.; Singh, P. Pudendal Nerve Entrapment Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Robert, R.; Prat-Pradal, D.; Labat, J.J.; Bensignor, M.; Raoul, S.; Rebai, R.; Leborgne, J.; Lardoux, M.C.; Thiodet, J. Anatomic Basis of Chronic Perineal Pain: Role of the Pudendal Nerve. Surg. Radiol. Anat. 1998, 20, 93–98. [Google Scholar] [CrossRef]
- Leibovitch, I.; Mor, Y. The Vicious Cycling: Bicycling Related Urogenital Disorders. Eur. Urol. 2005, 47, 277–286; discussion 286–287. [Google Scholar] [CrossRef]
- Lien, K.-C.; Morgan, D.M.; Delancey, J.O.L.; Ashton-Miller, J.A. Pudendal Nerve Stretch during Vaginal Birth: A 3D Computer Simulation. Am. J. Obstet. Gynecol. 2005, 192, 1669–1676. [Google Scholar] [CrossRef]
- Leslie, S.W.; Antolak, S.; Feloney, M.P.; Soon-Sutton, T.L. Pudendal Neuralgia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ramsden, C.E.; McDaniel, M.C.; Harmon, R.L.; Renney, K.M.; Faure, A. Pudendal Nerve Entrapment as Source of Intractable Perineal Pain. Am. J. Phys. Med. Rehabil. 2003, 82, 479–484. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Neuropathic Pain in Adults: Pharmacological Management in Non-Specialist Settings; National Institute for Health and Care Excellence (NICE): London, UK, 2020; ISBN 978-1-4731-0328-3. [Google Scholar]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Levesque, A.; Bautrant, E.; Quistrebert, V.; Valancogne, G.; Riant, T.; Beer Gabel, M.; Leroi, A.; Jottard, K.; Bruyninx, L.; Amarenco, G.; et al. Recommendations on the Management of Pudendal Nerve Entrapment Syndrome: A Formalised Expert Consensus. Eur. J. Pain 2022, 26, 7–17. [Google Scholar] [CrossRef]
- Basol, G.; Kale, A.; Gurbuz, H.; Gundogdu, E.C.; Baydilli, K.N.; Usta, T. Transvaginal Pudendal Nerve Blocks in Patients with Pudendal Neuralgia: 2-Year Follow-up Results. Arch. Gynecol. Obstet. 2022, 306, 1107–1116. [Google Scholar] [CrossRef]
- Charlotte, W.; Sebastian, D.; Viviane, T.; Luc, B. Selection Criteria for Surgical Treatment of Pudendal Neuralgia. Neurourol. Urodyn. 2017, 36, 663–666. [Google Scholar] [CrossRef]
- Wray, J.K.; Dixon, B.; Przkora, R. Radiofrequency Ablation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hoang Roberts, L.; Vollstedt, A.; Gilleran, J.; Peters, K.M. Bladder Dysfunction and Pelvic Pain: The Role of Sacral, Tibial, and Pudendal Neuromodulation. In Female Genitourinary and Pelvic Floor Reconstruction; Martins, F.E., Holm, H.V., Sandhu, J.S., McCammon, K.A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 255–273. ISBN 978-3-031-19597-6. [Google Scholar]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic Pain: A Maladaptive Response of the Nervous System to Damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, J.; Chen, J.; Gu, Y.; Shi, L.; Ni, H. Therapeutic Efficacy and Safety of Radiofrequency Ablation for the Treatment of Trigeminal Neuralgia: A Systematic Review and Meta-Analysis. J. Pain Res. 2019, 12, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Xie, E.; Garzon-Muvdi, T.; Bender, M.; Doshi, T.; Carson, B.; Lim, M.; Bettegowda, C. Association Between Radiofrequency Rhizotomy Parameters and Duration of Pain Relief in Trigeminal Neuralgia Patients with Recurrent Pain. World Neurosurg. 2019, 129, e128–e133. [Google Scholar] [CrossRef]
- Krijnen, E.A.; Schweitzer, K.J.; Van Wijck, A.J.M.; Withagen, M.I.J. Pulsed Radiofrequency of Pudendal Nerve for Treatment in Patients with Pudendal Neuralgia. A Case Series with Long-Term Follow-Up. Pain Practice 2021, 21, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Petrov-Kondratov, V.; Chhabra, A.; Jones, S. Pulsed Radiofrequency Ablation of Pudendal Nerve for Treatment of a Case of Refractory Pelvic Pain. Pain Physician 2017, 20, E451–E454. [Google Scholar] [CrossRef]
- Collard, M.D.; Xi, Y.; Patel, A.A.; Scott, K.M.; Jones, S.; Chhabra, A. Initial Experience of CT-Guided Pulsed Radiofrequency Ablation of the Pudendal Nerve for Chronic Recalcitrant Pelvic Pain. Clin. Radiol. 2019, 74, 897.e17–897.e23. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-L.; Song, T. The Clinical Efficacy of High-Voltage Long-Duration Pulsed Radiofrequency Treatment in Pudendal Neuralgia: A Retrospective Study. Neuromodulation 2022, 25, 1372–1377. [Google Scholar] [CrossRef]
- Wadhwa, V.; Scott, K.M.; Rozen, S.; Starr, A.J.; Chhabra, A. CT-Guided Perineural Injections for Chronic Pelvic Pain. Radio Graph. 2016, 36, 1408–1425. [Google Scholar] [CrossRef]
- Cesmebasi, A.; Yadav, A.; Gielecki, J.; Tubbs, R.S.; Loukas, M. Genitofemoral Neuralgia: A Review. Clin. Anat. 2015, 28, 128–135. [Google Scholar] [CrossRef]
- Zuckerbraun, B.S.; Cyr, A.R.; Mauro, C.S. Groin Pain Syndrome Known as Sports Hernia: A Review. JAMA Surg. 2020, 155, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Schwartz, R.; Herman, J.; Berger, A.A.; Lee, D.; Lee, C.; Zamarripa, A.M.; Slovek, A.; Habib, K.; Manchikanti, L.; et al. A Comprehensive Update of the Superior Hypogastric Block for the Management of Chronic Pelvic Pain. Curr. Pain Headache Rep. 2021, 25, 13. [Google Scholar] [CrossRef] [PubMed]
- Starzec-Proserpio, M.; Frawley, H.; Bø, K.; Morin, M. Effectiveness of Nonpharmacological Conservative Therapies for Chronic Pelvic Pain in Women: A Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2025, 232, 42–71. [Google Scholar] [CrossRef]
- Patil, A.S.; Gupta, M.; Knezevic, N.N.; Oehlermarx, W.; Diwan, S.; Abdallah, R.T.; Sanapati, M.; Soin, A.; Abd-Elsayed, A.A. A Comprehensive Review of Treatment Approaches to Ilioinguinal Neuralgia. Pain Physician 2025, 28, 197–205. [Google Scholar]
- Gangopadhyay, N.; Pothula, A.; Yao, A.; Geraghty, P.J.; Mackinnon, S.E. Retroperitoneal Approach for Ilioinguinal, Iliohypogastric, and Genitofemoral Neurectomies in the Treatment of Refractory Groin Pain After Inguinal Hernia Repair. Ann. Plast. Surg. 2020, 84, 431–435. [Google Scholar] [CrossRef]
- Chen, D.C.; Hiatt, J.R.; Amid, P.K. Operative Management of Refractory Neuropathic Inguinodynia by a Laparoscopic Retroperitoneal Approach. JAMA Surg. 2013, 148, 962–967. [Google Scholar] [CrossRef]
- Kastler, A.; Aubry, S.; Barbier-Brion, B.; Jehl, J.; Kastler, B. Radiofrequency Neurolysis in the Management of Inguinal Neuralgia: Preliminary Study. Radiology 2012, 262, 701–707. [Google Scholar] [CrossRef]
- Makharita, M.Y.; Amr, Y.M. Pulsed Radiofrequency for Chronic Inguinal Neuralgia. Pain Physician 2015, 18, E147–E155. [Google Scholar] [CrossRef] [PubMed]
- Kastler, A.; Aubry, S.; Piccand, V.; Hadjidekov, G.; Tiberghien, F.; Kastler, B. Radiofrequency Neurolysis versus Local Nerve Infiltration in 42 Patients with Refractory Chronic Inguinal Neuralgia. Pain Physician 2012, 15, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Parris, D.; Fischbein, N.; Mackey, S.; Carroll, I. A Novel CT-Guided Transpsoas Approach to Diagnostic Genitofemoral Nerve Block and Ablation. Pain Med. 2010, 11, 785–789. [Google Scholar] [CrossRef]
- Sondekoppam, R.V.; Tsui, B.C.H. Factors Associated With Risk of Neurologic Complications After Peripheral Nerve Blocks: A Systematic Review. Anesth. Analg. 2017, 124, 645–660. [Google Scholar] [CrossRef]
- Sayed, D.; Grider, J.; Strand, N.; Hagedorn, J.M.; Falowski, S.; Lam, C.M.; Tieppo Francio, V.; Beall, D.P.; Tomycz, N.D.; Davanzo, J.R.; et al. The American Society of Pain and Neuroscience (ASPN) Evidence-Based Clinical Guideline of Interventional Treatments for Low Back Pain. J. Pain Res. 2022, 15, 3729–3832. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, J.; Yang, Y.; Ye, L.; Wang, X. Clinical Effect and Safety of Pulsed Radiofrequency Treatment for Pudendal Neuralgia: A Prospective, Randomized Controlled Clinical Trial. J. Pain Res. 2018, 11, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Hetta, D.F.; Mahran, A.M.; Kamal, E.E. Pulsed Radiofrequency Treatment for Chronic Post-Surgical Orchialgia: A Double-Blind, Sham-Controlled, Randomized Trial: Three-Month Results. Pain Physician 2018, 21, 199–205. [Google Scholar] [CrossRef]
- Lee, K.S.; Sin, J.M.; Patil, P.P.; Hanna, A.S.; Greenberg, J.A.; Zea, R.D.; Brace, C.L. Ultrasound-Guided Microwave Ablation for the Management of Inguinal Neuralgia: A Preliminary Study with 1-Year Follow-Up. J. Vasc. Interv. Radiol. 2019, 30, 242–248. [Google Scholar] [CrossRef]


| Nerve | Pudendal | Ilioinguinal | Genitofemoral |
|---|---|---|---|
| Total Patients Per Nerve | 49 | 15 | 14 |
| Age (years) | 61.7 ± 14.1 | 57.3 ± 14.1 | 59.7 ± 18.4 |
| Males | 19 | 6 | 6 |
| Females | 30 | 9 | 8 |
| Body Mass Index | 26.3 ± 4.90 | 28.8 ± 7.0 | 29.9 ± 9.2 |
| Total Number of Procedures | 186 | 47 | 49 |
| Laterality | |||
| Left | 15 | 8 | 4 |
| Right | 10 | 5 | 6 |
| Bilateral | 24 | 2 | 4 |
| Etiology | Pudendal | Ilioinguinal | Genitofemoral |
|---|---|---|---|
| Idiopathic/Unspecified | 11 | 1 | 3 |
| Trauma | 8 | 2 | 1 |
| Childbirth | 8 | 1 | 0 |
| Chronic Constipation | 1 | 0 | 0 |
| Pelvic Tumor/Mass | 2 | 3 | 2 |
| Repetitive Stress Injury (long car drive, mountain biking, sedentary desk job, etc.) | 12 | 4 | 5 |
| Post-Surgical | 7 | 3 | 3 |
| Number of Injections | Pudendal | Ilioinguinal | Genitofemoral | Number of Ablations | Pudendal | Ilioinguinal | Genitofemoral |
|---|---|---|---|---|---|---|---|
| One Injection | 24 | 7 | 6 | One Ablation | 31 | 11 | 10 |
| Two Injections | 13 | 5 | 3 | Two Ablations | 13 | 4 | 5 |
| Three Injections | 9 | 1 | 3 | Three Ablations | 6 | 0 | 0 |
| Four Injections | 3 | 2 | 2 | Four Ablations | 2 | 0 | 0 |
| Five Injections | 0 | 0 | 0 | ||||
| Six Injections | 1 | 0 | 0 | ||||
| Seven Injections | 0 | 0 | 0 | ||||
| Eight Injections | 1 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaidi, Z.; Attia, S.; Wahid, M.; Xi, Y.; Sangha, H.; Scott, K.; Kumar, R.; Silva, F.D.; Chhabra, A. Recalcitrant Pelvic Pain: Evaluating the Effectiveness of Radiofrequency Ablation for Pudendal, Genitofemoral, and Ilioinguinal Neuropathy. Radiation 2025, 5, 28. https://doi.org/10.3390/radiation5040028
Zaidi Z, Attia S, Wahid M, Xi Y, Sangha H, Scott K, Kumar R, Silva FD, Chhabra A. Recalcitrant Pelvic Pain: Evaluating the Effectiveness of Radiofrequency Ablation for Pudendal, Genitofemoral, and Ilioinguinal Neuropathy. Radiation. 2025; 5(4):28. https://doi.org/10.3390/radiation5040028
Chicago/Turabian StyleZaidi, Zuhair, Sarah Attia, Muaz Wahid, Yin Xi, Hareena Sangha, Kelly Scott, Rupali Kumar, Flavio Duarte Silva, and Avneesh Chhabra. 2025. "Recalcitrant Pelvic Pain: Evaluating the Effectiveness of Radiofrequency Ablation for Pudendal, Genitofemoral, and Ilioinguinal Neuropathy" Radiation 5, no. 4: 28. https://doi.org/10.3390/radiation5040028
APA StyleZaidi, Z., Attia, S., Wahid, M., Xi, Y., Sangha, H., Scott, K., Kumar, R., Silva, F. D., & Chhabra, A. (2025). Recalcitrant Pelvic Pain: Evaluating the Effectiveness of Radiofrequency Ablation for Pudendal, Genitofemoral, and Ilioinguinal Neuropathy. Radiation, 5(4), 28. https://doi.org/10.3390/radiation5040028






