1. Introduction
Prostate cancer is a prevalent cancer in men, accounting for roughly 15% of cancer cases worldwide, with even higher rates in sub-Saharan Africa, where it constitutes 23% of male cancers [
1]. Projections suggest that the number of new prostate cancer cases will surge from 1.4 million in 2020 to 2.9 million by 2040, underscoring the need for proactive government strategies alongside lifestyle and public health initiatives to manage this increase [
2]. Radiation therapy (RT) has advanced significantly, becoming a cornerstone of cancer treatment as both a primary and adjunctive modality [
3,
4,
5,
6,
7]. The COVID-19 pandemic has highlighted the importance of reassessing treatment strategies and bringing hypofractionated radiotherapy (HFRT) to the forefront as an effective approach that reduces hospital visits and infection risks while maintaining efficacy [
8].
Hypofractionation (HF) delivers higher fractionated doses of radiation in fewer sessions, optimising therapeutic outcomes [
9]. While some studies have demonstrated its successful implementation, ongoing debates surround the optimal fractionation schemes and the underlying radiobiological mechanisms [
10,
11,
12]. Comprehensive in vitro investigations are essential to further our understanding of HFRT and its application [
13]. The complexity of radiobiological mechanisms is influenced by tissue sensitivity, fractionation and dose delivery, with in vitro studies providing vital insights into biological responses to radiation [
14]. These factors are critical in understanding HF within variable cancer contexts, where financial constraints, limited access to technology, and disparities in healthcare can impact treatment decisions [
15]. Radiotherapy centres in Africa have adapted their approaches to hypofractionated dosing, prioritising patient care and safety [
16,
17], reflecting a broader global response to the pandemic that emphasises flexibility and preparedness [
18].
Studies, including the CHHiP trial and the RTOG 0415 trial, have enhanced the understanding of hypofractionated radiation therapy for prostate cancer, indicating that its efficacy is comparable to conventional therapy, particularly in terms of disease-free survival for low-risk patients [
19,
20,
21]. Definitive conclusions about hypofractionation’s effectiveness for prostate cancer remain pending until ongoing large randomised trials are completed and published, despite low alpha–beta ratios of 1 to 3 Gy suggesting that hypofractionated radiotherapy could enhance treatment efficacy [
22]. However, current data from randomised clinical trials indicate that ultra-hypofractionation is a safe approach, with no clinically significant differences in functional outcomes and health-related quality of life compared to CF, although further follow-up is required to fully assess its efficacy compared to standard fractionation [
23,
24]. In addition, a comparison of sexual function after hypofractionated radiotherapy versus conventional fractionation in prostate cancer patients revealed no significant differences in erectile function between the two treatment modalities, although hormone-naïve patients reported significantly higher orgasmic function scores at three years post-hypofractionation [
25]. Furthermore, stereotactic radiotherapy (SRT) has emerged as a promising modality for the management of prostate cancer recurrence, demonstrating improved biochemical control rates and reduced treatment-related toxicity compared to conventional therapies [
26]. The integration of advanced imaging techniques, such as PSMA PET/CT, allows for the precise localisation of recurrent diseases, further optimizing treatment planning and patient outcomes [
26,
27]. Evaluating the interplay of tissue sensitivity, fractionation, and dose delivery is essential for assessing treatment outcomes and toxicity profiles in prostate cancer radiotherapy [
23,
24]. Advanced modalities such as intensity-modulated radiation therapy (IMRT) and stereotactic body radiotherapy (SBRT) have been shown to enhance outcomes while minimising exposure to healthy tissue [
25,
26].
The linear-quadratic (LQ) model is fundamental for understanding the biological response to radiation and recognising the interplay between lethal and potentially lethal effects [
27,
28,
29,
30,
31,
32,
33,
34]. The α/β ratio derived from this model is critical for predicting treatment outcomes and understanding radiosensitivity and repair capacity [
8,
29,
30]. A high α/β ratio indicates greater sensitivity to higher doses per fraction, informing clinical strategies for hypofractionation regimens through biological effective dose (BED) modelling [
31,
32,
33,
34].
The clonogenic survival assay (CSA) is a key method for evaluating cellular responses and determining α/β values, providing insights into the biological mechanisms underpinning treatment responses [
35]. Notably, research into DNA repair processes, including non-homologous end joining (NHEJ) and homologous recombination repair (HRR), has crucial implications for understanding hypofractionated radiotherapy outcomes [
36,
37,
38,
39]. Additionally, radiation impacts cell growth and apoptosis, with the p53 gene playing a pivotal role in modulating these processes, particularly in the context of cancer behaviour [
40,
41].
Lactate dehydrogenase (LDH), a key enzyme in the glycolytic pathway, serves as an important biomarker for monitoring cancer treatment outcomes, correlating with tumour progression and response to therapy [
42,
43,
44,
45,
46]. Elevated LDH levels often indicate aggressive disease behaviour and poor prognosis, offering insights into tumour metabolism and treatment response [
47,
48,
49].
This study aimed to elucidate the radiobiological principles underlying HF and conventionally fractionated (CF) radiation therapy. Key concepts such as the α/β ratio, BED, and radiobiological assays were explored to investigate the differential effects of HF and CF radiation doses on tumour and normal cell responses. A hypofractionated regimen was designed to deliver a single larger dose in one treatment session, theoretically achieving a BED similar to conventional fractionation. Cellular responses to radiation were examined through clonogenic survival assays, DNA damage assessment, migration, invasion, adaptive response, and LDH secretion. By integrating historical perspectives with contemporary research findings, this study seeks to enhance the understanding of the radiobiological effects of hypofractionation. Ultimately, it aims to inform the development of more effective treatment protocols. However, it is crucial that clinical implementation be guided not only by in vitro evidence but also by robust in vivo data, ensuring that treatments are both effective and safe for patient populations. This approach will contribute to the growing body of knowledge in this area while prioritising patient outcomes.
2. Materials and Methods
A systematic investigation was conducted to examine the effects of HF and CF radiation on prostate cell lines. Radiation doses were calculated based on clonogenic survival assays and adjusted to achieve a comparable BED between the two models. Radiation-induced responses were evaluated by monitoring cell growth and conducting radiobiological assays aimed at elucidating the effects of HF and CF on cellular behaviour and informing optimal treatment strategies. Specifically, the use of in vitro models with isolated cells allowed for the control of various variables and the isolation of the effects of different fractionation schedules. For HF, a single fraction was chosen to represent the higher dose delivered, as demonstrated by previous studies [
50,
51].
2.1. Prostate Cell Lines
This study utilised the following cell lines: cancerous cell lines (PC-3 and DU-145) and a non-cancerous cell line (BPH-1). The PC-3 cell line was cultured in Ham’s F-12 medium (Catalogue number 21765029) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (100 U/mL penicillin and 100 μg/mL streptomycin) under standard culture conditions (37 °C, 5% CO
2). BPH-1 was maintained in Roswell Park Memorial Institute (RPMI-1640) medium (Catalogue number 21875034) supplemented with 10% FBS, 1% penicillin-streptomycin, and 2 mM L-glutamine under similar conditions (37 °C, 5% CO
2). DU-145 was cultured in Eagle’s minimum essential medium (EMEM) (Catalogue number 21575022) supplemented with 10% FBS and 1% penicillin-streptomycin, also under standard conditions (37 °C, 5% CO
2). All media and supplements were obtained from LTC Tech South Africa (Pty) Ltd. (Fairland, Randburg, 2030, South Africa). The cancerous cell lines PC-3 and DU-145 are characterised by mutant p53 and high metastatic potential. PC-3 is androgen receptor-negative (AR-) and serves as a model for castration-resistant prostate cancer (CRPC), as it grows independently of androgens. DU-145, while often considered to have reduced functional androgen receptor signalling, exhibits altered androgen receptor expression and is mostly suited for studying hormone-refractory prostate cancer. In contrast, BPH-1 is a non-cancerous prostate epithelial cell line exhibiting wild-type p53 [
52,
53,
54].
2.2. Doubling Time, Adaptive Response, and Clonogenic Survival Assay (CSA)
The doubling time of the cell lines was calculated using a standard formula [
55,
56]. The cells were seeded at a predetermined density, and initial counts were recorded. Subsequent counts were taken at 24 h intervals over 72 h to monitor proliferation. Prior to experimentation, the cells were cultured for several weeks to ensure active growth. A priming dose of 0.5 Gy was administered to assess the adaptive doubling time, with additional serial counts performed to evaluate the effect of low-dose radiation (0.5 Gy) on cell proliferation. X-ray irradiation was conducted using a Precision X-Ray 320 kV irradiator (Madison, CT, USA) operated at 250 kV with a dose rate of 0.69 Gy/min.
The Clonogenic survival assay (CSA) was used to assess the surviving fraction of cells following exposure to varying doses of radiation [
57,
58,
59,
60,
61,
62]. The CSA served as the primary endpoint for evaluating radiation sensitivity and α/β ratios of the cell lines. Each cell line was seeded in 6-well plates at densities corresponding to each radiation dose and subsequently irradiated. A range of doses from 2 Gy to 10 Gy was administered in increments of 2 Gy. A total of 3 technical replicates were performed for each experimental condition, alongside 6 biological replicates per cell line and endpoint to ensure the reliability of the results. After incubating the cell cultures for six doubling times to facilitate colony formation, the surviving fractions at all doses were calculated. The survival data were fitted to the linear-quadratic (LQ) model using non-linear regression analysis with the Marquardt–Levenberg algorithm [
63] to generate survival curves. The LQ model equation was employed to model the relationship between radiation dose and survival fraction:
where
S(
D) represents the surviving fraction at dose
D, and
α and
β are parameters extracted from the curve fit. The fitted curve allowed for the extraction of the LQ model parameters (α) and (β), representing the linear and quadratic components of cell killing, respectively. Additionally, D
50, the absorbed radiation dose required for 50% cell killing, was calculated using the LQ model equation. The relative sensitivity (RS), indicating the ratio of D
50 values between non-cancerous and cancerous cell lines, was also determined. The extracted α and β values were interpreted in the context of radiosensitivity, with higher α/β ratio values suggesting greater sensitivity to radiation.
2.3. Biological Effective Dose (BED)
The biologically effective dose (BED) quantifies the biological effectiveness of radiation therapy by considering the dose per fraction and the radiobiological parameters, alpha (α) and beta (β), across different regimens [
64]. The BED provides a standardised measure for comparing the effects of radiation doses delivered over varying schedules, thereby facilitating the optimisation of radiation therapy protocols. In the HF model, the
BED for a single fraction was calculated using Equation (2):
where D is the total dose, d is the dose per fractions, and α/β is the ratio of alpha to beta [
64]. By substituting the calculated BED from the conventional fractionation of 2 Gy per fraction over 4 fractions (totalling 8 Gy), the required dose for hypofractionation (D = d for a single fraction) can be determined. In this context, the dose (D) for hypofractionation can be calculated as the only unknown variable in the BED equation, given that all other variables have been established using the conventional fractionation scheme.
2.4. Split Dose Experiment and the Clonogenic Survival Assay
To investigate the effects of varying time gaps between radiation fractions on cell survival, cells were seeded in T25 flasks and irradiated with a total dose of 2 Gy, divided into 2 fractions with intervals of 1 to 6 h. The cells first received 1 Gy, followed by incubation for the respective times. After this, a second 1 Gy fraction was delivered, and the plates were incubated for a duration equivalent to six doubling times. Following six doubling times to allow for colony formation, surviving fractions were determined. For staining, a 5% Crystal Violet solution was prepared by dissolving 500 mg of Crystal Violet (Merck Life Science, Lethabong, South Africa, Catalogue number: 179337) in a mixture of 25 mL AR-grade methanol (Merck Life Science, South Africa, Catalogue number: 179335) and 75 mL distilled water. This solution was utilised after the cells were fixed using ice-cold 100% methanol for 10 min. The cells were washed with cold PBS and then incubated with 3 mL of 0.5% Crystal Violet in 25% methanol for 10 min at room temperature. Excess dye was removed, and the plates were rinsed with water to halt the staining process, followed by air drying. Colonies were manually counted by visually identifying distinct morphologically viable colonies. The surviving fraction at each interval was compared to that of cells irradiated with the full 2 Gy dose without time gaps. The repair factor was calculated as the percentage change in survival due to each time interval, providing insight into the cells’ capacity to repair DNA damage.
2.5. Gamma-H2AX Foci Assay
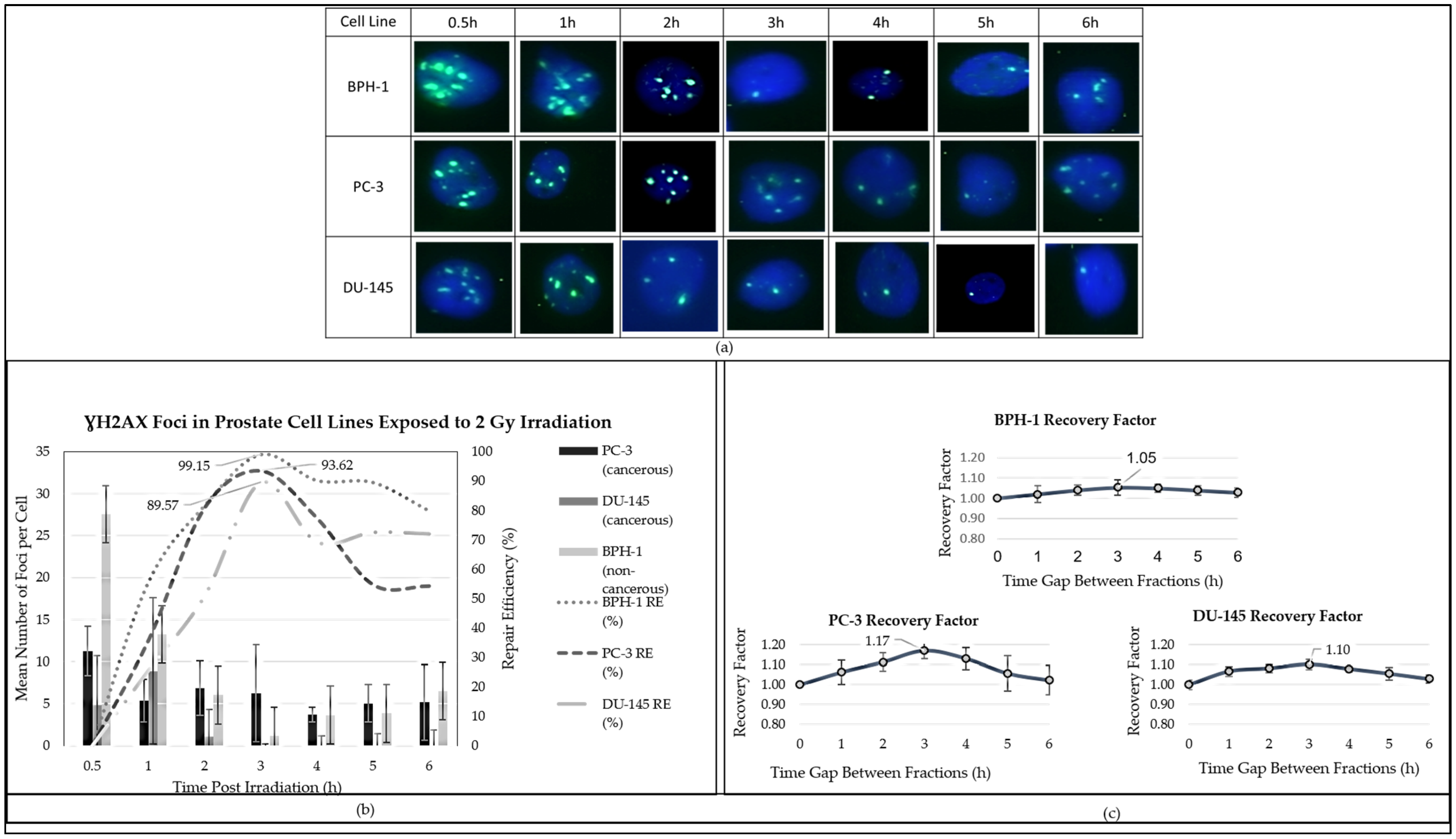
The γ-H2AX foci assay, as described by Nair et al. (2021), was employed to investigate DNA damage and repair kinetics following radiation exposure [
64]. One million cells were irradiated with 2 Gy and incubated for 0–6 h. Following the harvesting and trypsinisation of one million cells, they were irradiated with a dose of 2 Gy and incubated for time intervals ranging from 0 to 6 h. For fixation, the cells were treated with PBS containing 3% paraformaldehyde (PFA, freshly prepared) for 20 min, followed by overnight storage in PBS with 0.5% PFA. Immunostaining was performed using a primary anti-γ-H2AX antibody (catalogue number: 613402, Biocom Africa, South Africa) and a secondary rabbit anti-mouse FITC antibody (catalogue number: 31561, LTC Tech, South Africa) prior to quantification. The average number of γ-H2AX foci per cell was evaluated using the MetaCyte software module of the Metafer version 4 scanning system (MetaSystems, Altlussheim, Germany). A minimum of three slides were scored for each exposure condition.
To illustrate the repair processes of DNA damage induced by radiation, the efficiency of radiation-induced DNA damage repair was calculated using Equation (3):
where
REt represents the repair efficiency at timepoint (
t),
γ-H2
AXmax represents the maximum amount of foci observed in response to 2 Gy, and
γ-H2
AXt the amount of foci observed at the given timepoint in response to 2 Gy.
This methodology outlines the experimental steps and analysis involved in assessing the DNA damage response through immunostaining of γ-H2AX foci following 2 Gy irradiation at different timepoints. The repair efficiency (RE) was calculated based on the methodology outlined by Panek and Miszczyk (2019), with slight modifications to accommodate our experimental conditions [
65].
2.6. Migration Assay
The migration assay was conducted to investigate the migratory behaviour of the BPH-1 cell line, which serves as a non-cancerous model for benign prostatic hyperplasia (BPH). By using a non-cancerous cell line, this study aims to elucidate the effects of radiation on normal prostate tissue, thereby providing valuable insights into the response of non-cancerous cells in contrast to malignant counterparts. Understanding these dynamics is crucial for developing effective radiotherapy strategies that minimise damage to non-cancerous cells while effectively targeting tumour cells. Cells were seeded in T25 flasks at a density that ensured 100% confluence after 24 h. Cell suspensions were prepared and added to each well of the Culture-Insert 2 Well. After confluence was reached, the insert (Culture-Insert 2 Well ibidi® GmbH, Catalogue number: 80209-150, Gräfelfing, Germany) was carefully removed, and non-attached cells and debris were washed away with cell-free medium.
Irradiation was administered at a dose of 2 Gy per fraction, totalling 8 Gy delivered in 4 fractions with a 3 h interval between each conventional fractionation. For the hypofractionation approach, a single dose of 4.93 Gy was applied. Both fractionation schemes resulted in a similar biological effective dose (BED) of 13.93 Gy, allowing for a comparative analysis of the effects on cell migration under different radiotherapy regimens. Following irradiation, the cells were monitored using the CytoSMART™ System, a compact live cell imaging system acquired from Lonza (supplied locally by Whitehead Scientific, Cape Town, South Africa), which enables time-lapse observation every 4 h without disturbing the cultures. Images were captured at regular intervals with a 10× objective lens and analysed using ImageJ software (Version 1.54i) to quantify cell migration dynamics. The change in the gap area over time was plotted, and linear regression analysis was conducted to calculate the slope of the linear phase, representing the migration speed of the cells.
2.7. Invasion Assay
The invasion assay is a crucial tool in cancer research for assessing the invasive potential of tumour cells, mimicking the intricate process of cancer metastasis [
62]. This assay provides insights into the molecular and cellular determinants of tumour invasion. The transwell invasion assay was employed to investigate the invasive properties of prostate cancer cell lines (PC-3 and DU-145), using Geltrex
® (catalogue number: A1413202), obtained from LTC Tech South Africa (Pty) Ltd., as a substrate. The assay was performed per the manufacturer’s instructions, involving preparation, staining, and quantification. Briefly, irradiated cells were treated with a total dose of 8.00 Gy in four fractions for both PC-3 and DU-145 cell lines, with each fraction delivered at 2.00 Gy and a 3 h gap between fractions, while in the hypofractionation scheme, cells received single doses of 5.15 Gy and 4.85 Gy, respectively, before being added to the upper chamber, while complete media with 10% FBS was added to the lower well. The insert (LTC Tech South Africa Pty Ltd., catalogue number 140640) was incubated for 24 h at 37 °C. After incubation, the insert was removed, washed with PBS, fixed with 4% paraformaldehyde, and stained with Crystal Violet. Invaded cells were quantified at 10× magnification using the CytoSMART Lux 10 imaging system (Version 2, Lonza, Basel, Switzerland supplied locally by Whitehead Scientific) and expressed as a percentage of the total number of seeded cells.
2.8. Lactate Dehydrogenase (LDH) Assay
The LDH assay, a well-established method for evaluating cellular health, was used to assess cytotoxicity by quantifying lactate dehydrogenase enzyme release into the culture supernatant. LDH, a ubiquitous cytoplasmic enzyme, indicates cellular damage when found extracellularly [
62]. The LDH-Cytox Assay Kit (Biocom Africa, Catalogue: 426401) was employed according to the manufacturer’s instructions, which included meticulous cell preparation and incubation with test substances. Both cancerous (DU-145, PC-3) and non-cancerous (BPH-1) prostate cell lines were cultured in complete growth medium under standard conditions (37 °C, 5% CO
2) and prepared at a concentration of 5 × 10
5 cells/mL. These cells were then subjected to either conventional fractionation (2.00 Gy × 4 fractions for a total of 8.00 Gy with a 3 h inter-fractional interval) or hypofractionation (single doses of 4.93 Gy for BPH-1, 4.85 Gy for DU-145, and 5.15 Gy for PC-3). To determine the baseline LDH release from unirradiated cells, a control group of cells that were not exposed to radiation was included, and their LDH levels were measured using the LDH-Cytox Assay Kit.
The percentage of LDH release was calculated by comparing the absorbance values obtained from the test groups to the absorbance from the control group of unirradiated cells. Specifically, LDH release percentage refers to the amount of LDH released from the treated cells relative to the maximum release (referred to as ‘high control’), which was defined by a control in which the cells were completely lysed to release all LDH into the supernatant. This allowed for calculating the amount of LDH released in the treated samples as a percentage of the total LDH release. Following irradiation, the released LDH levels were measured using the LDH-Cytox Assay Kit (Biocom Africa, Centurion, South Africa, Catalogue: 426401) according to the manufacturer’s instructions, and absorbance was determined at 490 nm using a microplate reader (Berthold Technologies, Bad Wildbad, Germany, Version 2.2.2.1).
2.9. Data Analysis
Data analysis was conducted meticulously to ensure the reliability and reproducibility of the results. This study aimed to compare the responses of various cell lines to two radiotherapy techniques: conventional fractionation and hypofractionation. Multiple assays were performed to assess the effects of these radiotherapy methods on prostate cell lines in vitro. Microsoft Excel (Microsoft Office Standard 2019) was utilised for initial data organisation and basic statistical analysis. Means and standard deviations for each experimental condition were calculated to describe the variability in cell responses, with standard deviation derived from the formula that quantifies the dispersion of data points around the mean.
Logarithmic plots were generated using GraphPad Prism (version 10.0.0 for Windows), with the radiation dose plotted on the x-axis and the survival fraction plotted on the y-axis. To analyse the survival fraction data, nonlinear regression analysis was performed using the Marquardt–Levenberg algorithm in GraphPad Prism. This approach facilitated the fitting of survival curves to the LQ model, effectively modelling the relationship between radiation dose and cell survival. During this analysis, the estimated parameters (α and β) were derived, providing critical insights into the cellular responses to radiation exposure. The α and β values obtained from the survival curves were compared across the various cell lines. Statistical analyses were performed using analysis of variance (ANOVA) to assess differences among the different fractionation conditions. For all statistical tests, p-values were calculated to assess the significance of the observed differences. A p-value of less than 0.05 indicated that the differences in radiation response metrics among the treatment conditions were unlikely to have occurred by chance, thereby allowing for meaningful conclusions regarding the effects of conventional versus hypofractionation radiotherapy.
4. Discussion
The distinct radiobiological characteristics of prostate cell lines are elucidated through the analysis of their α and β values, which inform their radiosensitivity and potential responses to radiation therapy (see
Table 2). Among the tested cell lines, PC-3 cells exhibited the highest α value of 0.12 Gy
−1, indicating a greater sensitivity to radiation-induced cell death compared to BPH-1 (α = 0.08 Gy
−1) and DU-145 (α = 0.10 Gy
−1). Notably, while PC-3 cells shared the same β value of 0.03 Gy
−2 as BPH-1, Du-145 cells demonstrated a higher β value of 0.04 Gy
−2, indicating more efficient DNA repair mechanisms. Consequently, the lowest α/β ratio of the PC-3 cells highlights their heightened radiosensitivity, which is attributed to its increased susceptibility to radiation. The low α/β ratios observed in both PC-3 and DU-145 further indicate responsiveness to fractionation, where higher doses per fraction may overwhelm repair mechanisms, facilitating increased cell death or growth inhibition. Regarding neuroendocrine differentiation, PC-3 exhibits characteristics consistent with neuroendocrine differentiation, which is associated with a poor prognosis, while DU-145 generally lacks these characteristics. Jayakumar et al. (2014) reported reduced radiosensitivity in DU-145 cells compared to PC-3 cells, indicating that individual cellular characteristics significantly influence treatment outcomes [
66]. Though, Lövey et al. (2013) noted that although PC-3 cells demonstrate comparable radiosensitivity at lower doses (0.5 and 2 Gy), their surviving fraction declines more steeply relative to DU-145 as the radiation dose increases, thereby highlighting the complex relationship between radiation dose and cellular response [
67,
68]. Despite these differences, the relative sensitivity (RS) values of PC-3 and DU-145 compared to BPH-1 showed no statistically significant differences, suggesting that both cancerous lines have similarly elevated sensitivity to radiation. In terms of fractionation schemes, the α/β ratios provide valuable insights into potential responses, with PC-3 displaying the highest overall ratio and thus indicating greater sensitivity than both DU-145 and BPH-1. Importantly, the fractionation time gap between radiation fractions was influenced by both repair efficiency, as determined from γ-H2AX foci analysis, and the recovery factor derived from the survival fraction. However, this assessment has its limitations, as it relies heavily on the γ-H2AX method for measuring DNA damage repair, which may not capture the complete picture of the cellular response.
The concept of a “therapeutic window” pertains to radiation doses that effectively target and kill cancer cells while minimising damage to normal tissues. The observed RS values, coupled with the narrower therapeutic window in cancerous cell lines, underscore the potential for enhanced treatment efficacy [
69]. Predominant β values of 0.03 Gy
−2 across all cell lines imply comparable DNA repair efficiencies, revealing that variations in radiation sensitivity are primarily governed by differences in α values. These initial findings align with the work of van Leeuwen et al. (2018), where α values across prostate cell lines varied from 0.1 to 0.3 Gy
−1, and β values ranged from 0.01 to 0.06 Gy
−2 [
27]. The observed RS values may also be influenced by the roles of non-homologous end joining (NHEJ) and homologous recombination repair (HRR) in DNA repair, which are critical for understanding the cellular response to radiation, especially in the context of hypofractionated radiotherapy.
NHEJ, which is the predominant pathway for repairing double-strand breaks (DSBs) in mammalian cells, occurs throughout the cell cycle and is especially crucial during the G1 phase. This pathway allows for rapid repair by directly ligating broken DNA ends, although it may lead to errors due to the potential loss of genetic information at the joining sites. In our findings, the rapid appearance of foci post-irradiation can likely be attributed to the engagement of the NHEJ pathway, which mobilises repair proteins to the site of damage swiftly, consequently preventing cell death. Conversely, HRR operates primarily during the S and G2 phases and employs a sister chromatid as a template for error-free repair, making it more accurate than NHEJ. The eventual disappearance of foci could indicate a shift towards HRR after initial NHEJ processing, suggesting a dynamic interplay of repair mechanisms. The combined evidence of lower α/β ratios, reduced D50 values, and consistent β values in cancerous prostate cell lines supports the hypothesis that hypofractionated radiotherapy could possibly leverage the differential responses between cancerous and non-cancerous prostate cells. Furthermore, the interplay between NHEJ and HRR may influence therapeutic outcomes, as variations in repair proficiency could lead to distinct survival rates of cancerous versus normal cells. This necessitates further inquiry into optimal fractionation regimens for treating prostate cancer, taking into account the dynamics of radiation-induced cell death and the activation of these DNA repair mechanisms.
Interestingly, the intrinsic survival estimated through the LQ model did not consistently align with the observed survival following a single hypofractionated dose (see
Table 3). For instance, the intrinsic survival of BPH-1 was calculated at 32.5 ± 3.5%, while the observed survival posts at a 4.93 Gy dose were lower at 21.3 ± 4.0%, implying that the LQ model underestimates the radiosensitivity of these cells. Conversely, PC-3 cells exhibited an observed surviving rate of 25.5 ± 2.0% after a 5.15 Gy application, which surpassed predicted values, indicating an overestimation of their radiosensitivity by the LQ model. In contrast, DU-145 cells displayed an observed survival closely resembling their intrinsic survival at 22.9 ± 2.0%, thus indicating a more accurate prediction using the LQ model. Statistically significant differences were found between the predicted and observed survivals for BPH-1 cells (
p =
0.0012), while no significant divergence was observed for PC-3 or DU-145 (
p =
0.5612 and
p =
0.3148, respectively). These results underscore the limitations of the LQ model in predicting radiosensitivity, suggesting that more nuanced models may be necessary to accurately account for cellular responses in specific contexts. Investigations by Cui et al. (2020) indicate that converting conventionally fractionated doses to a single high dose may necessitate the use of alternative formulas or a higher α/β ratio within standard LQ models [
69].
Following the administration of a 0.5 Gy priming dose, significant adaptive responses were noted across the prostate cell lines, indicative of inherent radioresistance (
p <
0.05). The CF and HF with a 0.5 Gy priming dose resulted in increased survival percentages for all tested cell lines, specifically BPH-1 (15.28 to 17.36%,
p <
0.01), PC-3 (19.08 to 22.88,
p <
0.05), and DU-145 (17.23 to 20.27%,
p <
0.05). These results suggest robust DNA repair mechanisms among prostate cell lines, as illustrated in
Figure 1a, and imply that efficient repair of DNA damage correlates with increased survival rates. Prostate cancer cells such as PC-3 and DU-145 typically present alterations in cell cycle regulation and apoptosis pathways, including mutations in tumour suppressor genes (notably p53) and overexpression of anti-apoptotic proteins. This allows them to evade programmed cell death [
70]. Diverging from the expected outcomes, the adaptive responses observed in both BPH-1 and PC-3 cells imply that factors beyond the p53 status contribute to their observed radioresistance [
71]. Integral to cancer treatment strategies, these findings indicate that non-cancerous BPH-1 cells exhibited enhanced survival rates and adaptive response under HF, highlighting pronounced repair mechanisms (
p <
0.001). Meanwhile, the aggressive PC-3 cells exhibited a statistically significant preference for CF over HF (
p <
0.05), suggesting that HF strategies may benefit normal prostate tissue, whereas CF should be prioritised for aggressive cancer cells such as PC-3. This advocates for an integrated therapeutic approach designed to protect healthy tissues while optimising treatment outcomes based on tumour aggressiveness. Notably, the addition of a 0.5 Gy priming dose to CF enhanced the survival rate in non-cancerous BPH-1 cells, indicating a potentially beneficial effect of priming on these cells (
p <
0.01). In contrast, the inclusion of a 0.5 Gy priming dose to CF and HF failed to reduce survival rates in PC-3 and DU-145 cells, suggesting that priming may not have a significant effect on the survival of these cancerous cells (
p <
0.05). These findings suggest that a personalised approach to radiation therapy may be necessary, considering the specific characteristics of each tumour type and its sensitivity to different fractionation schemes and priming doses. This study underscores the importance of accounting for individual differences in DNA repair mechanisms, cell cycle regulation, and responses to cellular stress in developing radiation therapy protocols for prostate cancer. Combining HF and CF may improve therapeutic outcomes by leveraging the differential adaptive responses of non-cancerous and aggressive cancerous prostate cell lines. Therefore, a personalised approach to radiation therapy is essential, considering the unique characteristics of each tumour type and its sensitivity to varying fractionation schemes and priming doses.
The migration assay conducted on BPH-1 prostate cells provided valuable insights into the impact of radiation therapy on cell motility. The non-irradiated group exhibited rapid migration, whereas both the CF-treated and HF-treated groups displayed reduced speeds. This observation implies that radiation exposure inhibits the migration ability of non-cancerous cells and aligns with findings from other studies [
68,
69]. The comparison of migration rates between the CF and HF treatment groups indicated significant differences in slope values (
p =
0.0002), suggesting that fractionation regimens differentially affect prostate cell motility. Importantly, both irradiation groups exhibited significantly slower migration rates compared to the control group (
p <
0.001), underscoring the impact of radiation therapy on cell migration regardless of the fractionation regimen employed. The slower migration observed in the HF group may be attributed to enhanced cellular processes, such as activated repair mechanisms and metabolic activity, facilitating recovery despite reduced motility. Furthermore, the influence of radiation on cell adhesion is crucial in understanding the overall effects of radiation therapy on cellular behaviour within prostate tissue, particularly the observed reductions in motility alongside potential increases in tissue integrity and cellular communication [
70,
71,
72,
73,
74,
75].
The invasion assay conducted on prostate cancer cell lines (PC-3 and DU-145) revealed their invasive potential following exposure to different radiation fractionation regimes. While both cell lines are characterised by p53 mutations and originate from metastatic prostate adenocarcinoma, they inherently display a high degree of aggressiveness and a propensity for invasion. The irradiated groups showed increased invasion compared to the control groups, highlighting the potential impact of radiation on enhancing invasive capabilities. These findings corroborate those reported by Chang et al. (2014), who observed increased invasive behaviour in prostate cell lines following irradiation [
76]. Although both PC-3 and DU-145 exhibited slightly higher invasion capacities under CF compared to HF, these differences were not statistically significant. This suggests that the invasiveness of these cell lines may not be significantly influenced by the radiation fractionation schedule. However, it is important to note that these findings are limited to the PC-3 and DU-145 cell lines and may not comprehensively represent the diverse spectrum of prostate cancer types or their responses to fractionation changes, particularly concerning survival and invasion capabilities. The irradiation of immortalised cells may exacerbate p53 mutations while impairing the regulatory pathways that control cell cycle arrest and DNA repair [
71,
77]. This suggests that radiation may enhance the invasive potential of cells by disrupting key cellular mechanisms, although the lack of a significant difference between CF and HF indicates that both fractionation schemes may similarly impact their invasive behaviour due to shared underlying molecular pathways.
In summary, lactate dehydrogenase (LDH) assays demonstrate varying responses to radiation fractionation schemes among non-cancerous and cancerous prostate cell lines. For BPH-1 cells, the LDH release showed significant differences between CF and HF treatments (24% vs. 25%, p < 0.01), indicating that HF induces lower levels of cytotoxicity. This excess survival observed under HF suggests enhanced DNA repair capability among non-cancerous cells, making HF a potentially less damaging option and reducing the risk of adverse effects. Conversely, for the cancerous cell lines DU-145 and PC-3, LDH release did not show significant differences between CF and HF. DU-145 cells exhibited 19% LDH release under HF and 21% under CF (p = 0.084), while PC-3 cells showed 18% under HF and 19% under CF (p = 0.314). The lack of significant differences in LDH release might be attributable to their p53 mutant status, which could lead to consistent levels of membrane damage, irrespective of the fractionation scheme. However, the notable increase in surviving fraction under HF for PC-3 suggests a more effective repair of radiation-induced damage, thereby reducing cytotoxic effects.
Moreover, the potential involvement of senescence in LDH release among cancerous cell lines warrants investigation, as radiation has been shown to increase the expression of γ-H2AX, a marker for senescence [
76]. The similar LDH release observed despite increased survival for HF may indicate a rise in senescence within CF-treated cancer cells. This increase in senescence could lead to altered metabolic activity, which may not be immediately reflected in LDH assays that measure acute cytotoxicity. These findings highlight the complexity of cellular responses to different fractionation schemes and underscore the need for tailored treatment strategies that consider the specific characteristics of each tumour type and their sensitivity to varying fractionation schemes. In this context, the role of imaging techniques becomes crucial. PSMA PET/CT imaging has emerged as a valuable tool in the diagnosis of and therapy for aggressive prostate cancer [
78]. By providing highly sensitive detection of prostate-specific membrane antigen (PSMA) expression, PSMA PET/CT allows for more accurate localisation of cancerous lesions and aids in the assessment of disease burden. This enhanced precision in treatment planning, including radiation therapy, is critical in this setting [
77]. Furthermore, the integration of PSMA PET/CT into clinical practice plays a significant role in identifying candidates for more aggressive treatment approaches. It can help determine which patients may benefit from dose escalation strategies, particularly in cases of recurrent or metastatic disease. This integration can be particularly beneficial for tailoring hypofractionated radiation therapy, as it aligns treatment more closely with the biology of the tumour and its metastatic spread. Ultimately, this approach aims to improve therapeutic efficacy by optimising treatment based on individual tumour characteristics. However, it is crucial to emphasise that recommendations for personalised radiation therapy approaches should be grounded in robust in vivo data, as conclusions drawn solely from in vitro studies may not adequately reflect the complexities of tumour behaviour in a living organism. Further research is required to fully understand the long-term effects and potential therapeutic implications of different fractionation strategies, particularly concerning their roles in influencing senescence and cancer progression. Only through comprehensive in vivo studies can the safety, efficacy, and practicality of personalised treatment regimens be validated. Optimising radiation therapy protocols for metastatic prostate cancer remains a critical endeavour, as it has the potential to significantly improve therapeutic outcomes based on the unique responses of the respective cell lines. Notably, the PC-3 cell line demonstrated significantly increased survival under HF treatment, indicating its resilience in a castration-resistant context. In contrast, DU-145 exhibited no significant survival differences between the CF and HF treatments, suggesting HF non-inferiority to CF. However, these cell lines represent only two types of prostate cancer, each with distinct biological characteristics and implications for treatment strategies, and do not comprehensively represent the diverse spectrum of prostate cancer types. PC-3 is characterised by neuroendocrine differentiation, which is often associated with a poor prognosis, while DU-145 generally lacks these neuroendocrine traits. Understanding the differential responses between these cell lines is crucial, particularly as they model aspects of hormone-refractory prostate cancer and the complexities of CRPC. By tailoring radiation therapy protocols to these unique cellular responses, researchers may enhance therapeutic efficacy and improve outcomes for patients suffering from advanced metastatic prostate cancer.
5. Conclusions
Prostate cancer is the second most common cancer diagnosis globally among men, accounting for over 1.4 million new cases each year. In the United States alone, it is estimated that 1 in 9 men will be diagnosed with prostate cancer during their lifetime. In sub-Saharan Africa, prostate cancer accounts for 23% of all male cancers, a significant burden in regions where resources are already strained. The field of cancer treatment faces numerous challenges, particularly when it comes to managing prostate cancer. One significant issue is the risk of excessive pre-treatment radiation doses inducing a radiation adaptive response, which can lead to increased radioresistance in some prostate cell types. This underscores the need for individualised treatment approaches to effectively counteract unintended radiation exposure. In response, advancements in radiation therapy, such as the adoption of shortened fractionation regimens, aim to improve oncologic outcomes and patient comfort. This highlights the importance of tailoring radiation therapy approaches to the unique characteristics of prostate cancer. The findings suggest that HF may be a more effective treatment option for preserving non-cancerous prostate cells, while CF may be more suitable for controlling aggressive prostate cancer subtypes, particularly castration-resistant (PC-3) variants. In contrast, no significant differences were observed between CF and HF for hormone-refractory (DU-145) variants. While PC-3 cells showed higher survival fractions under hypofractionation, reflecting a robust capacity for DNA repair that could enable tumours to endure treatment, it is important to note that the study may not comprehensively represent the diverse spectrum of prostate cancer types. The observed differences in survival rates, migration, and invasion patterns between non-cancerous and cancerous prostate cells underscore the need for personalised radiation therapy strategies.
HF can result in lower LDH release and higher survival fractions in non-cancerous prostate cells, suggesting reduced cytotoxicity and better preservation of cell integrity. In contrast, cancerous cell lines exhibited varying responses to fractionation schemes, with PC-3 cells showing higher survival fractions under HF and DU-145 cells exhibiting no significant difference in survival. These findings have important implications for the development of optimal treatment strategies for prostate cancer patients.
In light of these results, it is recommended that healthcare providers consider personalised radiation therapy approaches for patients with prostate cancer, taking into account the unique characteristics of each patient’s cancer. However, these preliminary in vitro data should be confirmed in in vivo studies in any subset of high-risk prostate cancer. To improve treatment outcomes for prostate cancer patients, it is essential to prioritise timely and effective access to radiation therapy, particularly in resource-constrained settings. Further research is needed to investigate the long-term effects of radiation therapy on patients with prostate cancer, including the potential for delayed effects and impact on quality of life. Additionally, cost-effective strategies must be developed to deliver radiation therapy to underserved communities, ensuring equitable access to this important treatment modality. However, it is important to note that the current study’s findings are limited by the use of PC-3 and DU-145 cell lines, which may not comprehensively represent the diverse spectrum of prostate cancer types.