Chernobyl’s Aftermath: Multiple Manifestations of Basalioma in a Patient after Radioactive Contamination in 1986
Abstract
Simple Summary
Abstract
1. Introduction
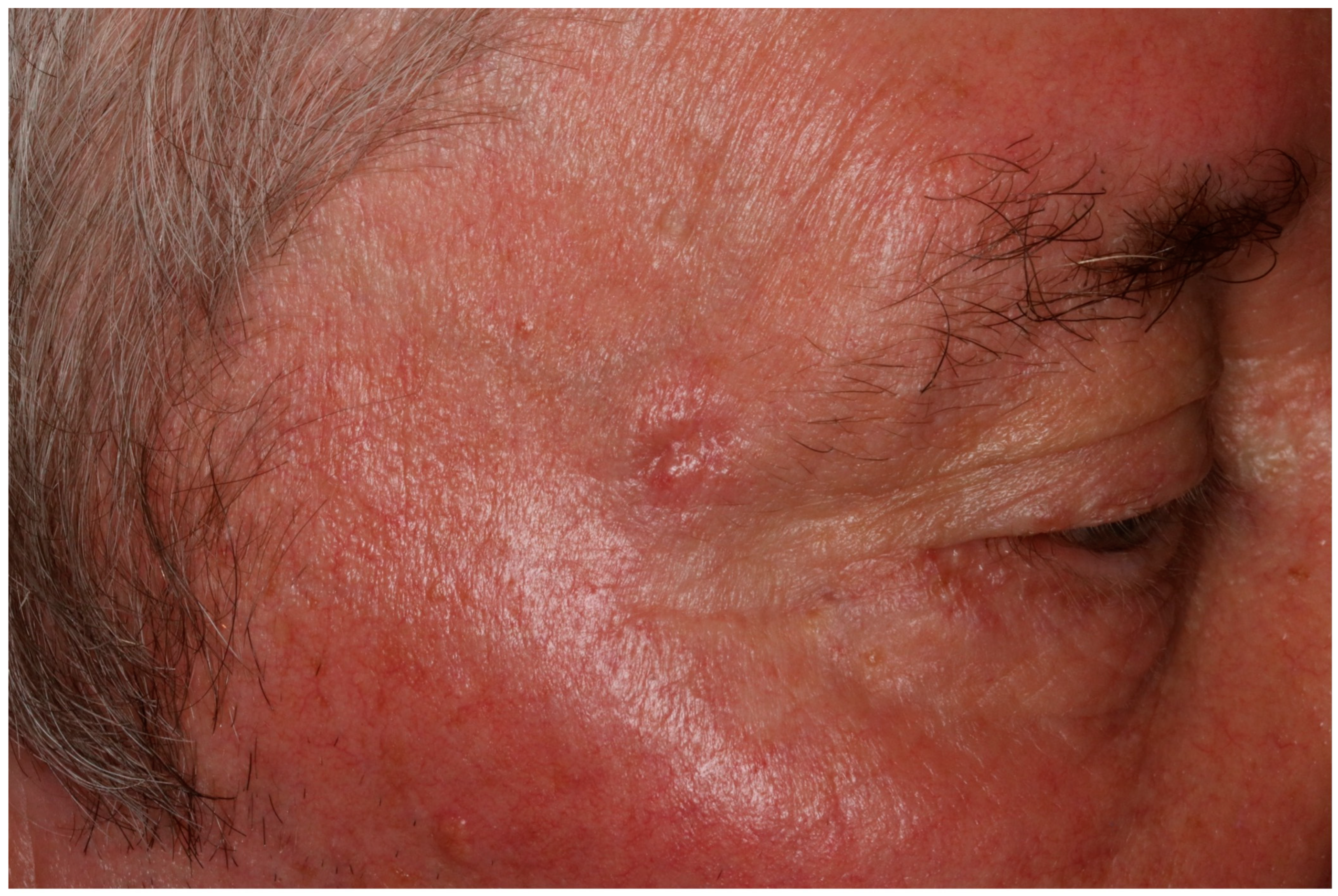
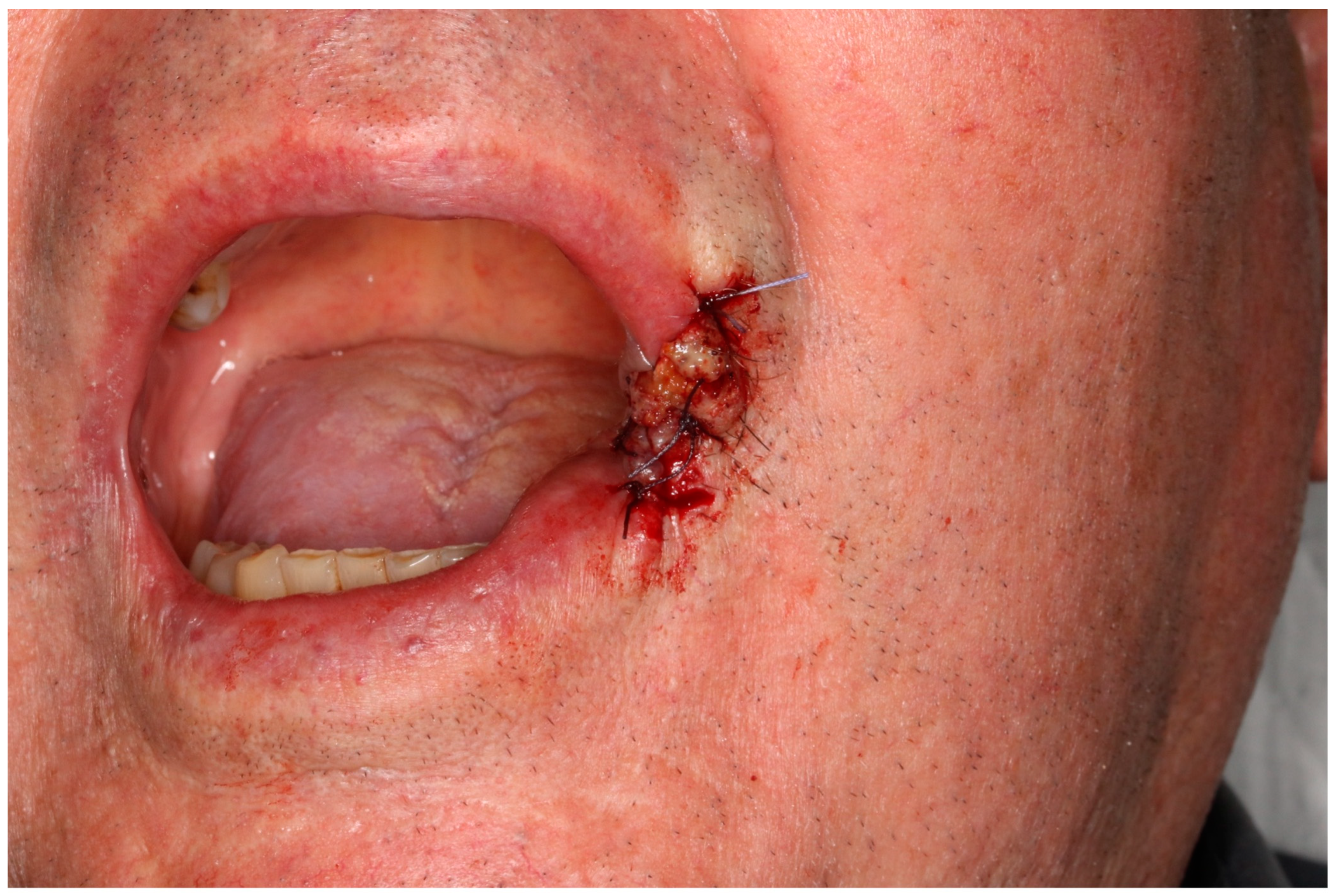
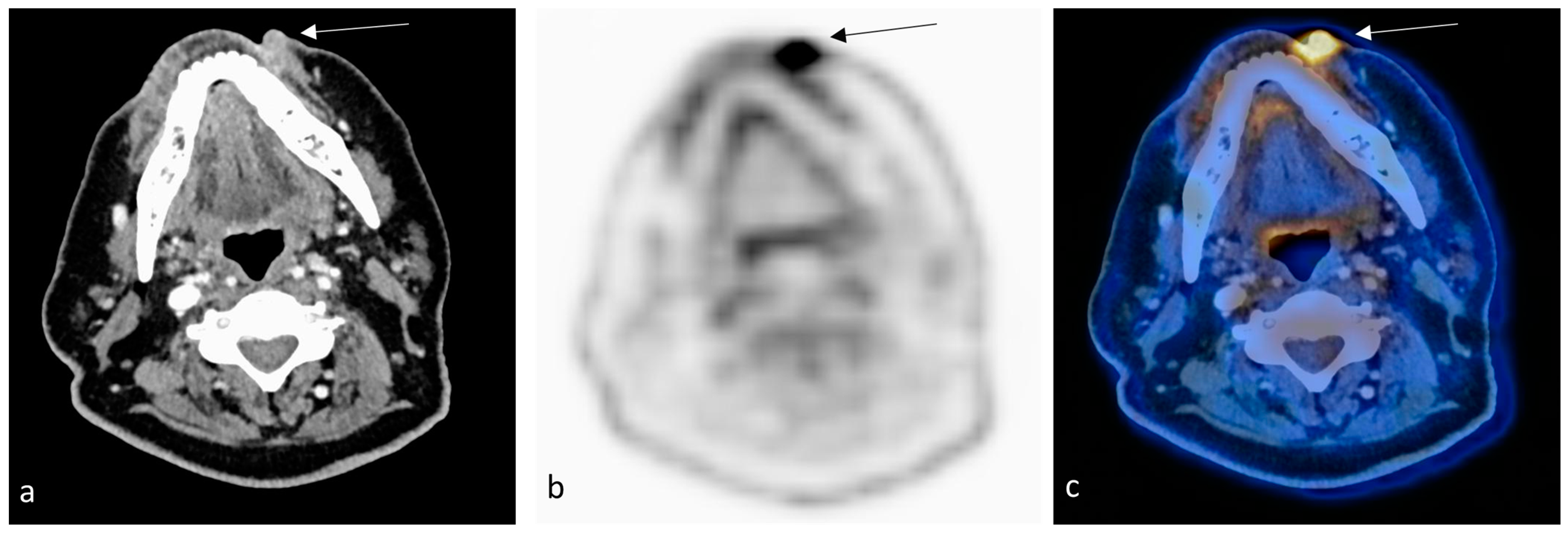
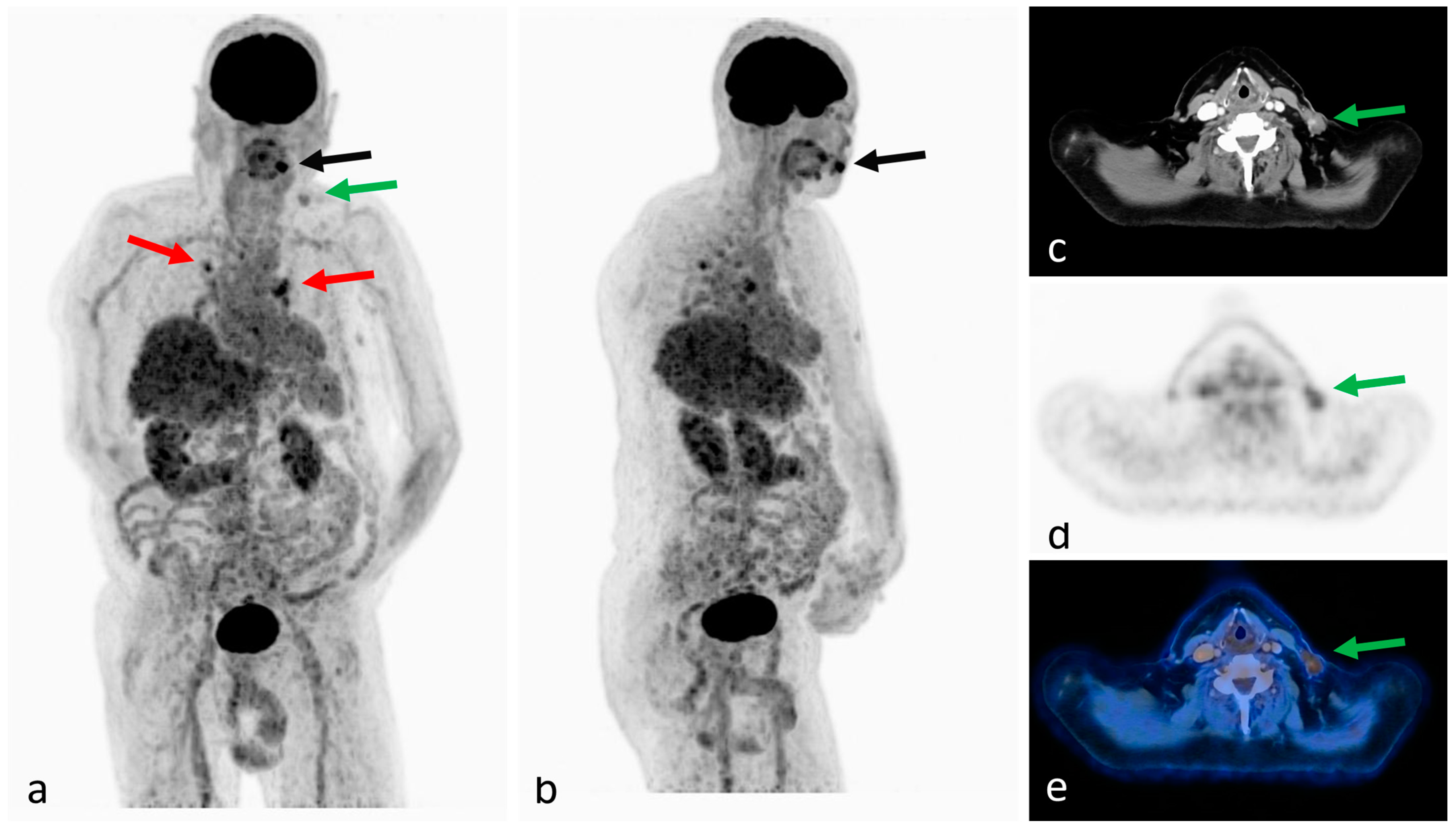
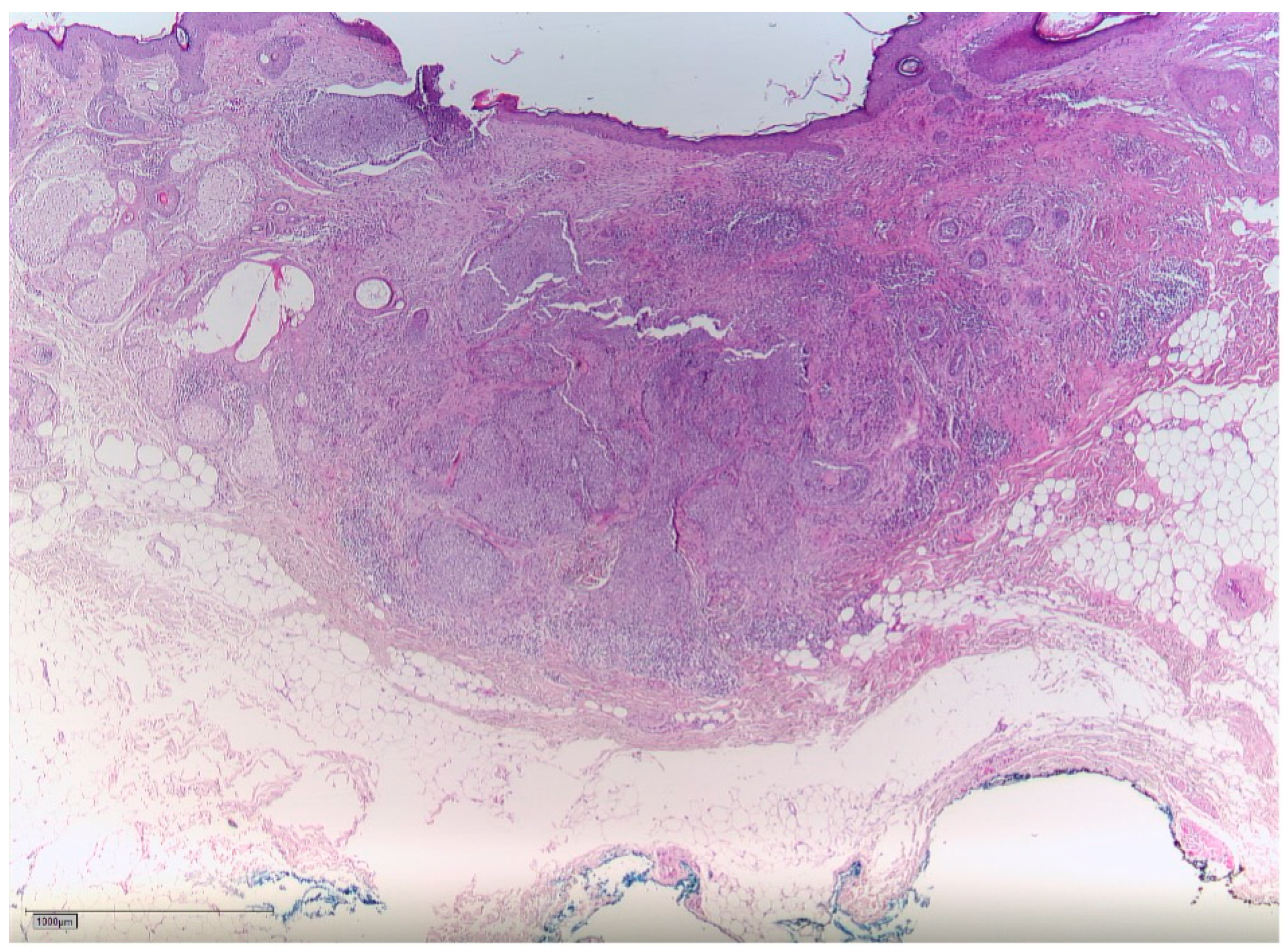
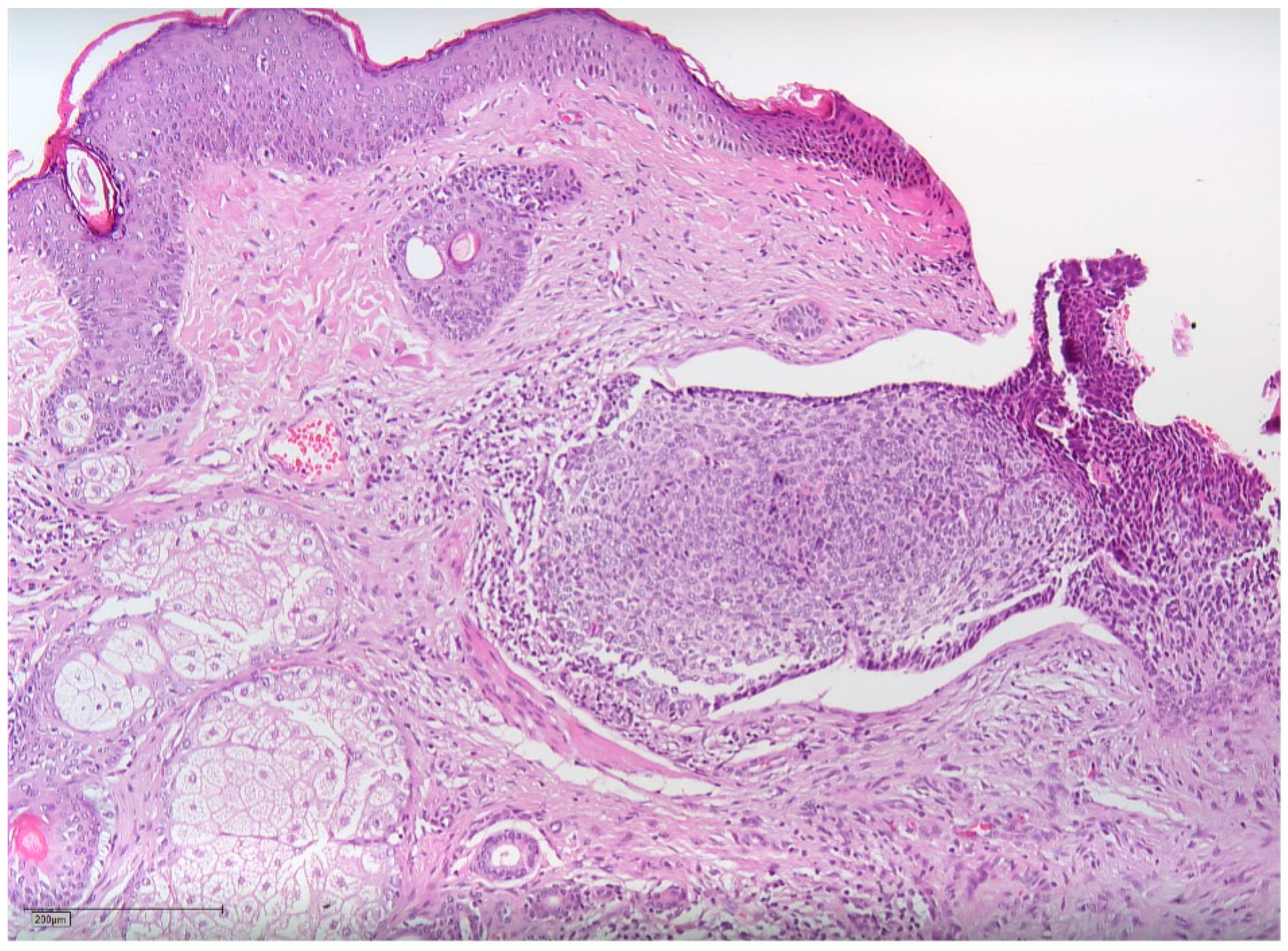
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Atomic Energy Agency. The Chernobyl Accident: Updating of INSAG-1. A Report by the International Nuclear Safety Advisory Group; Safety Series; International Atomic Energy Agency: Vienna, Austria, 1992; Volume INSAG-7, 136p. [Google Scholar]
- McCall, C. Chernobyl disaster 30 years on: Lessons not learned. Lancet 2016, 387, 1707–1708. [Google Scholar] [CrossRef]
- Sugiyama, H.; Misumi, M.; Kishikawa, M.; Iseki, M.; Yonehara, S.; Hayashi, T.; Soda, M.; Tokuoka, S.; Shimizu, Y.; Sakata, R.; et al. Skin Cancer Incidence among Atomic Bomb Survivors from 1958 to 1996. Radiat. Res. 2014, 181, 531–539. [Google Scholar] [CrossRef]
- Karagas, M.R.; McDonald, J.A.; Greendberg, E.R.; Stukel, T.A.; Weiss, J.E.; Baron, J.A.; Stevens, M.M. For the Skin Cancer Prevention Study Group Risk of Basal Cell and Squamous Cell Skin Cancers After Ionizing Radiation Therapy. JNCI J. Natl. Cancer Inst. 1996, 88, 1848–1853. [Google Scholar] [CrossRef]
- Eagan, J.T., Jr.; Jones, C.T. Cutaneous Cancers in an Interventional Cardiologist: A Cautionary Tale: Cutaneous cancers in an interventional cardiologist. J. Intervent. Cardiol. 2011, 24, 49–55. [Google Scholar] [CrossRef]
- Shore, R.E.; Albert, R.E.; Pasternack, B.S. Follow-up Study of Patients Treated by X-ray Epilation for Tinea Capitis: Resurvey of Post-Treatment Illness and Mortality Experience. Arch. Environ. Health Int. J. 1976, 31, 21–28. [Google Scholar] [CrossRef]
- Mizuno, T.; Tokuoka, S.; Kishikawa, M.; Nakashima, E.; Mabuchi, K.; Iwamoto, K.S. Molecular basis of basal cell carcinogenesis in the atomic-bomb survivor population: p53 and PTCH gene alterations. Carcinogenesis 2006, 27, 2286–2294. [Google Scholar] [CrossRef][Green Version]
- Baheti, A.D.; Tirumani, S.H.; Giardino, A.; Rosenthal, M.H.; Tirumani, H.; Krajewski, K.; Ramaiya, N.H. Basal Cell Carcinoma: A Comprehensive Review for the Radiologist. Am. J. Roentgenol. 2015, 204, W132–W140. [Google Scholar] [CrossRef]
- Lang, B.; Balermpas, P.; Bauer, A. S2k-Leitlinie Basalzellkarzinom der Haut (Aktualisierung 2017/2018). J. Dtsch. Dermatol. Ges. 2019, 17, 94–104. [Google Scholar]
- Fosko, S.W. Positron Emission Tomography for Basal Cell Carcinoma of the Head and Neck. Arch. Dermatol. 2003, 139, 1141. [Google Scholar] [CrossRef]
- Weiss, G.J.; Korn, R.L. Metastatic basal cell carcinoma in the era of hedgehog signaling pathway inhibitors: Metastatic Basal Cell Carcinoma. Cancer 2012, 118, 5310–5319. [Google Scholar] [CrossRef] [PubMed]
- Leisenring, W.; Friedman, D.L.; Flowers, M.E.D.; Schwartz, J.L.; Deeg, H.J. Nonmelanoma Skin and Mucosal Cancers After Hematopoietic Cell Transplantation. J. Clin. Oncol. 2006, 24, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Prysyazhnyuk, A.; Gristchenko, V.; Fedorenko, Z.; Gulak, L.; Fuzik, M.; Slipenyuk, K.; Tirmarche, M. Twenty years after the Chernobyl accident: Solid cancer incidence in various groups of the Ukrainian population. Radiat. Environ. Biophys. 2007, 46, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bouville, A.; Likhtarev, I.A.; Kovgan, L.N.; Minenko, V.F.; Shinkarev, S.M.; Drozdovitch, V.V. Radiation dosimetry for highly contaminated belarusian, russian and ukrainian populations, and for less contaminated populations in europe. Health Phys. 2007, 93, 487–501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romanenko, A.; Morimura, K.; Wanibuchi, H.; Salim, E.I.; Kinoshita, A.; Kaneko, M.; Vozianov, A.; Fukushima, S. Increased oxidative stress with gene alteration in urinary bladder urothelium after the Chernobyl accident. Int. J. Cancer 2000, 86, 790–798. [Google Scholar] [CrossRef]
- El Ghissassi, F.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part D: Radiation. Lancet Oncol. 2009, 10, 751–752. [Google Scholar] [CrossRef]
- Williams, D. Radiation carcinogenesis: Lessons from Chernobyl. Oncogene 2008, 27, S9–S18. [Google Scholar] [CrossRef]
- Cardis, E.; Krewski, D.; Boniol, M.; Drozdovitch, V.; Darby, S.C.; Gilbert, E.S.; Akiba, S.; Benichou, J.; Ferlay, J.; Gandini, S.; et al. Estimates of the cancer burden in Europe from radioactive fallout from the Chernobyl accident. Int. J. Cancer 2006, 119, 1224–1235. [Google Scholar] [CrossRef]
- Gottlöber, P.; Steinert, M.; Weiss, M.; Bebeshko, V.; Belyi, D.; Nadejina, N.; Stefani, F.H.; Wagemaker, G.; Fliedner, T.M.; Peter, R.U. The Outcome of Local Radiation Injuries: 14 Years of Follow-up after the Chernobyl Accident. Radiat. Res. 2001, 155, 409–416. [Google Scholar] [CrossRef]
- Athar, M.; Li, C.; Kim, A.L.; Spiegelman, V.S.; Bickers, D.R. Sonic Hedgehog Signaling in Basal Cell Nevus Syndrome. Cancer Res. 2014, 74, 4967–4975. [Google Scholar] [CrossRef]
- Epstein, E.H. Basal cell carcinomas: Attack of the hedgehog. Nat. Rev. Cancer 2008, 8, 743–754. [Google Scholar] [CrossRef]
- Kasper, M.; Jaks, V.; Hohl, D.; Toftgård, R. Basal cell carcinoma—Molecular biology and potential new therapies. J. Clin. Investig. 2012, 122, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, S.; Mancuso, M.; Tanori, M.; Atkinson, M.J.; Merola, P.; Rebessi, S.; Di Majo, V.; Covelli, V.; Hahn, H.; Saran, A. Modulation of Patched-Associated Susceptibility to Radiation Induced Tumorigenesis by Genetic Background. Cancer Res. 2004, 64, 3798–3806. [Google Scholar] [CrossRef] [PubMed]
- Beinke, C.; Braselmann, H.; Meineke, V. Establishment of an x-ray standard calibration curve by conventional dicentric analysis as prerequisite for accurate radiation dose assessment. Health Phys. 2010, 98, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Abend, M.; Azizova, T.; Müller, K.; Dörr, H.; Senf, S.; Kreppel, H.; Rusinova, G.; Glazkova, I.; Vyazovskaya, N.; Schmidl, D.; et al. Gene Expression Analysis in Mayak Workers With Prolonged Occupational Radiation Exposure. Health Phys. 2014, 106, 664–676. [Google Scholar] [CrossRef]
- Tebel, K.; Boldt, V.; Steininger, A.; Port, M.; Ebert, G.; Ullmann, R. GenomeCAT: A versatile tool for the analysis and integrative visualization of DNA copy number variants. BMC Bioinform. 2017, 18, 19. [Google Scholar] [CrossRef]
- Chlebicka, I.; Stefaniak, A.; Matusiak, Ł.; Szepietowski, J. Basal cell carcinoma: What new can be learned aboutthe most common human cancer? A cross-sectionalprospective study of 180 cases in a single centre. Adv. Dermatol. Allergol. 2021, 38, 1086–1091. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebeling, M.; Steinestel, K.; Grunert, M.; Schramm, A.; Wilde, F.; Pietzka, S.; Sakkas, A. Chernobyl’s Aftermath: Multiple Manifestations of Basalioma in a Patient after Radioactive Contamination in 1986. Radiation 2023, 3, 203-210. https://doi.org/10.3390/radiation3040016
Ebeling M, Steinestel K, Grunert M, Schramm A, Wilde F, Pietzka S, Sakkas A. Chernobyl’s Aftermath: Multiple Manifestations of Basalioma in a Patient after Radioactive Contamination in 1986. Radiation. 2023; 3(4):203-210. https://doi.org/10.3390/radiation3040016
Chicago/Turabian StyleEbeling, Marcel, Konrad Steinestel, Michael Grunert, Alexander Schramm, Frank Wilde, Sebastian Pietzka, and Andreas Sakkas. 2023. "Chernobyl’s Aftermath: Multiple Manifestations of Basalioma in a Patient after Radioactive Contamination in 1986" Radiation 3, no. 4: 203-210. https://doi.org/10.3390/radiation3040016
APA StyleEbeling, M., Steinestel, K., Grunert, M., Schramm, A., Wilde, F., Pietzka, S., & Sakkas, A. (2023). Chernobyl’s Aftermath: Multiple Manifestations of Basalioma in a Patient after Radioactive Contamination in 1986. Radiation, 3(4), 203-210. https://doi.org/10.3390/radiation3040016







