Proton Therapy and Gliomas: A Systematic Review
Abstract
:1. Background
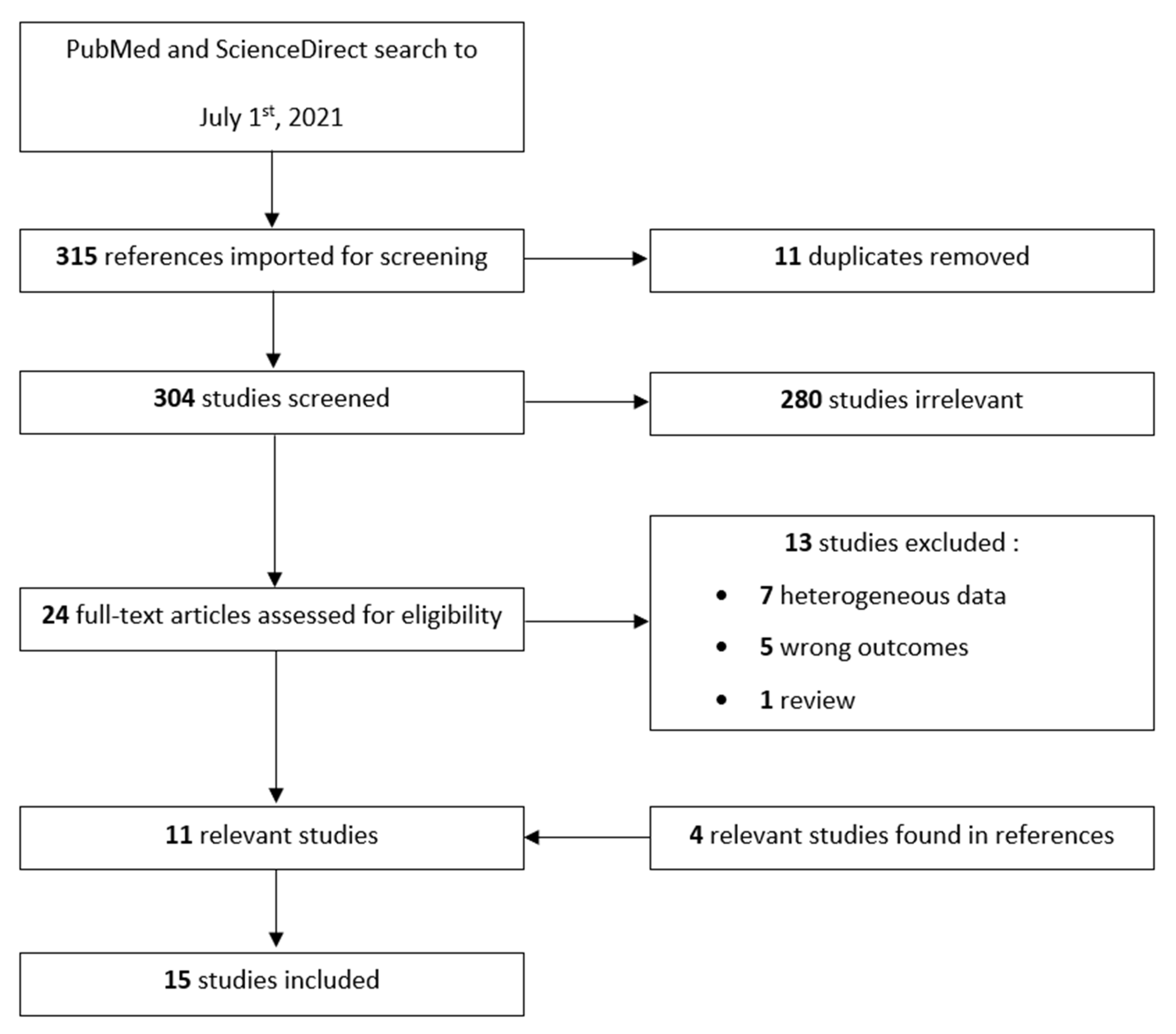
2. Methods
3. Results
3.1. Low-Grade Gliomas
3.1.1. Children
Disease Outcomes
Toxicity Outcomes
Pseudoprogression
3.1.2. Adults
Disease Outcomes
Toxicity Outcomes
Pseudoprogression
3.1.3. Dosimetry Data
3.2. High-Grade Gliomas
3.2.1. Children
3.2.2. Adults
3.2.3. Dosimetry Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D-CRT | Conventional three-dimensional RT |
| CTV | Clinical target volume |
| DIPG | Diffuse intrinsic pontine glioma |
| EUD | Equivalent uniform dose |
| FLAIR | Fluid-attenuated inversion recovery |
| GBM | Glioblastoma multiforme |
| Gy RBE | Gray radiobiological equivalent |
| GTV | Gross tumor volume |
| HGGs | High-grade gliomas |
| IMPT | Intensity-modulated proton therapy |
| IMRT | Intensity-modulated RT |
| IDH | Isocitrate dehydrogenase |
| LET | Linear energy transfer |
| LC | Local control |
| LGGs | Low-grade gliomas |
| MGMT | Methyl-guanine methyl transferase |
| NTCP | Normal tissue complication probability |
| OAR | Organs at risk |
| OS | Overall survival |
| PTV | Planning target volume |
| PFS | Progression-free survival |
| PRT | Proton therapy |
| PsP | Pseudoprogression |
| QoL | Quality of life |
| RT | Radiation therapy |
| RBE | Relative biological effectiveness |
| SOBP | Spread-out Bragg peak |
| TMZ | Temozolomide |
| TOMO | Tomotherapy |
| VMAT | Volumetric modulated arc therapy |
| WHO | World Health Organization |
References
- Stupp, R.; Brada, M.; van den Bent, M.J.; Tonn, J.-C.; Pentheroudakis, G. ESMO Guidelines Working Group High-Grade Glioma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25 (Suppl. 3), iii93–iii101. [Google Scholar] [CrossRef] [PubMed]
- Kleihues, P.; Burger, P.C.; Scheithauer, B.W. The New WHO Classification of Brain Tumours. Brain Pathol. Zurich Switz. 1993, 3, 255–268. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Reifenberger, G.; Wirsching, H.-G.; Knobbe-Thomsen, C.B.; Weller, M. Advances in the Molecular Genetics of Gliomas—Implications for Classification and Therapy. Nat. Rev. Clin. Oncol. 2017, 14, 434–452. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Tseng, Y.; Halasz, L. Description of Proton Therapy. In Principles of Neurological Surgery; Ellenbogen, R.G., Sekhar, L.N., Kitchen, N., Eds.; Elsevier Health: Amsterdam, The Netherlands, 2018; pp. 736–744.e3. ISBN 978-0-323-43140-8. [Google Scholar]
- Eaton, B.R.; Yock, T. The Use of Proton Therapy in the Treatment of Benign or Low-Grade Pediatric Brain Tumors. Cancer J. Sudbury Mass 2014, 20, 403–408. [Google Scholar] [CrossRef]
- Noël, G.; Antoni, D. [Proton therapy]. Cancer Radiother. J. Soc. Francaise Radiother. Oncol. 2016, 20, 508–512. [Google Scholar] [CrossRef]
- van der Weide, H.L.; Kramer, M.C.A.; Scandurra, D.; Eekers, D.B.P.; Klaver, Y.L.B.; Wiggenraad, R.G.J.; Méndez Romero, A.; Coremans, I.E.M.; Boersma, L.; van Vulpen, M.; et al. Proton Therapy for Selected Low Grade Glioma Patients in the Netherlands. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 154, 283–290. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Journy, N.; Indelicato, D.J.; Withrow, D.R.; Akimoto, T.; Alapetite, C.; Araya, M.; Chang, A.; Chang, J.H.-C.; Chon, B.; Confer, M.E.; et al. Patterns of Proton Therapy Use in Pediatric Cancer Management in 2016: An International Survey. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 132, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Zetterling, M.; Roodakker, K.R.; Berntsson, S.G.; Edqvist, P.-H.; Latini, F.; Landtblom, A.-M.; Pontén, F.; Alafuzoff, I.; Larsson, E.-M.; Smits, A. Extension of Diffuse Low-Grade Gliomas beyond Radiological Borders as Shown by the Coregistration of Histopathological and Magnetic Resonance Imaging Data. J. Neurosurg. 2016, 125, 1155–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smoll, N.R.; Gautschi, O.P.; Schatlo, B.; Schaller, K.; Weber, D.C. Relative Survival of Patients with Supratentorial Low-Grade Gliomas. Neuro-Oncology 2012, 14, 1062–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indelicato, D.J.; Rotondo, R.L.; Uezono, H.; Sandler, E.S.; Aldana, P.R.; Ranalli, N.J.; Beier, A.D.; Morris, C.G.; Bradley, J.A. Outcomes Following Proton Therapy for Pediatric Low-Grade Glioma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Greenberger, B.A.; Pulsifer, M.B.; Ebb, D.H.; MacDonald, S.M.; Jones, R.M.; Butler, W.E.; Huang, M.S.; Marcus, K.J.; Oberg, J.A.; Tarbell, N.J.; et al. Clinical Outcomes and Late Endocrine, Neurocognitive, and Visual Profiles of Proton Radiation for Pediatric Low-Grade Gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Le Fèvre, C.; Lhermitte, B.; Ahle, G.; Chambrelant, I.; Cebula, H.; Antoni, D.; Keller, A.; Schott, R.; Thiery, A.; Constans, J.-M.; et al. Pseudoprogression versus True Progression in Glioblastoma Patients: A Multiapproach Literature Review: Part 1—Molecular, Morphological and Clinical Features. Crit. Rev. Oncol. Hematol. 2020, 157, 103188. [Google Scholar] [CrossRef] [PubMed]
- Mannina, E.M.; Bartlett, G.K.; McMullen, K.P. Extended Volumetric Follow-up of Juvenile Pilocytic Astrocytomas Treated with Proton Beam Therapy. Int. J. Part. Ther. 2016, 3, 291–299. [Google Scholar] [CrossRef]
- Ludmir, E.B.; Mahajan, A.; Paulino, A.C.; Jones, J.Y.; Ketonen, L.M.; Su, J.M.; Grosshans, D.R.; McAleer, M.F.; McGovern, S.L.; Lassen-Ramshad, Y.A.; et al. Increased Risk of Pseudoprogression among Pediatric Low-Grade Glioma Patients Treated with Proton versus Photon Radiotherapy. Neuro-Oncol. 2019, 21, 686–695. [Google Scholar] [CrossRef]
- Shih, H.A.; Sherman, J.C.; Nachtigall, L.B.; Colvin, M.K.; Fullerton, B.C.; Daartz, J.; Winrich, B.K.; Batchelor, T.T.; Thornton, L.T.; Mancuso, S.M.; et al. Proton Therapy for Low-Grade Gliomas: Results from a Prospective Trial. Cancer 2015, 121, 1712–1719. [Google Scholar] [CrossRef]
- Tabrizi, S.; Yeap, B.Y.; Sherman, J.C.; Nachtigall, L.B.; Colvin, M.K.; Dworkin, M.; Fullerton, B.C.; Daartz, J.; Royce, T.J.; Oh, K.S.; et al. Long-Term Outcomes and Late Adverse Effects of a Prospective Study on Proton Radiotherapy for Patients with Low-Grade Glioma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 137, 95–101. [Google Scholar] [CrossRef]
- Bronk, J.K.; Guha-Thakurta, N.; Allen, P.K.; Mahajan, A.; Grosshans, D.R.; McGovern, S.L. Analysis of Pseudoprogression after Proton or Photon Therapy of 99 Patients with Low Grade and Anaplastic Glioma. Clin. Transl. Radiat. Oncol. 2018, 9, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Dworkin, M.; Mehan, W.; Niemierko, A.; Kamran, S.C.; Lamba, N.; Dietrich, J.; Martinez-Lage, M.; Oh, K.S.; Batchelor, T.T.; Wen, P.Y.; et al. Increase of Pseudoprogression and Other Treatment Related Effects in Low-Grade Glioma Patients Treated with Proton Radiation and Temozolomide. J. Neurooncol. 2019, 142, 69–77. [Google Scholar] [CrossRef]
- Dennis, E.R.; Bussiere, M.R.; Niemierko, A.; Lu, M.W.; Fullerton, B.C.; Loeffler, J.S.; Shih, H.A. A Comparison of Critical Structure Dose and Toxicity Risks in Patients with Low Grade Gliomas Treated with IMRT versus Proton Radiation Therapy. Technol. Cancer Res. Treat. 2013, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Harrabi, S.B.; Bougatf, N.; Mohr, A.; Haberer, T.; Herfarth, K.; Combs, S.E.; Debus, J.; Adeberg, S. Dosimetric Advantages of Proton Therapy over Conventional Radiotherapy with Photons in Young Patients and Adults with Low-Grade Glioma. Strahlenther. Onkol. Organ Dtsch. Rontgenges. Al 2016, 192, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Eekers, D.B.P.; Roelofs, E.; Cubillos-Mesías, M.; Niël, C.; Smeenk, R.J.; Hoeben, A.; Minken, A.W.H.; Granzier, M.; Janssens, G.O.; Kaanders, J.H.A.M.; et al. Intensity-Modulated Proton Therapy Decreases Dose to Organs at Risk in Low-Grade Glioma Patients: Results of a Multicentric in Silico ROCOCO Trial. Acta Oncol. Stockh. Swed. 2019, 58, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Hartmann, C.; Hentschel, B.; Simon, M.; Westphal, M.; Schackert, G.; Tonn, J.C.; Loeffler, M.; Reifenberger, G.; Pietsch, T.; von Deimling, A.; et al. Long-Term Survival in Primary Glioblastoma with versus without Isocitrate Dehydrogenase Mutations. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 5146–5157. [Google Scholar] [CrossRef] [Green Version]
- Preusser, M.; Charles Janzer, R.; Felsberg, J.; Reifenberger, G.; Hamou, M.-F.; Diserens, A.-C.; Stupp, R.; Gorlia, T.; Marosi, C.; Heinzl, H.; et al. Anti-O6-Methylguanine-Methyltransferase (MGMT) Immunohistochemistry in Glioblastoma Multiforme: Observer Variability and Lack of Association with Patient Survival Impede Its Use as Clinical Biomarker. Brain Pathol. Zurich Switz. 2008, 18, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Muroi, A.; Mizumoto, M.; Ishikawa, E.; Ihara, S.; Fukushima, H.; Tsurubuchi, T.; Sakurai, H.; Matsumura, A. Proton Therapy for Newly Diagnosed Pediatric Diffuse Intrinsic Pontine Glioma. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2020, 36, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Petr, J.; Platzek, I.; Hofheinz, F.; Mutsaerts, H.J.M.M.; Asllani, I.; van Osch, M.J.P.; Seidlitz, A.; Krukowski, P.; Gommlich, A.; Beuthien-Baumann, B.; et al. Photon vs. Proton Radiochemotherapy: Effects on Brain Tissue Volume and Perfusion. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 128, 121–127. [Google Scholar] [CrossRef]
- Brown, P.D.; Chung, C.; Liu, D.D.; McAvoy, S.; Grosshans, D.; Al Feghali, K.; Mahajan, A.; Li, J.; McGovern, S.L.; McAleer, M.-F.; et al. A Prospective Phase II Randomized Trial of Proton Radiotherapy vs Intensity-Modulated Radiotherapy for Patients with Newly Diagnosed Glioblastoma. Neuro-Oncology 2021, 23, 1337–1347. [Google Scholar] [CrossRef]
- Adeberg, S.; Harrabi, S.B.; Bougatf, N.; Bernhardt, D.; Rieber, J.; Koerber, S.A.; Syed, M.; Sprave, T.; Mohr, A.; Abdollahi, A.; et al. Intensity-Modulated Proton Therapy, Volumetric-Modulated Arc Therapy, and 3D Conformal Radiotherapy in Anaplastic Astrocytoma and Glioblastoma: A Dosimetric Comparison. Strahlenther. Onkol. Organ Dtsch. Rontgenges. Al 2016, 192, 770–779. [Google Scholar] [CrossRef]
- van den Bent, M.J.; Afra, D.; de Witte, O.; Ben Hassel, M.; Schraub, S.; Hoang-Xuan, K.; Malmström, P.-O.; Collette, L.; Piérart, M.; Mirimanoff, R.; et al. Long-Term Efficacy of Early versus Delayed Radiotherapy for Low-Grade Astrocytoma and Oligodendroglioma in Adults: The EORTC 22845 Randomised Trial. Lancet Lond. Engl. 2005, 366, 985–990. [Google Scholar] [CrossRef]
- Cherlow, J.M.; Shaw, D.W.W.; Margraf, L.R.; Bowers, D.C.; Huang, J.; Fouladi, M.; Onar-Thomas, A.; Zhou, T.; Pollack, I.F.; Gajjar, A.; et al. Conformal Radiation Therapy for Pediatric Patients with Low-Grade Glioma: Results from the Children’s Oncology Group Phase 2 Study ACNS0221. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Gnekow, A.K.; Falkenstein, F.; von Hornstein, S.; Zwiener, I.; Berkefeld, S.; Bison, B.; Warmuth-Metz, M.; Driever, P.H.; Soerensen, N.; Kortmann, R.-D.; et al. Long-Term Follow-up of the Multicenter, Multidisciplinary Treatment Study HIT-LGG-1996 for Low-Grade Glioma in Children and Adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro-Oncol. 2012, 14, 1265–1284. [Google Scholar] [CrossRef]
- Jhaveri, J.; Cheng, E.; Tian, S.; Buchwald, Z.; Chowdhary, M.; Liu, Y.; Gillespie, T.W.; Olson, J.J.; Diaz, A.Z.; Voloschin, A.; et al. Proton vs. Photon Radiation Therapy for Primary Gliomas: An Analysis of the National Cancer Data Base. Front. Oncol. 2018, 8, 440. [Google Scholar] [CrossRef]
- Proton Radiation Therapy for Gliomas—Tabular View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT01358058 (accessed on 9 January 2021).
- Jalali, R.; Mallick, I.; Dutta, D.; Goswami, S.; Gupta, T.; Munshi, A.; Deshpande, D.; Sarin, R. Factors Influencing Neurocognitive Outcomes in Young Patients with Benign and Low-Grade Brain Tumors Treated with Stereotactic Conformal Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 974–979. [Google Scholar] [CrossRef] [PubMed]
- NRG Oncology. A Phase II Randomized Trial of Proton vs. Photon Therapy (IMRT) for Cognitive Preservation in Patients With IDH Mutant, Low to Intermediate Grade Gliomas. 2020. Available online: clinicaltrials.gov (accessed on 3 September 2021).
- Merchant, T.E.; Conklin, H.M.; Wu, S.; Lustig, R.H.; Xiong, X. Late Effects of Conformal Radiation Therapy for Pediatric Patients with Low-Grade Glioma: Prospective Evaluation of Cognitive, Endocrine, and Hearing Deficits. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 3691–3697. [Google Scholar] [CrossRef] [Green Version]
- Taphoorn, M.J.; Heimans, J.J.; van der Veen, E.A.; Karim, A.B. Endocrine Functions in Long-Term Survivors of Low-Grade Supratentorial Glioma Treated with Radiation Therapy. J. Neurooncol. 1995, 25, 97–102. [Google Scholar] [CrossRef]
- Paganetti, H.; Athar, B.S.; Moteabbed, M.; Adams, J.; Schneider, U.; Yock, T.I. Assessment of Radiation-Induced Second Cancer Risks in Proton Therapy and IMRT for Organs inside the Primary Radiation Field. Phys. Med. Biol. 2012, 57, 6047–6061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.S.; Yock, T.I.; Nelson, K.; Xu, Y.; Keating, N.L.; Tarbell, N.J. Incidence of Second Malignancies among Patients Treated with Proton versus Photon Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; Welby, J.P.; Laack, N.N.; Mahajan, A.; Daniels, D.J. Pseudoprogression after Radiation Therapies for Low Grade Glioma in Children and Adults: A Systematic Review and Meta-Analysis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 142, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Fèvre, C.; Constans, J.-M.; Chambrelant, I.; Antoni, D.; Bund, C.; Leroy-Freschini, B.; Schott, R.; Cebula, H.; Noël, G. Pseudoprogression versus True Progression in Glioblastoma Patients: A Multiapproach Literature Review. Part 2—Radiological Features and Metric Markers. Crit. Rev. Oncol. Hematol. 2021, 159, 103230. [Google Scholar] [CrossRef] [PubMed]
- NRG Oncology. Randomized Phase II Trial of Hypofractionated Dose-Escalated Photon IMRT or Proton Beam Therapy Versus Conventional Photon Irradiation with Concomitant and Adjuvant Temozolomide in Patients with Newly Diagnosed Glioblastoma. 2021. Available online: clinicaltrials.gov (accessed on 3 September 2021).
| Database | MeSH Search Expression |
|---|---|
| PubMed | (Protons OR proton therapy) AND (glioma) |
| ScienceDirect | Title, abstract, keywords: protons AND glioma AND brain tumors |
| Author | Year | Type Of Study | Population | Median Age at RT (Years (Range)) | Grade | Number of Patients | Prescription | Median RT Dose (Gy RBE (Range)) | Volume | Number of Patients with Pre-RT Chemotherapy | Median Follow-Up (Years (Range)) | Clinical Outcomes | Toxicity Outcomes | PsP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Greenberger et al. | 2014 | Retrospective | Children | 11.0 (2.7–21.5) | LGG | 32 | NA | 52.2 | CTV = GTV + 3–5 mm PTV = CTV + 8–12 mm | 16 | 7.6 (3.2–18.2) | 8-year PFS and OS rates = 83% and 100%, respectively | Significant decline in children < 7 years and those with higher dose to the left temporal lobe and hippocampus | NA |
| Mannina et al. | 2016 | Retrospective | Children | 10.9 (4–20) | LGG | 15 | NA | 54 (50.4–59.4) | NA | 9 | 4.6 | NA | NA | 3 patients (20%), the maximum volume was observed 3 to 8 months after PRT and regressed after 18 months |
| Indelicato et al. | 2019 | Prospective | Children | 9 (2–21) | LGG | 174 | 129 treated with 54 Gy RBE45 treated with <54 Gy RBE | NA | CTV = GTV + 5 mm PTV = CTV + 3 mm | 74 | 4.4 (0.5–11.4) | 5-year PFS and OS rates = 84% and 92%, respectively | 12.6% nausea or vomiting; 1.1% headaches; 2.9% sensorineural troubles; 22% neuroendocrine deficiency | 56 patients (32%) |
| Ludmir et al. | 2019 | Retrospective | Children | 10.0 (1.0–17.6) | LGG | 83 | NA | 50.4 (45–59.4) | NA | 32 | 5.6 | Improved local control for PBT patients (HR 0.34, 95% CI: 0.10–1.18, p = 0.099) | NA | RT modality was found to predict PsP, with a higher cumulative incidence of PsP among PBT patients (23/51, 45%) than IMRT patients (8/32, 25%) (p = 0.048) |
| Shih et al. | 2015 | Prospective | Adult | 37.5 (22–56) | LGG | 20 | 54 GyRBE in 30 fractions | NA | CTV = GTV + 15 mm PTV = CTV + 8 mm | NA | 5.1 (3.3–5.2) | 5-year PFS and OS rates = 40% and 84%, respectively | Patients with LGG tolerate proton therapy well, and a subset develops neuroendocrine deficiencies. There is no evidence for overall decline in cognitive function or QOL | NA |
| Bronk et al. | 2018 | Retrospective | Adult | 47 (24–71) Oligo 46 (26–53) Astro | LGG | 36 | NA | 54 (40–57) Oligo 50.4 (50.4–57) Astro | CTV = GTV + 10–15 mm | NA | NA | NA | NA | Same incidence of PsP in both groups (17%). The median time of PsP detection was 33 days (range, 18–116 days) |
| Tabrizi et al. | 2019 | Prospective | Adult | 37.5 (22–56) | LGG | 20 | 54 GyRBE in 30 fractions | NA | CTV = GTV + 15 mm PTV = CTV + 8 mm | NA | 6.8 (1.8–11.5) | Median PFS = 4.5 years | The majority of patients with LGG who received proton therapy retained stable cognitive and neuroendocrine function | NA |
| Dworkin et al. | 2019 | Retrospective | Adult | 37 (18–68) | LGG | 119 | NA | 54 (54–60) | NA | NA | 4.8 | NA | NA | 43.6%, the median time of PsP detection was 7.6 months (range 0.6–65.8 months). There was an increased risk of PsP following PRT + TMZ vs. PRT-alone (HR = 2.2, p = 0.006) |
| Muroi et al. | 2020 | Retrospective | Children | 5.8 (4–9.9) | HGG | 12 | 54 GyRBE in 30 fractions | NA | CTV = GTV + 5–10 mm PTV = CTV + 2–3 mm | NA | NA | Median PFS = 5 months (range 1–11 months), and median OS = 9 months (range 4–48 months) | The most reported toxicities were grade ≤ 2 and included alopecia in the irradiated area (n = 12), nausea(n = 4), a decreased lymphocyte count (n = 4), vomiting (n = 2), bullous dermatitis (n = 1), and allergic reaction (n = 1) | NA |
| Petr et al. | 2017 | Retrospective | Adult | (54.9 ±14.0 years) | HGG | 67 | 60 GyRBE in 30 fractions | NA | CTV = GTV + 20 mm PTV = CTV + 5 mm | NA | NA | NA | NA | NA |
| Brown et al. | 2021 | Prospective | Adult | 53 (26–82) IMRT 54.5 (33–72) PRT | HGG | 67 | 60 Gy or GyRBE in 30 fractions | NA | CTV = GTV + 20 mm PTV50 = CTV + 3–5 mm and PTV60 = GTV + 3–5 mm | NA | 48.7 (7.1–66.7) | Median PFS = 8.9 months in IMRT vs. 6.6 months in PRT (p = 0.24), and médian OS = 21.2 months in IMRT vs. 24.5 months in PRT (p = 0.60) | There was no significant difference in time to cognitive failure between treatment arms. PRT was associated with a lower rate of fatigue | NA |
| Author | Year | Type of Study | Population | Median Age at RT (Years (Range)) | Grade | Number of Patients | Prescription | Median RT Dose (GyRBE (Range)) | Volume | Target Volume | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Harrabi et al. | 2016 | In silico | Children and adult | 31.2 (2.0–64.2) | LGG | 74 | 54 GyRBE in 30 fractions | 54.0 (50.4–60) | CTV = GTV + 10 mm | Median = 185.2 cc (range 11.8–709.6) | Reduction in dose in critical neurologic structures with PRT, with similar target volume coverage in both plans |
| Eekers et al. | 2018 | In silico | Children | NA | LGG | 25 | 50.4 GyRBE in 30 fractions | NA | CTV = GTV + 10 mm PTV = CTV + 2 mm | Mean = 240 cc (range 92–456) | IMPT was better than the other modalities to spare OAR, especially those located contralateral to the target volume |
| Dennis et al. | 2013 | In silico | Adult | NA | LGG | 11 | 54 GyRBE in 30 fractions | NA | CTV = GTV + 15 mm PTV = CTV + 3 mm | Mean = 162.2 cc (range 22.5–390.3) | Equivalent uniform dose (EUD) between 10 and 20 GyRBE lower with PRT to crucial neuronal structures, including optic nerves, hippocampus, cochlea, and pituitary |
| Adeberg et al. | 2016 | In silico | Adult | 36.5 (26–63) | HGG | 12 | 60 GyRBE in 30 fractions | 60 (56.0–60.0) | CTV = GTV + 20–30 mm | NA | Statistically significant reductions of mean dose (Dmean) with IMPT in neurosensorial structures, neuroendocrine structures, and critical organs of neurocognition (p < 0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chambrelant, I.; Eber, J.; Antoni, D.; Burckel, H.; Noël, G.; Auvergne, R. Proton Therapy and Gliomas: A Systematic Review. Radiation 2021, 1, 218-233. https://doi.org/10.3390/radiation1030019
Chambrelant I, Eber J, Antoni D, Burckel H, Noël G, Auvergne R. Proton Therapy and Gliomas: A Systematic Review. Radiation. 2021; 1(3):218-233. https://doi.org/10.3390/radiation1030019
Chicago/Turabian StyleChambrelant, Isabelle, Jordan Eber, Delphine Antoni, Hélène Burckel, Georges Noël, and Romane Auvergne. 2021. "Proton Therapy and Gliomas: A Systematic Review" Radiation 1, no. 3: 218-233. https://doi.org/10.3390/radiation1030019
APA StyleChambrelant, I., Eber, J., Antoni, D., Burckel, H., Noël, G., & Auvergne, R. (2021). Proton Therapy and Gliomas: A Systematic Review. Radiation, 1(3), 218-233. https://doi.org/10.3390/radiation1030019






