Functional Herkogamy and Pollination Biology in Passiflora cincinnata Mast.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Germplasm
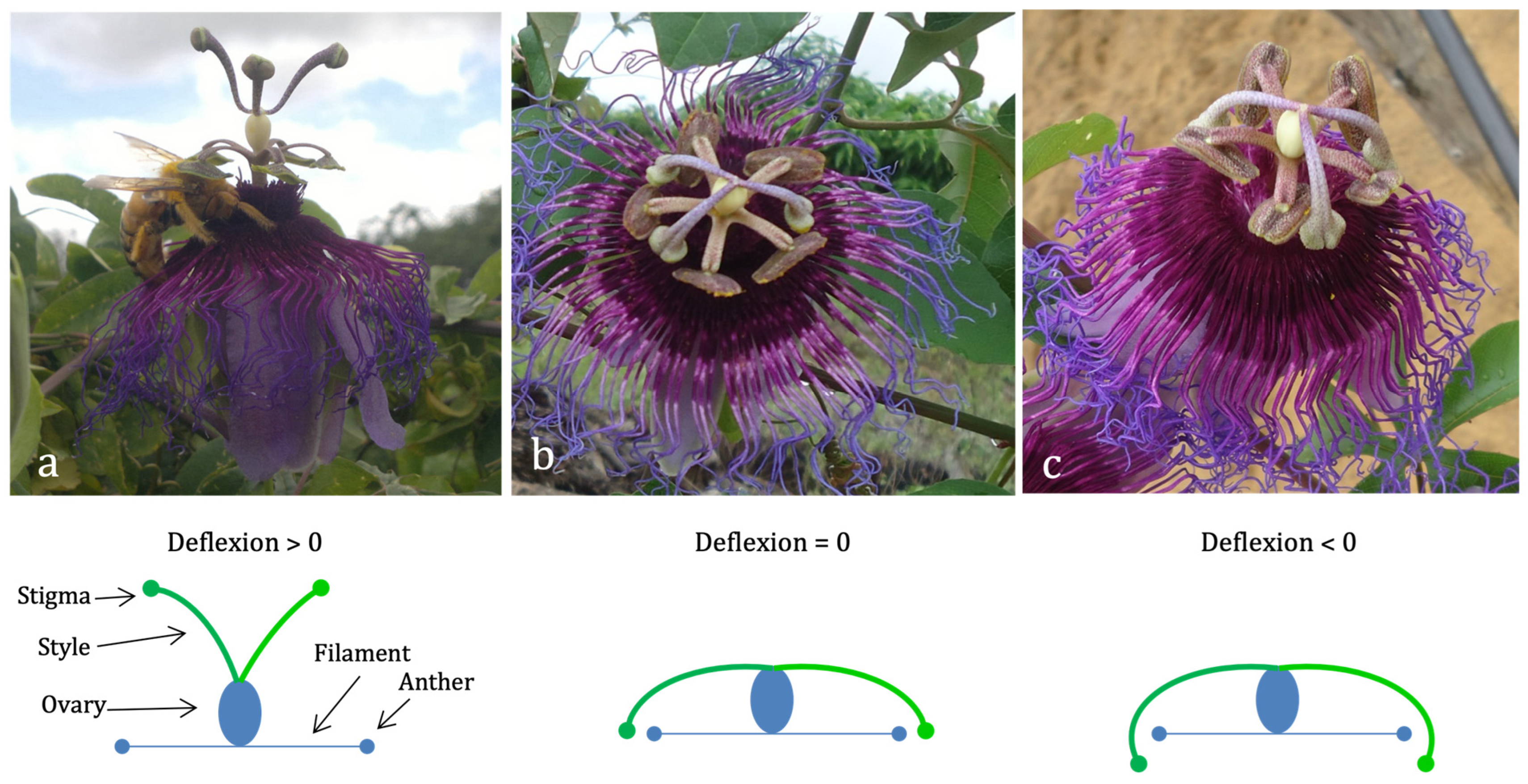
2.2. Floral Morphometrics and Stigma–Anther Curvature Classes
2.3. Pollination Treatments
2.4. Pollen Viability Assessment
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernacci, L.C.; Nunes, T.S.; Mezzonato, A.C.; Milward-de-Azevedo, M.A.; Imig, D.C.; Cervi, A.C. Passiflora . In Flora do Brasil 2020; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2020. Available online: https://floradobrasil2020.jbrj.gov.br/reflora/floradobrasil/FB12508 (accessed on 23 November 2025).
- Kiill, L.H.P.; Siqueira, K.M.M.; Araújo, F.P.; Trigo, S.P.M.; Feitoza, E.A.; Lemos, I.B. Biologia reprodutiva de Passiflora cincinnata Mast. (Passifloraceae) na região de Petrolina (Pernambuco, Brazil). Oecol. Aust. 2010, 14, 115–127. [Google Scholar] [CrossRef]
- Amela García, M.T. Breeding system and related floral features under natural and experimental conditions of Passiflora suberosa (Passifloraceae). Bol. Soc. Argent. Bot. 2008, 43, 83–93. [Google Scholar]
- Webb, C.J.; Lloyd, D.G. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkogamy. N. Z. J. Bot. 1986, 24, 163–178. [Google Scholar] [CrossRef]
- Dai, C.; Galloway, L.F. Do dichogamy and herkogamy reduce sexual interference in a self-incompatible species? Funct. Ecol. 2011, 25, 271–278. [Google Scholar] [CrossRef]
- Dai, C.; Liang, X.; Ren, J.; Liao, M.; Li, J.; Galloway, L.F. The mean and variability of a floral trait have opposing effects on fitness traits. Ann. Bot. 2016, 117, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.G.; Webb, C.J. The evolution of heterostyly. In Evolution and Function of Heterostyly; Barrett, S.C.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 151–178. [Google Scholar]
- Armbruster, W.S.; Bolstad, G.H.; Hansen, T.F.; Keller, B.; Conti, E.; Bjerknes, Ø.; Pélabon, C. The measure and mismeasure of reciprocity in heterostylous flowers. New Phytol. 2009, 183, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C.H. “A most complex marriage arrangement”: Recent advances on heterostyly and unresolved questions. New Phytol. 2019, 224, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Ángel-Coca, C.; Nates-Parra, G.; Ospina-Torres, R.; Melo Ortiz, C.D.; Amaya, M. Biología floral y reproductiva de la gulupa Passiflora edulis Sims f. edulis. Caldasia 2011, 33, 433–451. [Google Scholar]
- Beavon, M.A.; Kelly, D. Invasional meltdown: Pollination of the invasive liana Passiflora tripartita var. mollissima (Passifloraceae) in New Zealand. N. Z. J. Ecol. 2012, 36, 100–107. [Google Scholar]
- Amela García, M.T.; Hoc, P.S. Biología floral de Passiflora foetida (Passifloraceae): Fases florales, polinizadores y sistema reproductivo. Rev. Biol. Trop. 1998, 46, 191–201. [Google Scholar]
- Barrett, S.C.H. The evolution of plant sexual diversity. Nat. Rev. Genet. 2002, 3, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C.H.; Shore, J.S. New insights on heterostyly: Comparative biology, ecology and genetics. In Self-Incompatibility in Flowering Plants: Evolution, Diversity, and Mechanisms; Franklin-Tong, V.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–32. [Google Scholar] [CrossRef]
- Ferrero, V.; de Vega, C.; Stafford, G.I.; van Staden, J.; Johnson, S.D. Heterostyly and reciprocal herkogamy promote pollen transfer in Nerine humilis (Amaryllidaceae). Ann. Bot. 2011, 108, 797–807. [Google Scholar] [CrossRef]
- Glover, B.J. Understanding Flowers and Flowering: An Integrated Approach, 2nd ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Moro, M.F.; Amorim, V.O.; de Queiroz, L.P.; da Costa, L.R.F.; Maia, R.P.; Taylor, N.P.; Zappi, D.C. Biogeographical districts of the Caatinga dominion: A proposal based on geomorphology and endemism. Bot. Rev. 2024, 90, 376–429. [Google Scholar] [CrossRef]
- Harder, L.D.; Barrett, S.C.H. Ecology and Evolution of Flowers; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Radford, A.E.; Dickison, W.C.; Massey, J.R.; Bell, C.R. Vascular Plant Systematics; Harper & Row: New York, NY, USA, 1974. [Google Scholar]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Barrett, S.C.H.; Hough, J. Sexual dimorphism in flowering plants. J. Exp. Bot. 2013, 64, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Dorken, M.E.; Barrett, S.C.H. Sex determination and the evolution of dioecy from monoecy in angiosperms. Proc. R. Soc. B Biol. Sci. 2021, 288, 20211189. [Google Scholar]
- Cobra, S.S.O.; Silva, C.A.; Krause, W.; Lage, L.A. Availability of floral resources in yellow passion fruit cultivars. Commun. Sci. 2017, 8, 555–561. [Google Scholar] [CrossRef]


| Deflexion Category | Total Flowers | Percentage (%) |
|---|---|---|
| d < 0 | 238 | 41.75 |
| d = 0 | 150 | 26.32 |
| d > 0 | 182 | 31.93 |
| ♀ ♂ | d < 0 | d = 0 | d > 0 |
|---|---|---|---|
| d < 0 | 20 | 5 | 0 |
| d = 0 | 19 | 2 | 0 |
| d > 0 | 16 | 0 | 0 |
| Total | 55 (73.3%) | 7 (0.02%) | 0 (0.0%) |
| Style Deflexion Type | Viability (Acetic Carmine) | Viability (Alexander Stain) | Mean Pollen Grain Viability (%) | Total Grains Counted |
|---|---|---|---|---|
| d < 0 | 96.41 | 97.01 | 96.71 | 1620 |
| d = 0 | 96.09 | 96.81 | 96.45 | 1333 |
| d > 0 | 96.42 | 96.62 | 96.52 | 1082 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Nery, L.P.C.; Santos, T.C.d.; Ribeiro, J.M.; Melo, N.F.d. Functional Herkogamy and Pollination Biology in Passiflora cincinnata Mast. J. Zool. Bot. Gard. 2026, 7, 2. https://doi.org/10.3390/jzbg7010002
Nery LPC, Santos TCd, Ribeiro JM, Melo NFd. Functional Herkogamy and Pollination Biology in Passiflora cincinnata Mast. Journal of Zoological and Botanical Gardens. 2026; 7(1):2. https://doi.org/10.3390/jzbg7010002
Chicago/Turabian StyleNery, Lucas Peixinho Campos, Tatiane Cezário dos Santos, Juliana Martins Ribeiro, and Natoniel Franklin de Melo. 2026. "Functional Herkogamy and Pollination Biology in Passiflora cincinnata Mast." Journal of Zoological and Botanical Gardens 7, no. 1: 2. https://doi.org/10.3390/jzbg7010002
APA StyleNery, L. P. C., Santos, T. C. d., Ribeiro, J. M., & Melo, N. F. d. (2026). Functional Herkogamy and Pollination Biology in Passiflora cincinnata Mast. Journal of Zoological and Botanical Gardens, 7(1), 2. https://doi.org/10.3390/jzbg7010002






