Abstract
In southern white rhinoceroses (Ceratotherium simum simum), pant calls are well-studied contact vocalisations, whereas the function of frequently emitted snorts remains unclear. We conducted playback experiments with 15 rhinoceroses at three European zoos. The first experiment tested responses to conspecific versus heterospecific snorts, comparing pulsed and non-pulsed acoustic structures. The second experiment contrasted conspecific snorts with conspecific pants from males differing in age and faecal testosterone metabolite (fTM) levels. Behavioural responses—including body orientation, approach toward loudspeaker, locomotion, and vocalisations—were analysed. Snorts, regardless of sender species or pulsation, elicited uniformly low-intensity responses, suggesting limited communicative function. In contrast, pants evoked significantly stronger responses depending on sex and group setting. While males showed increased locomotion, females vocalised more, reflecting the species’ social dynamics. Individuals tested alone displayed overall heightened vigilance and vocal activity compared to those tested in pairs, emphasising the role of social context. No evidence was found for discrimination between pant calls differing in fTM levels. Our findings reinforce the communicative relevance of pants in conveying social cues while indicating that snorts may either lack species-specific acoustic markers or not be socially salient. Playback experiments thus appear as valuable tools for assessing acoustic communication in zoo-housed mammals.
1. Introduction
Understanding the function of animal vocalisations requires careful assessment of both the behavioural and environmental contexts in which they are produced and the behavioural response they induce in the receiver. While recording and analysing the vocal behaviour of the sender provides first indications about the communicative function of a vocalisation, playback experiments have proven to be a powerful tool to experimentally test these hypotheses by simulating natural vocal interactions and examining how the receiver responds to specific sounds under controlled conditions [1,2,3].
Vocalisations that serve a communicative function by conveying information relevant to the receiver are typically expected to elicit clear, measurable responses in listeners. Being produced with the potential to influence the behaviour of others, these signals are considered social features [4]. Common examples include contact and mating calls that facilitate reproduction, agonistic calls used to defend resources, and alarm calls that warn conspecifics of potential threats [5]. Because these call types are critical for survival and reproductive success, they often encode rich information about the sender, enabling receivers to discern individual identity ([6,7,8,9], reviewed in [10]), kinship [11,12,13], sex [14,15,16], and to discriminate between species [17,18,19,20,21].
In contrast, other acoustic outputs do not necessarily serve an immediate communicative purpose, even though they can be frequently produced. These include signals such as sighs, sneezes or snorts, which are often emitted independently of a specific audience and may primarily fulfil non-communicative physiological functions [22,23,24,25].
In recent years, snorts specifically have received growing attention due to emerging evidence across a broad range of taxa suggesting that, beyond their presumed physiological origins, they may also carry functional relevance in emotional and social contexts. Across species, snorts have been linked to a variety of arousal states, including positive affective states [26,27], frustration and anxiety [28], aggression [29], and alertness and irritation [30,31,32], as well as to behavioural contexts such as alarm signalling [33,34,35], reproduction [36], and close-range affiliative interactions [37]. Within the order of Perissodactyla, snorts occur in all three extant families. In horses (Equus ferus caballus), for example, snorts are considered to be an acoustic marker of positive emotional states [26,27], while in plains zebras (Equus quagga), long snorts are associated with contentment, whereas short ones are emitted in alarm contexts [38]. South American tapirs (Tapirus terrestris) utter snorts in agonistic contexts [39], while in the Indian rhinoceros (Rhinoceros unicornis), snorts serve as contact calls [40]. Taken together, this cross-species evidence suggests that while snorts may be structurally similar across taxa, their function is likely shaped by emotional state, context, and species-specific social systems.
In southern white rhinoceroses (Ceratotherium simum simum), a semi-social species with highly territorial males and females that form temporarily stable social groups, snorts are the most frequently produced call type [41] but remain the least understood. Acoustically, snorts in white rhinoceroses, similar to those in horses [27], are characterised as noisy nasal exhalations comprising two structural subtypes, a non-pulsed and pulsed variation [42], that are produced by individuals of all ages and both sexes [41,43]. While snorts have been consistently described in various studies on the vocal repertoire [42,43,44] and have primarily been documented in non-social contexts such as feeding and resting [42,43], their communicative value, irrespective of the subtype, remains unknown.
In contrast, the communicative function of the pant call and its social and behavioural contexts are well established. Characterised as a repetitive sequence of inhalations and exhalations, pants primarily function as contact calls during social approaches or separations [43,44,45]. They have been shown to exhibit the highest degree of individual distinctiveness among white rhinoceros call types [45,46] and to convey various information about the sender, including sex [47] and territorial status [48]. Playback experiments have also demonstrated that pants enable species-specific discrimination, with individuals able to differentiate between the two white rhinoceros subspecies based on acoustic cues [47]. Moreover, a male-specific variation in the pant, known as the “Hic,” occurs during interactions with receptive females, further underscoring the call’s social relevance and functional plasticity in reproductive contexts [44,47,48]. While these findings highlight the diverse types of information encoded in pants, further playback experiments are required to assess whether additional acoustic correlates, such as indicators of age or testosterone levels, are also present in this call type.
The aim of this study was to gain deeper insight into the communicative function of vocalisations in southern white rhinoceroses. Specifically, we investigated the function of snort vocalisations, which, despite being the most prevalent call type, lack a clear functional interpretation. To this end, we conducted a series of playback experiments to assess behavioural responses to both conspecific and heterospecific snorts, as well as to compare reactions to snorts versus pants. Given the high production rate of snorts and the observed structural variation within this call type, our approach aimed to determine whether snorts serve a specific communicative function and whether they contribute to species recognition.
We hypothesised that if snorts carried communicative value, the study animals would show stronger behavioural responses to conspecific snorts than to heterospecific snorts or control stimuli. Furthermore, if pants function as socially relevant calls coordinating interactions within and between individuals, we expected the animals to respond more strongly to pants than to snorts, which have primarily been observed in non-social contexts. In addition, we explored whether behavioural responses to pants differed depending on the characteristics of the sender, particularly comparing pants produced by juvenile males versus adult males with varying testosterone levels.
2. Materials and Methods
2.1. Subjects and Study Sites
The playback study was conducted on a total of 15 adult southern white rhinoceroses housed in three zoological institutions: Planète Sauvage in France (n = 4), Serengeti-Park Hodenhagen in Germany (n = 7), and Givskud Zoo in Denmark (n = 4) (Table 1).

Table 1.
Information on study animals. M = male, F = female, age in years, SPB = snort playback, PPB = pant playback.
At Planète Sauvage, playback experiments were carried out with four white rhinoceroses. Individuals were housed in two groups (Jambo with Sana and Dinari with Goliath), which were alternated between two non-connected outdoor enclosures, allowing the playback experiments to be conducted in the smaller one of the two (approx. 200 m2).
At Serengeti-Park Hodenhagen, seven study animals were tested. The playback experiments were carried out in an outdoor enclosure (approx. 230 m2) adjacent to the stables and the larger outdoor area. As a result, the study animals typically maintained visual, acoustic, and/or olfactory contact with the other conspecifics. Adjusting to the typical rotation schedule of the animals between the different compartments, the two study males, Martin and Ekozu, and the females Makena and Moana, were tested separately, whereas Claudia, Kianga, and Uzuri were tested together with their respective calves.
At Givskud Zoo, playback experiments were conducted with four study females in an outdoor enclosure (approx. 450 m2). While Mazumba and Samia were tested separately, Sofie and Inger were tested together.
All playback experiments were conducted during the daily housing routine, and all study animals had continuous access to water and hay and/or pasture.
2.2. Recordings and Preparation of Playback Stimuli
Playback stimuli for white rhinoceros vocalisations were obtained from recordings of previous studies [41,42,46]. Playback stimuli for horses were provided from the Ethology Department at the University of Rennes. All senders were unfamiliar to the study animals. All playback stimuli were prepared using Praat software (version 6.0.52, [49]). For the snort playbacks (SPBs), five different sets were created, each containing one stimulus per category (a white noise control, a pulsed and non-pulsed conspecific snort of a white rhinoceros, and a pulsed and non-pulsed heterospecific snort of a horse). Single snorts were cut with a duration of approximately 0.83 s (±0.15 s) and ramped by a 0.3 s fade-in ramp at the beginning to prevent abrupt signal truncation by the loudspeaker. The white noise stimulus was synthesised with a length of 0.6 s. Each stimulus consisted of one single presentation of the sound (Figure 1).
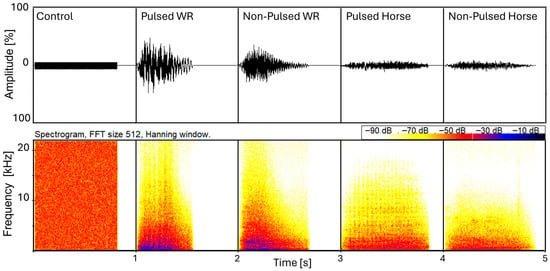
Figure 1.
Oscillograms and spectrograms for each of the five stimuli of set 3 of the snort playback. WR: white rhinoceros. Spectrograms were produced using BatSound Pro (version 3.31, Pettersson Elektronik AB, Uppsala, Sweden; settings: FFT 512, Hanning window).
For the pant playbacks (PPBs), two different sets were created, each containing one conspecific stimulus per category: a non-pulsed snort call as control, a juvenile pant, a high-testosterone pant (high T-pant), and a low-testosterone pant (low T-pant). The faecal testosterone metabolite (fTM) levels of the sender males had been determined in a previous study [50]: The average fTM concentration in adult, reproductively successful males selected as high-T pant senders was 25.85 ng/g faeces. Non-reproductive adult males selected as low-T pant senders had an average concentration of 11.74 ng/g faeces, while juvenile males selected to represent the juvenile pant stimulus exhibited a mean fTM level of 10.67 ng/g faeces.
Pants were selected with a duration of approximately 1.78 s (±0.65 s). Since the pilot tests of the snort playbacks revealed a low response intensity, the pant playbacks were also designed as a control to estimate whether the low response intensity was due to the single presentation or the low sound level of the played stimuli. Thus, for the pant playbacks, a stimulus chain consisting of three repetitions of a stimulus call with a 3 s pause between each repetition was created (Figure 2). Additionally, a 0.3 s ramp was added at the beginning of each stimulus to prevent abrupt signal truncation. In total, the stimulus chains had a duration of 11.29 s (±1.92 s).
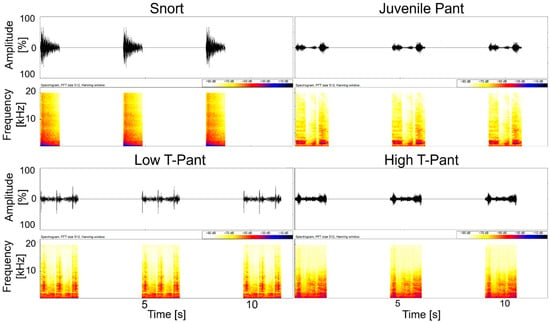
Figure 2.
Oscillograms and spectrograms for each of the four stimuli of set 1 of the pant playback. Low T-pant: low testosterone pant; high T-pant: high testosterone pant. Spectrgrams were produced using BatSound Pro (version 3.31, Pettersson Elektronik AB, Uppsala, Sweden; settings: FFT 512, Hanning window).
Stimuli for the snort playback were sound pressure levelled to 75 dB (±5 dB) re 20 μPa, and for the pant playback to 85 dB (±5 dB) re 20 μPa in a sound chamber at a distance of 1 m using a Precision Impulse Sound Level Type 2233 from Brüel & Kjaer (Nærum, Denmark). Due to a delay in loudspeaker activation, each stimulus/stimulus chain was preceded by a 300 ms 22 kHz sinusoidal tone outside the animals’ assumed hearing range, followed by a 20 ms silent interval before onset to guarantee a smooth playback.
2.3. Playback Setup and Procedure
The playback stimuli were played back from a laptop connected to a loudspeaker (JBL Xtreme 2, by Harman, Stamford, CT, USA). The response behaviour of the study animals was both video- and audio-recorded for five minutes following playback onset. Video recordings were made with two to three digital camcorders (Sony DCR-SR36E, Sony Corporation, Tokyo, Japan) covering the entire area of the outdoor enclosures. In Planète Sauvage, two digital camcorders were installed at the front and back of the outdoor enclosure. In Serengeti-Park Hodenhagen, three camcorders were installed along two sides of the enclosure, with an additional handheld camcorder used to adjust the focus on the study animals as needed. In Givskud Zoo, one camcorder (Panasonic HDC-SD600, Panasonic Holdings Corporation, Kadoma, Japan) was installed at the front of the outdoor enclosure, and an additional handheld camcorder (Sony HDR-CX240E, Sony Corporation, Tokyo, Japan) was used to adjust the focus on the study animals.
For audio recordings, an omnidirectional Sennheiser MKH 8020 microphone (Sennheiser electronic GmbH & Co. KG, Wedemark-Wennebostel, Germany; flat frequency response of 10–20,000 Hz ± 5 dB) or a directional Sennheiser ME64 microphone (flat frequency response: 40–20,000 Hz ± 2.5 dB) was used. Both microphones were fitted with a windshield and connected to a digital recording device (Sound Devices 702T State Recorder, Sound Devices LLC, Reedsburg, WI, USA; frequency response: 10–40,000 Hz; settings: 44.1 kHz sampling rate, 16-bit, uncompressed .wav format). In Givskud Zoo, a directional Sennheiser MKH 816 P48 microphone (Sennheiser electronic GmbH and Co. KG, Wedemark-Wennebostel, Germany; flat frequency response of 40–20,000 Hz ±5 dB) that was connected to a Marantz PMD561 audio recorder (Marantz, Carlsbad, CA, USA; frequency response: 20–24,000 Hz; settings: 44.1 kHz sampling rate, 16-bit, uncompressed .wav format) was used.
Except for the four females in Givskud Zoo, who were only presented with the pant playback, all study animals were tested in both the snort and the pant playback experiments (Table 1). The acoustic stimuli were played at a distance of one to three body lengths between the loudspeaker and the study animals at an angle of approximately 180 degrees. The loudspeaker was permanently installed in Planète Sauvage and Givskud Zoo, whereas in Serengeti-Park Hodenhagen, its position was adjusted based on the animals’ locations. The playback stimuli were presented in a randomised order with an inter-playback interval of at least ten minutes at all three of the study sites. The maximum number of playback stimuli presented to a study animal per day was three in Planète Sauvage and six in Serengeti-Park Hodenhagen and Givskud Zoo. Some playbacks were repeated to ensure optimal positioning of the animals relative to the loudspeaker and to maximise the general audibility of the stimuli if unexpected acoustic disturbance occurred.
2.4. Vocal and Behavioural Analysis
For behavioural coding, video recordings were synchronised with respective audio recordings and analysed using the Observer XT software (version 12, Noldus Information Technology, Wageningen, The Netherlands, Noldus, L. [51]). The analysis was conducted by two different observers (MH: Planète Sauvage, Serengeti-Park Hodenhagen 2021, Givskud Zoo; JJ: Serengeti-Park Hodenhagen 2022). The Cohen’s Kappa coefficient was determined among the observers by comparing eight randomly selected observations. The mean coefficient of 0.65 indicated a substantial agreement between the observers [52].
For each study animal that was presented with both the snort and the pant playback, a total of nine observations were analysed (five for SPB and four for PPB). For the four individuals from Givskud Zoo, four observations from the pant playbacks were analysed. Overall, 115 observations were included in the statistical analysis.
The following behaviours were coded during a five-minute observation period following the onset of the playback stimulus: ear movement, head movement, body turns towards the loudspeaker, approaching the loudspeaker, locomotion, and vocalisations (focusing on snort and pant vocalisations).
Based on a previous playback study on white rhinoceroses by Cinková and Policht [47], a 5-stage scoring scale was adopted to evaluate the intensity of the behavioural reaction of the study animals, with higher scores reflecting more intense behavioural responses (Table 2; for a detailed description, Supplementary Table S1). Focusing on the immediate reactions, only behavioural responses within the first 15 s after playback of the stimuli were analysed for the assessment of the score. Each individual was assigned the highest score based on the most intense behaviour displayed during this initial period.

Table 2.
Score scale of immediate behavioural response to playbacks in southern white rhinoceroses (within the first 15 s of observation) ordered according to increasing intensity.
In addition, the duration of locomotion (in seconds), the latency of approaching the loudspeaker (in seconds), and the call rate (per five minutes) were calculated as indicators for increased vigilance and attention in the study animals.
2.5. Statistical Analysis
Statistical tests were calculated in ‘RStudio’, version 2024.12.1.563 [53]. The significance level was set at p ≤ 0.05.
For the analysis of the effects of playbacks on the behavioural score and the call rate (= number of calls uttered in the 5 min observation period), we fitted generalised linear mixed-effects models using the ‘glmmTMB’ function (‘glmmTMB’ package, version 1.1.11), initially assuming a Poisson distribution (‘poisson’) appropriate for count data, e.g., [54]. We assessed model assumptions by simulating residuals with the ‘simulateResiduals’ function (‘DHARMa’ package, version 0.4.7). If over- or underdispersion were indicated, we refitted the model using a generalised Poisson distribution (‘genpois’). For both distribution families, the log link function was used. Further, we tested for zero inflation using the ‘testZeroInflation’ function (‘DHARMa’ package) and, when detected, accounted for it by specifying a zero-inflation formula (‘ziformula’) within the model. If a model failed to converge, we examined the variance components using the ‘VarCorr’ function and removed the random effect with an estimated variance of zero to simplify the model.
For the continuous response variables locomotion and latency to approach the loudspeaker, we used linear mixed-effects models fitted with the ‘lmer’ function (‘lme4’ package, version 1.1-37). We tested residuals for normality using the K-S test (‘DHARMa’ package). If the test revealed a significant deviation from normality, we applied a square root transformation to the response variable.
For the Snort playback experiments, we employed two complementary modelling approaches. In the first, we investigated the effects of stimulus type (control, horse, and rhinoceros) and group type (single or paired) as well as the sex of the receiver (male or female) on the behavioural variables. Animal ID and zoo were included as random effects to account for repeated measures and potential site-level variation. In the second approach, we investigated the effect of species (conspecific, heterospecific) and pulsation (pulsed, non-pulsed), excluding the control trials, which did not differ in pulsation. The response variable locomotion was square rooted to meet the assumption of normality.
For the pant playback experiments, we used the predictor variables stimulus type (snort, juvenile pant, low T-pant, and high T-pant), group type (single or paired), and sex of the receiver (female or male), again including animal ID and zoo as random effects.
All models were initially fitted with a full set of fixed effects and interaction terms. We applied backward elimination by sequentially removing the highest non-significant interaction term, using the ‘anova’ function to compare model performance at each step. This process continued until (a) only main effects remained, (b) all remaining interactions were significant, or (c) the reduced model significantly differed from the previous model.
Statistical significance of predictor variables was assessed using the ‘Anova’ function (‘car’ package, version 3.1-3). For models with a significant effect of stimulus type or an interaction term, we conducted post hoc comparisons using the ‘emmeans’ function (‘emmeans’ package, version 1.11.1, ‘fdr’ adjustment for multiple comparisons, ‘response’ type for back-transforming from log to response scale).
3. Results
3.1. Snort Playbacks
The final model assessing the effects on the behavioural score retained the three main terms—stimulus type, group type, and sex—as well as significant interaction between group type * sex (χ2 = 5.43, p = 0.020; Table 3). While stimulus type did not significantly affect the behavioural score (χ2 = 4.76, p = 0.092), the interaction revealed that females exhibited significantly higher behavioural scores when tested alone compared to when tested in the presence of conspecifics (Est. = 0.37, SE = 0.12, p = 0.002, Figure 3A). In contrast, males showed no such difference between testing conditions (Est. = 1.10, SE = 0.37, Z = 0.27, p = 0.788, Figure 3A).

Table 3.
Results of the final models for the snort playback testing the effect of stimulus type, group type, and sex on the response parameters: behavioural score, locomotion, and call rate; df = degree of freedom, * = statistically significant effect.
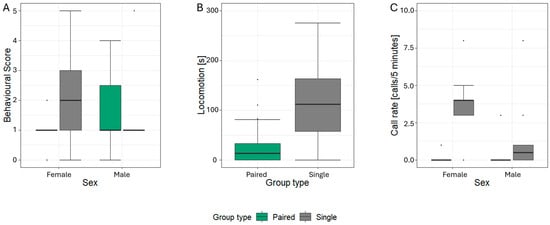
Figure 3.
Boxplots for the behavioural responses to snort playbacks dependent on group type for (A) behavioural score, (B) locomotion, and (C) call rate; black line = median, box = 25–75 % quartile, whisker = min-max non-outlier range.
In the analysis of locomotor activity following snort playbacks, the final model included only the main terms. Among these, group type emerged as a significant predictor (χ2 = 34.19, p < 0.001, Table 3), with animals tested alone displaying higher levels of locomotion compared to those tested in pairs (Figure 3B).
In only 17 out of 55 (30.9%) snort playback trials, vocalisations were recorded from seven study animals during the observation period. Analysing the overall call rate, the final model contained the main terms, the interaction term stimulus type * group type, and a significant interaction between group type * sex (χ2 =4.86, p = 0.027, Table 3, Figure 3C). However, post hoc comparisons of the interaction term revealed no significant differences (Est. = 0, SE ≤ 0.56, Z ≤ |1.85|, p ≥ 0.998). Stimulus type did not significantly affect call rate, either (χ2 = 3.43, p = 0.330).
Overall, snort vocalisations were more frequent among the study animals than pants (snort: control = 2, pulsed horse = 4, non-pulsed horse = 4, pulsed rhino = 3, non-pulsed rhino = 2; pant: control = 1, non-pulsed horse = 3, pulsed rhino = 2, non-pulsed rhino = 2). Three individuals produced both snorts and pants across different trials.
In only ten out of 55 (18.18%) playback trials did the individuals approach the loudspeaker (control = 1, pulsed horse = 2, non-pulsed horse = 3, pulsed rhino = 2, non-pulsed rhino = 2); in the remaining trials, they did not move towards the loudspeakers during the entire observation period, preventing a statistically meaningful evaluation of this response parameter.
Finally, when examining the effects of species and pulsation on snort perception, the final models only contained the main terms of species (conspecific vs. heterospecific) and pulsation (pulsed vs. non-pulsed). Neither factor had a significant effect on any of the measured behavioural responses (χ2 ≤ 2.72, p ≥ 0.099, Supplementary Table S2), indicating that the behavioural reactions of the study animals did not differ based on whether the snort stimulus originated from a conspecific or a heterospecific individual, nor on whether the call was pulsed or non-pulsed.
3.2. Pant Playbacks
The final model for the behavioural score included the three main terms stimulus type, group type, and sex, as well as a significant interaction between stimulus and group type (χ2 = 13.90, p = 0.003, Table 4). This interaction indicated that the effect of stimulus type on the behavioural response score varied depending on whether the study animals were tested alone or in the presence of a conspecific. In both group settings, the snort stimulus elicited significantly lower response scores than any of the pant stimuli (Est. ≤ 0.65, SE ≤ 0.12, Z ≥ |2.40|, p ≤ 0.033, Figure 4A). However, study animals tested together with a conspecific demonstrated more differentiation among the pant stimuli than those tested alone. Specifically, they showed significantly higher behavioural scores in response to the juvenile pant compared to the low-T pant (Est. = 1.59, SE = 0.25, Z = 2.92, p = 0.005, Figure 4A). No significant differences were found between the other pant stimuli (Est. ≥ 0.78, SE ≤ 0.18, Z ≤ |1.52|, p ≥ 0.147; Supplementary Table S3).

Table 4.
Results of the final models for the pant playback testing the effect of stimulus type, group type, and sex on the response parameters: behavioural score, locomotion, call rate, and latency to approach the loudspeaker; df = degree of freedom, * = statistically significant effect.
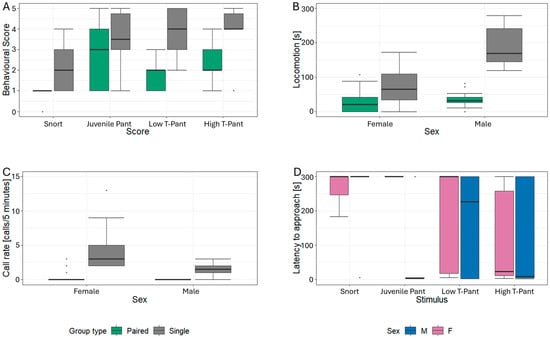
Figure 4.
Boxplots for the behavioural responses to pant playbacks dependent on group type for (A) behavioural score, (B) locomotion, (C) call rate, and (D) latency to approach the loudspeaker; black line = median, box = 25–75 % quartile, whisker = min-max non-outlier range.
Analysis of locomotor activity revealed that the final model included the main terms along with a significant interaction between group type and sex (χ2 = 13.05, p ≤ 0.001, Table 4). Among study animals tested alone, males exhibited significantly higher locomotor activity than females (Est. = −119.65, SE = 60.60, Z = −3.92, p = 0.003, Figure 4B). No such difference was observed in individuals that were tested in the presence of conspecifics (Est. = −8.63, SE = 24.7, Z = −0.349, p = 0.743).
In 28 of 60 (46.67%) pant playback trials, vocalisations were recorded from nine study animals during the observation period. Analysis of the overall call rate showed that the final model retained the main terms and a non-significant interaction between stimulus type and group type. Whereas stimulus type did not have a significant effect (χ2 = 6.63, p = 0.085), group type and sex had a significant impact on the call rate (χ2 ≥ 13.77, p ≤ 0.001, Table 4). Thus, study animals tested alone vocalised more than those tested with conspecifics, and females vocalised more than males (Figure 4C). In terms of occurring call types, snorts were uttered less frequently than pants and were more often elicited by the snort stimulus than by the pant stimuli (snort = 5, juvenile pant = 2, low T-pant = 3, high T-pant = 3). In contrast, pants were evenly distributed across all playback stimuli (snort = 5, juvenile pant = 6, low T-pant = 6, high T-pant = 6). Snorts were produced by five individuals and pants by seven, with three individuals producing both call types.
Study animals approached the loudspeaker during 24 of 60 (40%) playback trials within the observation interval (snort = 5, juvenile pant = 4, low T-pant = 7, high T-pant = 10). Analysis of latency to approach the loudspeaker revealed that the final model included the main terms as well as two interaction terms: stimulus type * sex and group type * sex (Table 4). Of these, only the interaction between stimulus type and sex was significant (χ2 = 15.86, p = 0.001, Figure 4D), indicating that the differences in the latency to approach the loudspeaker among the playback stimuli were highly affected by the sex of the study animals. Specifically, none of the females approached the loudspeaker following the juvenile pant stimulus, whereas males displayed their shortest approach latencies in response to this exact stimulus, resulting in a significant difference between the sexes (Est. = 283.30, SE = 63.70, Z = 4.45, p < 0.001, Figure 4D). Consequently, females exhibited significantly longer latencies following the juvenile pant compared to the high T-pant and the low T-pant (Est. ≥ 113.9, SE = 40.5, Z ≥ 2.816, p ≤ 0.015, Supplementary Table S4). In males, the juvenile pant elicited significantly shorter latencies to approach than the snort stimulus (ratio = 177.70, SE = 57.20, Z = 3.11, p = 0.021). In addition, it took females significantly longer to approach the loudspeaker following the snort stimuli compared to the high T-pant (ratio = 154.7, SE = 40.5, Z = 3.824, p = 0.001).
4. Discussion
In this study, we used controlled playback experiments to investigate the communicative function of snort and pant vocalisations in southern white rhinoceroses under captive conditions. By presenting conspecific and heterospecific snorts as well as male pants differing in the sender’s age and faecal testosterone levels, we aimed to assess how these cues are perceived by receivers. Our findings provide insights into the functional relevance of snorts and pants and the effects of social context and sex on behavioural responses. In the following, we discuss these findings in light of previous research, identify possible limitations, and propose directions for future studies.
4.1. Communicative Function of Snorts
The findings from the snort playback experiments indicate that snort vocalisations did not elicit differentiated behavioural responses depending on whether the calls originated from conspecific or heterospecific individuals. This outcome suggests that snort calls either lack species-specific acoustic signatures, making them acoustically ambiguous to the receiver, or that, irrespective of the sender species, snort calls convey minimal communicative information and therefore evoke similarly low levels of response behaviour under all conditions.
The first explanation of an inadequate species signature is supported by findings in previous studies describing snorts as being rather unspecific and exhibiting the lowest level of individual distinctiveness compared to other common call types in southern white rhinoceroses, such as pants, hisses, and grunts [45,46]. It is therefore likely that their acoustic structure also possesses few to no species-related properties, as no difference in any of the response behaviour parameters between the snorts and the white noise control was observed. At the same time, the lack of a difference compared to the white noise control can also be interpreted as an indicator for the snorts generally lacking social or contextual information. These observations are consistent with previous descriptions of snorts in southern white rhinoceroses, which attribute either a hygienic function to this call type [42] or one that occurs in non-social contexts such as during resting or feeding [43,44,46]. Corresponding to this are the descriptions of snorts in black rhinoceroses (Diceros bicornis), which, similar to the snorts in white rhinoceroses, do not seem to have a behavioural context and do not elicit any reactions from the conspecifics [55]. Meanwhile, in the Indian rhinoceros, the snort call has been described as an initial contact call that is most often directed at adult males [40]. While this observation suggests a clear communicative function for the snort, it should be noted that the description of the snort call type in Indian rhinoceros research does not necessarily correspond to the acoustic properties of the snorts described in other studies on the vocal repertoire in the Rhinocerotidae family: while Laurie [40] characterises the snort in the Indian rhinoceroses as a “series of quick bursts through the lips and nostrils”, the snort call type in the white rhinoceros has rather been associated with single air blows through the nostrils [42,43]. Thus, while the terms may be the same, they may describe different vocalisations depending on the study or species, and direct comparisons should therefore be made with caution.
Overall, the non-existing differences among the responses to different snort stimuli suggest that snorts do not have a distinct communicative function. This conclusion is supported by comparative data from the pant playback experiments, where snorts consistently elicited significantly lower response intensities than any of the pant stimuli. Notably, reactions to snorts rarely exceeded the body turn toward the loudspeaker on the behavioural response scale. In addition, only one-third of the study animals approached the loudspeaker at all following the snort stimulus, and only one of them actually did so immediately after the playback (within the first 15 s). These rather low-level responses suggest that, although the stimuli were perceived and may have briefly attracted attention due to their novelty, they failed to sustain interest or provoke further engagement, such as orienting or approaching the sound source.
Importantly, the consistency of low response levels to snorts across both playback designs affirms the validity of the experimental approach. The low reactivity cannot be attributed to the design itself—such as the use of single-call playback or the low sound level—but rather to the intrinsic nature of the snort as a stimulus. In addition, across a total of 55 snort playback sessions, observable behavioural responses (a score of 1 and higher on the behavioural response scale) were recorded in the vast majority of trials. In only seven sessions, no visible reaction to the acoustic stimulus could be observed. Given the high consistency of visible responses in the remaining sessions, it is reasonable to assume that the stimuli were clearly audible and perceived by the study animals. Thus, the few instances of no response are more likely attributable to the nature of the stimulus rather than to issues with stimulus delivery. This is further supported by the pant playback trials, where the only two instances of no observable behavioural response occurred following a snort stimulus, again reinforcing the notion that snorts elicit notably less engagement in the receiver compared to pants.
Nevertheless, it cannot be ruled out that snorts have an internal emotional function, signalling at least a relaxed state in southern white rhinoceros. Studies in horses showed that snort production is not random but seems to be related to emotional positive contexts and occur more often when welfare conditions increased [26,27]. Thus, further studies are needed to investigate the link between the internal emotional state and snort production rate.
4.2. Effect of Group Type
Group composition was found to significantly affect responses in both playback experiments. Individuals tested alone displayed higher response intensity, increased vigilance in the form of locomotion, and a greater frequency of vocalisations following playback. One possible explanation is that solitary animals were more aroused by the unexpected presence of vocalisations in the absence of visible conspecifics. In contrast, animals tested in pairs were likely habituated to conspecific vocalisations due to the constant presence of others, both before and during playback, resulting in a more attenuated reaction.
Similar effects of social environment have also been described in playback studies in other species. For instance, in rats (Rattus norvegicus), a higher heart rate, locomotor activity, and vocalisation rate after a 50 kHz appetitive stimulus compared to a 22 kHz aversive stimulus were particularly pronounced in solitarily housed animals [56]. Compared to the paired-housed group, singly housed rats also tended to spend more time close to the loudspeaker.
It is important to note that the present analysis did not differentiate between adult females housed with their calves and study animals paired with other adult conspecifics during the experiments. Maternal status can have a considerable influence on the behavioural responses, as shown in a playback study in black bears [57]: mother bears accompanied by their cubs advanced more frequently towards the sound source and emitted more grunts following a playback of a predatory competitor compared to solitary individuals. In a similar way, females with calves in the presented study might also have shown behavioural patterns distinct from non-maternal individuals, potentially reflected in differences in vigilance or call rate. However, for our dataset, the limited number of subjects in each subgroup did not allow for sufficient statistical power to assess such an effect reliably. Future research should consider maternal status and social pairing context more explicitly, as these factors may affect how individuals perceive and respond to social acoustic cues.
More broadly, these findings emphasise the importance of considering the social environment when interpreting playback responses. To date, much of the research on audience effects has focused on how the social environment shapes the signalling behaviour of the sender, e.g., [58,59], whereas comparatively few studies have addressed how social context and social cues modulate the responses of receivers. Investigating both perspectives is essential for a more comprehensive understanding of animal communication.
4.3. Effect of Sex
Sex also emerged as a significant predictor of response behaviour, but only in the pant playback experiment. Males exhibited greater activity levels than females, which correlates with the functional relevance of pant calls as mating or territorial signals. Since pants are typically associated with courting males approaching oestrous females [41,44,45,47,50], the playback may have triggered heightened movement behaviour in study males, potentially in an attempt to locate or monitor the unseen sender. Notably, increased locomotion occurred only in solitary males, which further supports the interpretation of an alarmed or investigative response toward a potential rival, unfamiliar caller, or intruder. These sex-specific differences in behavioural responses are consistent with recent findings from a playback study conducted in wild white rhinoceroses in Botswana, where males were reported to approach the loudspeaker more frequently than females following the playback of pant vocalisations, suggesting a heightened sensitivity or motivation among males toward these calls [60].
A sex-specific difference was also observed in call rate. However, in contrast to the other behavioural response parameters, it was the females that produced the specific pant vocalisation more frequently than males. This appears to contradict earlier results from Jenikejew et al. [41], who reported a higher pant call rate in males. One possible explanation for this discrepancy may lie in the age of the vocalising individuals. In this present study, the most vocal females were subadults or relatively young adults (between five and 14 years), potentially exhibiting greater social motivation or general vocal reactivity. Another relevant factor might be the testing condition: all four of these particularly vocal females were tested alone during the playback trials. It is therefore plausible that the increased pant production reflected attempts to re-establish contact with familiar conspecifics, given that the pant is commonly used as a contact call [42,43].
Findings from a recent playback study by Cinková and Shrader [61] suggest that oestrus can influence female response to pant vocalisations. They demonstrated that female white rhinoceroses that were not in oestrus exhibited limited vigilance and looking behaviour in response to the courtship-related variation in the pant call, known as the hic. These findings may help explain the comparatively low levels of movement and approaching activity observed among females in the present study. Although oestrus status was not systematically assessed for our study females, it could be confidently ruled out for approximately half of the females based on reproductive history, such as the presence of dependent calves or advanced age. Moreover, none of the females displayed behavioural indicators typically associated with oestrus, such as urine spraying or elevated tail posture [62].
At the same time, the observation that females showed higher call rates but less bodily movement and exploratory behaviour in response to pant playbacks, compared to males, may also reflect underlying social dynamics characteristic of the species. In the wild, females typically form temporally stable associations with other females and subadults [63,64] and thus may be more inclined to maintain vocal contact when separated from group members. In contrast, males are predominantly solitary and engage in extensive movement to establish and defend territories [65,66,67], a behavioural pattern that could manifest as greater locomotor activity during playback experiments. Taken together, these observations support the interpretation that the lack of strong approach responses among females may be linked to reproductive state as well as sex-specific social strategies. It is plausible that females in oestrus would show greater responsiveness to pant playbacks, potentially narrowing or reversing the observed sex-based differences in response behaviour. Future studies that explicitly account for oestrus status and social context would provide valuable insights into the modulatory role of reproductive condition and social organisation in shaping responses to vocal signals and help clarify the functional relevance of pant vocalisations in different behavioural contexts.
4.4. Effect of Androgen Level
No significant differences were found between behavioural responses to high T- and low T-pant stimuli. As a previous study showed that faecal testosterone levels were significantly higher in territorial white rhinoceros males than in nonterritorial ones [68], high T-pants—presumably representing more territorial or competitive males—were expected to elicit stronger behavioural responses in both female and male recipients. In line with that, a previous playback study on white rhinoceroses revealed that males were able to distinguish territorial from subordinate males based on their pant calls and reacted with higher attentiveness towards territorial senders [48]. Taken together, the two previous studies suggest that territorial and non-territorial white rhinoceros males differ in both their testosterone levels and the acoustic properties of their pant calls. In contrast, the results of the present study suggest that features potentially related to circulating testosterone levels, such as male quality or social dominance and thus, territoriality, were either not necessarily encoded in the acoustic properties of the pant stimuli or have simply not been perceived by the study animals. These contrasting results might be explained by the different experimental conditions: While both Rachlow et al. [68] and Cinková & Shrader [48] conducted their studies on free-ranging white rhinoceroses in natural settings of national parks or reserves in Southern Africa, the present study has been carried out under captive conditions.
In captive environments, male white rhinoceroses do not need to establish or defend territories, and consequently, cues related to territoriality may not be as distinctly encoded in their vocalisations compared to wild conspecifics. Furthermore, neither females nor males in the study population were likely to anticipate territorial intrusions, potentially reducing their attentiveness or responsiveness to any territoriality-related information embedded in pant calls.
A further consideration is the basis for stimulus selection. The high T- and low T-pant stimuli used in this study were classified solely based on the faecal testosterone metabolite (fTM) concentrations of the senders. On the one hand, it may be that the variation in fTM concentrations in the males available from the previous study (n = 16, [50]) was not broad enough to cover the range between actually low and high values. On the other hand, it remains unknown whether these hormonal profiles would reliably correspond to truly territorial versus non-territorial males under natural conditions. It is therefore possible that the androgen level differences between the selected stimuli were not sufficiently distinct to elicit clear behavioural discrimination among the receivers.
Future studies could provide deeper insights by employing playback stimuli from a larger sample size of wild individuals whose territorial status and androgen profiles are independently verified. Comparing responses to such stimuli could help clarify whether captive rhinoceroses retain sensitivity to social or territorial cues in pant vocalisations and whether such information is more salient when derived from naturally territorial males.
5. Conclusions
In summary, this study advances our understanding of vocal communication in southern white rhinoceroses by showing that snort vocalisations, despite their high production rate and structural variation, appear to have limited communicative value, as evidenced by consistently low and undifferentiated behavioural responses across experimental conditions. In contrast, pant calls elicited more pronounced response reactions influenced by both sex and social context: males exhibited greater activity levels, likely reflecting the role of pants in mating and territorial behaviour, while solitary individuals displayed heightened vigilance, possibly due to the unexpected presence of vocal signals in the absence of visible conspecifics. Meanwhile, females showed higher call rates in response to pant playbacks but engaged in less movement and exploration than males, a pattern that may reflect their social tendency to maintain vocal contact with group members rather than physically investigate potential intruders. The absence of discrimination between pants differing in testosterone levels suggests that, under captive conditions where territorial defence is not required, hormonal or dominance-related cues may not be strongly encoded or perceived. These findings highlight the value of playback experiments in disentangling the functional relevance of vocal signals and emphasise the importance of considering both intrinsic call features and extrinsic social factors when interpreting animal communication.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jzbg6040051/s1, Table S1: Ethogram for the analyses of the behavioural response towards the playback stimuli; Table S2: Results of the final models testing the effect of species and pulsation on the response parameters; Table S3: Results of the pairwise comparison of the playback stimuli for the behavioural score; Table S4: Results of the pairwise comparison of the playback stimuli for the latency to approach the loudspeaker.
Author Contributions
Conceptualization, J.J., M.S. (Mathilde Stomp), A.L., M.H. (Martine Hausberger), and M.S. (Marina Scheumann); methodology, J.J. and M.S. (Marina Scheumann); video analysis, J.J. and M.H. (Mascha Huelsewig); statistical analysis, J.J. and M.S. (Marina Scheumann); resources, A.L., M.H. (Martine Hausberger), I.A.-S., and M.B.; data collection, J.J., M.H. (Mascha Huelsewig), D.R., and M.S. (Marina Scheumann); writing—original draft preparation, J.J. and M.S. (Marina Scheumann); writing—review and editing, M.H. (Mascha Huelsewig), D.R., M.S. (Mathilde Stomp), A.L., M.H. (Martine Hausberger), I.A.-S., and M.B.; visualisation, M.S. (Marina Scheumann); supervision, M.S. (Marina Scheumann); project administration M.S. (Marina Scheumann); funding acquisition, A.L., M.H. (Martine Hausberger), and M.S. (Marina Scheumann) All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the PROCOPE programme of the German Academic Exchange Service (DAAD; grant number 57445356) and the German Research Foundation (SCHE 1927/2-1). We acknowledge financial support by the Open Access Publication Fund of the University of Veterinary Medicine Hannover Foundation.
Institutional Review Board Statement
The playbacks were conducted during the daily routine of the animals in their normal enclosures. No animal was taken out of its usual environment or physically manipulated by the authors. The authors received permission to record the animals’ data on the grounds of the respective zoo. For Givskud Zoo, research was additionally approved by the Animal Ethics Institutional Review Board at KU (No. 2025-01-BioEcoEv-005A).
Data Availability Statement
The raw dataset used for the manuscript is published in the Zenodo repository 10.5281/zenodo.17223266. Video and audio files are stored in the sound archive of the Institute of Zoology, University of Veterinary Medicine Hannover, and are available on reasonable request from Dr. Marina Scheumann.
Acknowledgments
We sincerely thank Sönke von den Berg and Alida Hasiniaina for their valuable assistance with data collection. We are also grateful to Planète Sauvage, Serengeti-Park Hodenhagen, and Givskud Zoo Zootopia for granting us access to their facilities and for their generous support throughout the study. In particular, we would like to thank Sabrina Wietzke, Oliver Kant, Kim Skalborg Simonsen, and all the dedicated animal keepers involved for their patience, cooperation, and encouragement.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SPB | Snort Playback |
| PPB | Pant Playback |
References
- McGregor, P.K. Playback experiments: Design and analysis. Acta Ethol. 2000, 3, 3–8. [Google Scholar] [CrossRef]
- McGregor, P.K. Playback and Studies of Animal Communication; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Fischer, J.; Noser, R.; Hammerschmidt, K. Bioacoustic field research: A primer to acoustic analyses and playback experiments with primates. Am. J. Primatol. 2013, 75, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Seyfarth, R.M.; Cheney, D.L. Signalers and receivers in animal communication. Annu. Rev. Psychol. 2003, 54, 145–173. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Watanabe, S. Contact calls: Information and social function. Jpn. Psychol. Res. 2009, 51, 197–208. [Google Scholar] [CrossRef]
- Charlton, B.D.; Ellis, W.A.; McKinnon, A.J.; Brumm, J.; Nilsson, K.; Fitch, W.T. Perception of male caller identity in koalas (Phascolarctos cinereus): Acoustic analysis and playback experiments. PLoS ONE 2011, 6, e20329. [Google Scholar] [CrossRef]
- Koren, L.; Geffen, E. Individual identity is communicated through multiple pathways in male rock hyrax (Procavia capensis) songs. Behav. Ecol. Sociobiol. 2011, 65, 675–684. [Google Scholar] [CrossRef]
- Pitcher, B.J.; Harcourt, R.G.; Charrier, I. Individual identity encoding and environmental constraints in vocal recognition of pups by Australian sea lion mothers. Anim. Behav. 2012, 83, 681–690. [Google Scholar] [CrossRef]
- Soltis, J.; Leong, K.; Savage, A. African elephant vocal communication II: Rumble variation reflects the individual identity and emotional state of callers. Anim. Behav. 2005, 70, 589–599. [Google Scholar] [CrossRef]
- Terry, A.M.; Peake, T.M.; McGregor, P.K. The role of vocal individuality in conservation. Front. Zool. 2005, 2, 10. [Google Scholar] [CrossRef]
- Wilson, D.R.; Goble, A.R.; Boutin, S.; Humphries, M.M.; Coltman, D.W.; Gorrell, J.C.; Shonfield, J.; McAdam, A.G. Red squirrels use territorial vocalizations for kin discrimination. Anim. Behav. 2015, 107, 79–85. [Google Scholar] [CrossRef]
- Kessler, S.E.; Scheumann, M.; Nash, L.T.; Zimmermann, E. Paternal kin recognition in the high frequency/ultrasonic range in a solitary foraging mammal. BMC Ecol. 2012, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Rendall, D.; Rodman, P.S.; Emond, R.E. Vocal recognition of individuals and kin in free-ranging rhesus monkeys. Anim. Behav. 1996, 51, 1007–1015. [Google Scholar] [CrossRef]
- Schuchmann, M.; Puechmaille, S.J.; Siemers, B.M. Horseshoe bats recognise the sex of conspecifics from their echolocation calls. Acta Chiropterol. 2012, 14, 161–166. [Google Scholar] [CrossRef]
- Rendall, D.; Owren, M.J.; Weerts, E.; Hienz, R.D. Sex differences in the acoustic structure of vowel-like grunt vocalizations in baboons and their perceptual discrimination by baboon listeners. J. Acoust. Soc. Am. 2004, 115, 411–421. [Google Scholar] [CrossRef]
- Rubow, J.; Cherry, M.I.; Sharpe, L.L. Dwarf mongooses use sex and identity cues in isolation calls to discriminate between callers. Anim. Behav. 2017, 127, 23–31. [Google Scholar] [CrossRef]
- Mielke, A.; Zuberbühler, K. A method for automated individual, species and call type recognition in free-ranging animals. Anim. Behav. 2013, 86, 475–482. [Google Scholar] [CrossRef]
- Rakotonirina, H.; Kappeler, P.M.; Fichtel, C. The role of acoustic signals for species recognition in redfronted lemurs (Eulemur rufifrons). BMC Evol. Biol. 2016, 16, 100. [Google Scholar] [CrossRef]
- Braune, P.; Schmidt, S.; Zimmermann, E. Acoustic divergence in the communication of cryptic species of nocturnal primates (Microcebus ssp.). BMC Biol. 2008, 6, 19. [Google Scholar] [CrossRef]
- Schuchmann, M.; Siemers, B.M. Behavioral evidence for community-wide species discrimination from echolocation calls in bats. Am. Nat. 2010, 176, 72–82. [Google Scholar] [CrossRef]
- Gwilliam, J.; Charrier, I.; Harcourt, R.G. Vocal identity and species recognition in male Australian sea lions, Neophoca cinerea. J. Exp. Biol. 2008, 211, 2288–2295. [Google Scholar] [CrossRef]
- Bartlett, D., Jr. Origin and regulation of spontaneous deep breaths. Respir. Physiol. 1971, 12, 230–238. [Google Scholar] [CrossRef]
- Tembrock, G. Acoustic behaviour of mammals. In Acoustic Behaviour of Animals; Busnel, R.-G., Ed.; Elsevier: London, UK, 1963; pp. 751–786. [Google Scholar]
- Li, P.; Yackle, K. Sighing. Curr. Biol. 2017, 27, R88–R89. [Google Scholar] [CrossRef]
- Soltysik, S.; Jelen, P. In rats, sighs correlate with relief. Physiol. Behav. 2005, 85, 598–602. [Google Scholar] [CrossRef]
- Stomp, M.; Leroux, M.; Cellier, M.; Henry, S.; Lemasson, A.; Hausberger, M. An unexpected acoustic indicator of positive emotions in horses. PLoS ONE 2018, 13, e0197898. [Google Scholar] [CrossRef] [PubMed]
- Stomp, M.; Leroux, M.; Cellier, M.; Henry, S.; Hausberger, M.; Lemasson, A. Snort acoustic structure codes for positive emotions in horses. Sci. Nat. 2018, 105, 57. [Google Scholar] [CrossRef] [PubMed]
- Déaux, É.C.; Clarke, J.A. Dingo (Canis lupus dingo) acoustic repertoire: Form and contexts. Behaviour 2013, 150, 75–101. [Google Scholar] [CrossRef]
- Gogoleva, S.; Volodin, J.; Volodina, E.; Trut, L. To bark or not to bark: Vocalizations by red foxes selected for tameness or aggressiveness toward humans. Bioacoustics 2008, 18, 99–132. [Google Scholar] [CrossRef]
- Sieber, O.J. Vocal communication in raccoons (Procyon lotor). Behaviour 1984, 90, 80–113. [Google Scholar] [CrossRef]
- Volodina, E.V.; Volodin, I.A.; Chelysheva, E.V.; Frey, R. Hiss and snort call types of wild-living giraffes Giraffa camelopardalis: Acoustic structure and context. BMC Res. Notes 2018, 11, 12. [Google Scholar] [CrossRef]
- Wong, J.; Stewart, P.D.; Macdonald, D.W. Vocal repertoire in the European badger (Meles meles): Structure, context, and function. J. Mammal. 1999, 80, 570–588. [Google Scholar] [CrossRef]
- Arnon, A.; Koyama, N.F.; Wronski, T. Vocalisation in wild-living mountain gazelles (Gazella gazella): Structure and context of acoustical signals. Behaviour 2024, 1, 731–751. [Google Scholar] [CrossRef]
- Bro-Jorgensen, J.; Pangle, W.M. Male topi antelopes alarm snort deceptively to retain females for mating. Am. Nat. 2010, 176, E33–E39. [Google Scholar] [CrossRef] [PubMed]
- Leuchtenberger, C.; Sousa-Lima, R.; Duplaix, N.; Magnusson, W.E.; Mourao, G. Vocal repertoire of the social giant otter. J. Acoust. Soc. Am. 2014, 136, 2861–2875. [Google Scholar] [CrossRef] [PubMed]
- Volodin, I.A.; Volodina, E.V.; Frey, R. Rutting vocal display in male impala (Aepyceros melampus) and overlap with alarm context. Front. Zool. 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.; Tonkin-Leyhausen, B.A. Evolution of acoustic communication signals of mammals: Friendly close-range vocalizations in Felidae (Carnivora). J. Mamm. Evol. 1999, 6, 129–159. [Google Scholar] [CrossRef]
- Hex, S.B.; Rubenstein, D.I. Using networks to visualize, analyse and interpret multimodal communication. Anim. Behav. 2024, 207, 295–317. [Google Scholar] [CrossRef]
- Hunsaker, D., II; Hahn, T.C. Vocalization of the South American tapir, Tapirus terrestris. Anim. Behav. 1965, 13, 69–74. [Google Scholar] [CrossRef]
- Laurie, A. Behavioural ecology of the Greater one-horned rhinoceros (Rhinoceros unicornis). J. Zool. 1982, 196, 307–341. [Google Scholar] [CrossRef]
- Jenikejew, J.; Chaignon, B.; Linn, S.; Scheumann, M. Proximity-based vocal networks reveal social relationships in the Southern white rhinoceros. Sci. Rep. 2020, 10, 15104. [Google Scholar] [CrossRef]
- Linn, S.N.; Boeer, M.; Scheumann, M. First insights into the vocal repertoire of infant and juvenile Southern white rhinoceros. PLoS ONE 2018, 13, e0192166. [Google Scholar] [CrossRef]
- Policht, R.; Tomášová, K.; Holečková, D.; Frynta, D. The vocal repertoire in Northern white rhinoceros Ceratotherium simum cottoni as recorded in the last surviving herd. Bioacoustics 2008, 18, 69–96. [Google Scholar] [CrossRef]
- Owen-Smith, R.N. The Behavioural Ecology of the White Rhinoceros; University of Wisconsin Madison: Madison, WI, USA, 1973. [Google Scholar]
- Cinkova, I.; Policht, R. Contact Calls of the Northern and Southern White Rhinoceros Allow for Individual and Species Identification. PLoS ONE 2014, 9, e98475. [Google Scholar] [CrossRef] [PubMed]
- Linn, S.N.; Schmidt, S.; Scheumann, M. Individual distinctiveness across call types of the southern white rhinoceros (Ceratotherium simum simum). J. Mammal. 2021, 102, 440–456. [Google Scholar] [CrossRef]
- Cinková, I.; Policht, R. Sex and species recognition by wild male southern white rhinoceros using contact pant calls. Anim. Cogn. 2016, 19, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Cinková, I.; Shrader, A.M. Rival assessment by territorial southern white rhinoceros males via eavesdropping on the contact and courtship calls. Anim. Behav. 2020, 166, 19–31. [Google Scholar] [CrossRef]
- Boersma, P.; Weenink, D. Praat: Doing phonetics by computer. Glot Int. 2021, 5, 341–345. Available online: https://www.praat.org (accessed on 2 May 2019).
- Jenikejew, J.; Wauters, J.; Dehnhard, M.; Scheumann, M. The female effect—How female receptivity influences faecal testosterone metabolite levels, socio-positive behaviour and vocalization in male southern white rhinoceroses. Conserv. Physiol. 2021, 9, coab026. [Google Scholar] [CrossRef]
- Noldus, L. The Observer: A software system for collection and analysis of observational data. Behav. Res. Methods Instrum. Comput. 1991, 23, 415–429. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef]
- Team Posit. RStudio: Integrated Development for R; Posit Software, PBC: Boston, MA, USA, 2025. [Google Scholar]
- Santon, M.; Korner-Nievergelt, F.; Michiels, N.K.; Anthes, N. A versatile workflow for linear modelling in R. Front. Ecol. Evol. 2023, 11, 1065273. [Google Scholar] [CrossRef]
- Budde, C.; Klump, G.M. Vocal repertoire of the black rhino Diceros bicornis ssp. and possibilities of individual identification. Mamm. Biol. 2003, 68, 42–47. [Google Scholar] [CrossRef]
- Olszyński, K.H.; Polowy, R.; Małż, M.; Boguszewski, P.M.; Filipkowski, R.K. Playback of alarm and appetitive calls differentially impacts vocal, heart-rate, and motor response in rats. IScience 2020, 23, 101577. [Google Scholar] [CrossRef]
- Suraci, J.P.; Clinchy, M.; Roberts, D.J.; Zanette, L.Y. Eavesdropping in solitary large carnivores: Black bears advance and vocalize toward cougar playbacks. Ethology 2017, 123, 593–599. [Google Scholar] [CrossRef]
- Zuberbühler, K. Audience effects. Curr. Biol. 2008, 18, R189–R190. [Google Scholar] [CrossRef]
- Matos, R.J.; Schlupp, I. Performing in front of an audience: Signallers and the social environment. In Animal Communication Networks; Mc Gregor, P.K., Ed.; Cambridge University Press: Cambridge, UK, 2005; pp. 63–83. [Google Scholar]
- Pfannerstill, V.; Balkenhol, N.; Bennitt, E.; Maboga, O.S.; Scheumann, M. Assessing the potential of conspecific playbacks as a post-translocation management tool for white rhinoceros. Conserv. Sci. Pract. 2023, 5, e12996. [Google Scholar] [CrossRef]
- Cinková, I.; Shrader, A.M. Individuality, species-specific features, and female discrimination of male southern white rhinoceros courtship calls. Anim. Cogn. 2022, 25, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Jenikejew, J.; Wauters, J.; Dehnhard, M.; Scheumann, M. Linking socio-sexual and vocal behaviour with faecal progesterone and oestrogen metabolite levels in Southern white rhinoceros females. Conserv. Physiol. 2021, 9, coab098. [Google Scholar] [CrossRef]
- Shrader, A.M.; Owen-Smith, R.N. The role of companionship in the dispersal of white rhinoceroses (Ceratotherium simum). Behav. Ecol. Sociobiol. 2002, 52, 255–261. [Google Scholar] [CrossRef]
- Owen-Smith, R.N. The social ethology of the White Rhinoceros Ceratotherium simum (Burchell 1817*). Z. Tierpsychol. 1975, 38, 337–384. [Google Scholar] [CrossRef]
- Owen-Smith, R.N. The social system of the white rhinoceros. In Proceedings of the Symposium on the Behaviour of Ungulates and its Relation to Management, Calgary, AB, Canada, 2–5 November 1971; Paper 15. IUCN: Morges, Switzerland, 1974; pp. 341–351. [Google Scholar]
- Owen-Smith, R.N. Territoriality in the white rhinoceros (Ceratotherium simum) Burchell. Nature 1971, 231, 294–296. [Google Scholar] [CrossRef]
- Rachlow, J.L.; Kie, J.G.; Berger, J. Territoriality and spatial patterns of white rhinoceros in Matobo National Park, Zimbabwe. Afr. J. Ecol. 1999, 37, 295–304. [Google Scholar] [CrossRef]
- Rachlow, J.L.; Berkeley, E.V.; Berger, J. Correlates of male mating strategies in white rhinos (Ceratotherium simum). J. Mammal. 1998, 79, 1317–1324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).