Can the Morphological Variation of Amazonian Bufonidae (Amphibia, Anura) Be Predicted by Their Habits and Habitats?
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Species and Morphological Trait Measurements
2.2. Habitat and Habit Characteristics
2.3. Statistical Analysis
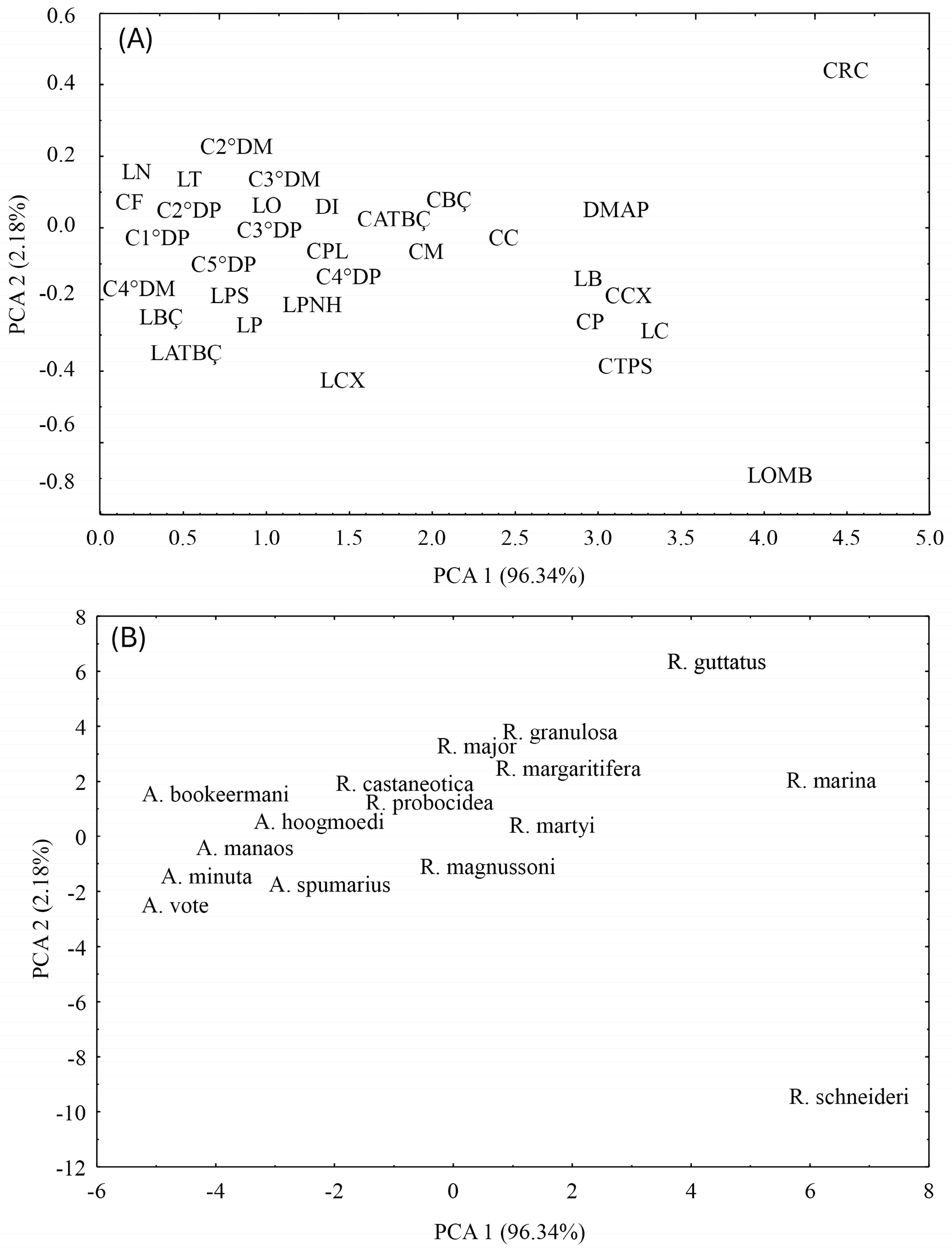
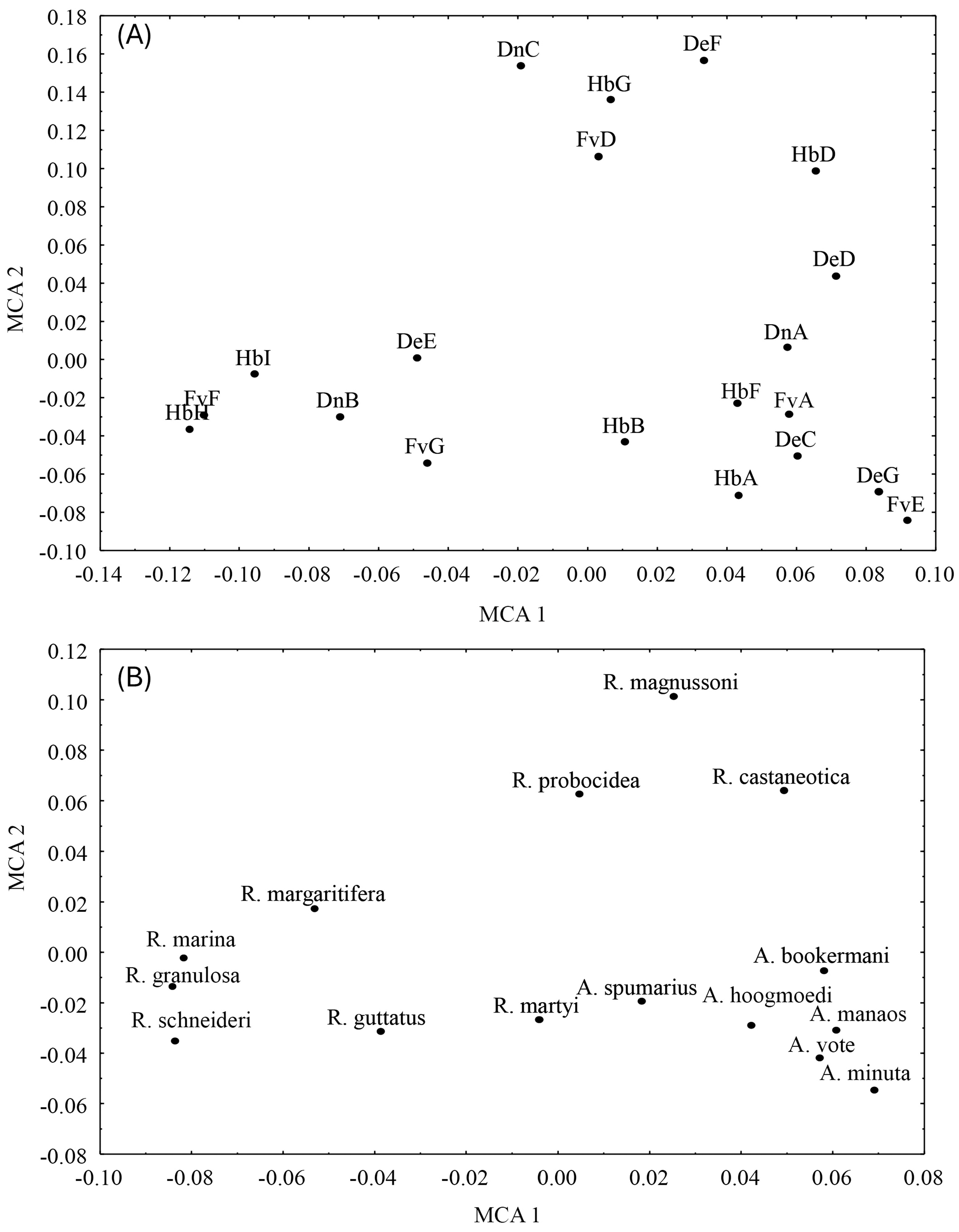
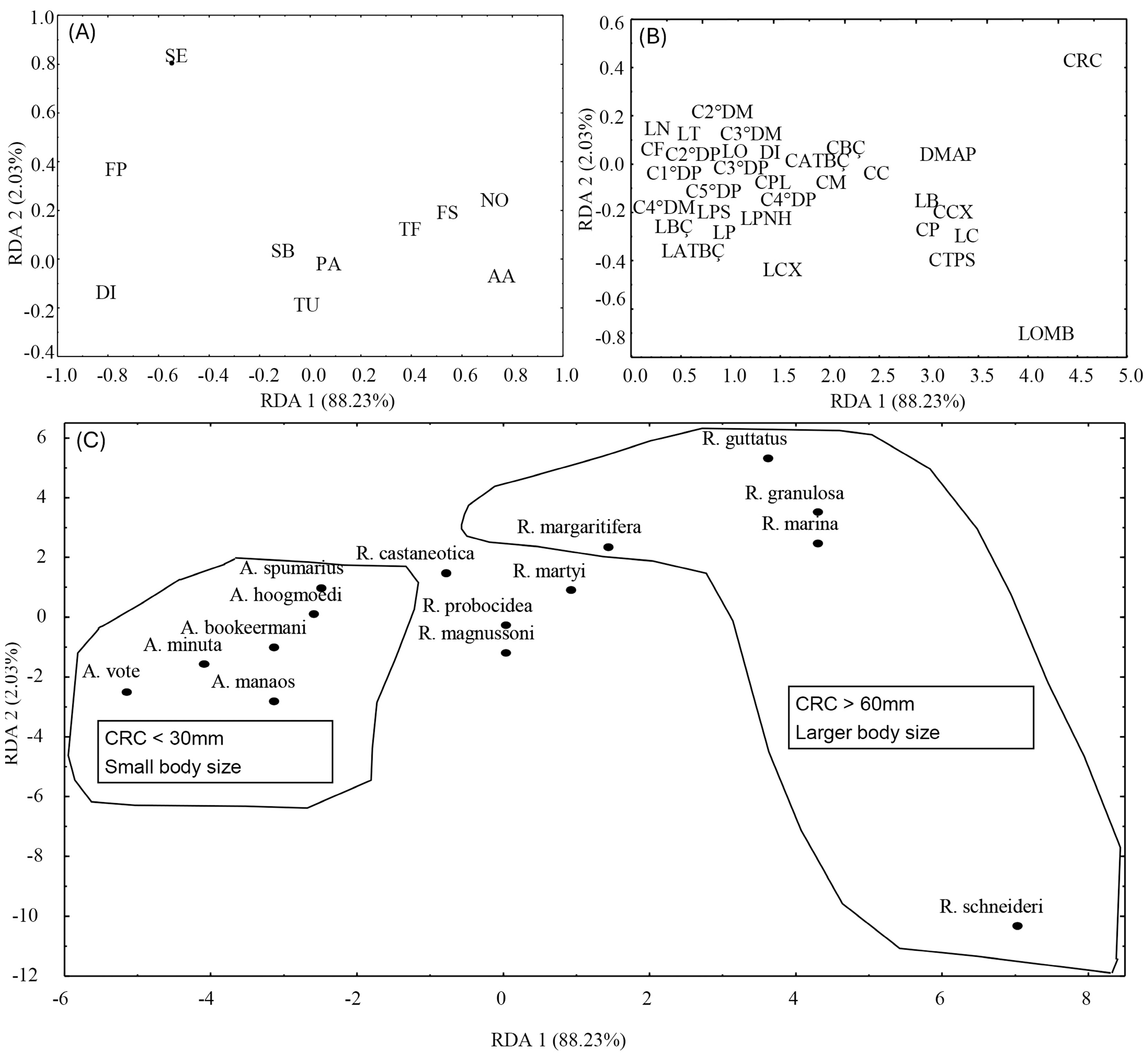
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wainwright, P.C. Ecomorphology: Experimental Functional Anatomy for Ecological Problems. Integr. Comp. Biol. 1991, 31, 680–693. [Google Scholar] [CrossRef]
- Geise, W.; Linsenmair, K.E. Adaptations of the Reed Frog Hyperolius Viridiflavus (Amphibia, Anura, Hyperoliidae) to Its Arid Environment—II. Some Aspects of the Water Economy of Hyperolius Viridiflavus Nitidulus under Wet and Dry Season Conditions. Oecologia 1986, 68, 542–548. [Google Scholar] [CrossRef]
- Irschick, D.J.; Losos, J.B. Do Lizards Avoid Habitats in Which Performance Is Submaximal? The Relationship between Sprinting Capabilities and Structural Habitat Use in Caribbean Anoles. Am. Nat. 1999, 154, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Korfiatis, K.J.; Stamou, G.P. Habitat Templets and the Changing Worldview of Ecology. Biol. Philos. 1999, 14, 375–393. [Google Scholar] [CrossRef]
- Citadini, J.M.; Brandt, R.; Williams, C.R.; Gomes, F.R. Evolution of Morphology and Locomotor Performance in Anurans: Relationships with Microhabitat Diversification. J. Evol. Biol. 2018, 31, 371–381. [Google Scholar] [CrossRef]
- Sherratt, E.; Anstis, M.; Keogh, J.S. Ecomorphological Diversity of Australian Tadpoles. Ecol. Evol. 2018, 8, 12929–12939. [Google Scholar] [CrossRef]
- Marques, N.C.S.; Rattis, L.; Nomura, F. Local Environmental Conditions Affecting Anuran Tadpoles’ Microhabitat Choice and Morphological Adaptation. Mar. Freshw. Res. 2019, 70, 395–401. [Google Scholar] [CrossRef]
- Annibale, F.S.; De Sousa, V.T.T.; De Sousa, C.E.; Venesky, M.D.; Rossa-Feres, D.d.C.; Nomura, F.; Wassersug, R.J. Influence of Substrate Orientation on Tadpoles’ Feeding Efficiency. Biol. Open 2019, 8, bio037598. [Google Scholar] [CrossRef]
- Annibale, F.S.; de Sousa, V.T.T.; de Sousa, C.E.; Venesky, M.D.; Rossa-Feres, D.d.C.; Wassersug, R.J.; Nomura, F. Smooth, Striated, or Rough: How Substrate Textures Affect the Feeding Performance of Tadpoles with Different Oral Morphologies. Zoomorphology 2020, 139, 97–110. [Google Scholar] [CrossRef]
- Vasconcelos, T.d.S.; dos Santos, T.G.; Rossa-Feres, D.d.C.; Haddad, C.F.B. Spatial and Temporal Distribution of Tadpole Assemblages (Amphibia, Anura) in a Seasonal Dry Tropical Forest of Southeastern Brazil. Hydrobiologia 2011, 673, 93–104. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World. Available online: https://amphibiansoftheworld.amnh.org/ (accessed on 19 November 2024).
- Segalla, M.V.; Berneck, B.; Canedo, C.; Caramaschi, U.; Cruz, A.G.C.; Garcia, P.C.A.; Grant, T.; Haddad, C.F.B.; Lourenço, A.C.; Mângia, S.; et al. Lista de Anfíbios Do Brasil. Herpetol. Bras. 2021, 10, 121–216. [Google Scholar] [CrossRef]
- Hillyard, S.D.; Hoff, K.V.S.; Propper, C. The Water Absorption Response: A Behavioral Assay for Physiological Processes in Terrestrial Amphibians. Physiol. Zool. 1998, 71, 127–138. [Google Scholar] [CrossRef]
- Navas, C.A. Implications of Microhabitat Selection and Patterns of Activity on the Thermal Ecology of High Elevation Neotropical Anurans. Oecologia 1996, 108, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Roznik, E.A.; Rodriguez-Barbosa, C.A.; Johnson, S.A. Hydric Balance and Locomotor Performance of Native and Invasive Frogs. Front. Ecol. Evol. 2018, 6, 159. [Google Scholar] [CrossRef]
- Freeland, W.J.; Kerin, S.H. Ontogenetic Alteration of Activity and Habitat Selection by Bufo Marinus. Wildl. Res. 1991, 18, 431–443. [Google Scholar] [CrossRef]
- Schwarzkopf, L.; Alford, R.A.; Alfordt, R.A. Desiccation and shelter-site use in a tropical amphibian: Comparing toads with physical models. Funct. Ecol. 1996, 10, 193–200. [Google Scholar] [CrossRef]
- Jaeger, R.G.; Hailman, J.P. Activity of Neotropical Frogs in Relation to Ambient Light. Biotropica 1981, 13, 59–65. [Google Scholar] [CrossRef]
- Cohen, M.P.; Alford, R.A. Herpetologists’ League Factors Affecting Diurnal Shelter Use by the Cane Toad. Herpetologica 1996, 52, 172–181. [Google Scholar]
- Hoffman, J.; Katz, U. The Ecological Significance of Burrowing Behaviour in the Toad (Bufo Viridis). Oecologia 1989, 81, 510–513. [Google Scholar] [CrossRef]
- Siqueira, C.C.; Van Sluys, M.; Ariani, C.V.; Rocha, C.F.D. Feeding Ecology of Thoropa Miliaris (Anura, Cycloramphidae) in Four Areas of Atlantic Rain Forest, Southeastern Brazil. J. Herpetol. 2006, 40, 520–525. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Legendre, L.; Legendre, P. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Team, R.D.C. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Goslee, S.C.; Urban, D.L. The Ecodist Package for Dissimilarity-Based Analysis of Ecological Data. J. Stat. Softw. 2007, 22, 1–19. [Google Scholar] [CrossRef]
- Roy, M. McDiarmid 1971 Comparative_Morphology_and_Evolution_Atelopus.Pdf. Bull. Los Angeles Cty. Mus. Nat. Hist. 1971, 12, 1–66. [Google Scholar]
- Noonan, B.P.; Gaucher, P. Phylogeography and Demography of Guianan Harlequin Toads (Atelopus): Diversification within a Refuge. Mol. Ecol. 2005, 14, 3017–3031. [Google Scholar] [CrossRef] [PubMed]
- Narvaes, P.; Rodrigues, M.T. Taxonomic Revision of Rhinella Granulosa Species Group (Amphibia, Anura, Bufonidae), with a Description of a New Species. Arq. Zool. 2009, 40, 1–73. [Google Scholar] [CrossRef]
- Menin, M.; Rodrigues, D.J.; Lima, A.P. The Tadpole of Rhinella Proboscidea (Anura: Bufonidae) with Notes on Adult Reproductive Behavior. Zootaxa 2006, 1258, 47–56. [Google Scholar] [CrossRef]
- de Melo, G.V.; Rossa-Feres, D.d.C.; Jim, J. Variação Temporal No Sítio de Vocalização Em Uma Comunidade de Anuros de Botucatu, Estado de São Paulo, Brasil. Biota Neotrop. 2007, 7, 93–102. [Google Scholar] [CrossRef]
- Brazil-Sousa, C.; Marques, R.M.; Albrecht, M.P. Segregação Alimentar Entre Duas Espécies de Heptapteridae No Rio Macaé, RJ. Biota Neotrop. 2009, 9, 31–37. [Google Scholar] [CrossRef]
- Bertoluci, J.; Rodrigues, M.T. Utilização de Habitats Reproduttvos e Micro-Habitats de Vocalização Em Uma Taxocenose de Anuros (Amphibia) Da Mata Atlântic a Do Sudeste Do Brasil. Pap. Avulsos Zool. 2002, 42, 287–297. [Google Scholar] [CrossRef]
- Batista, R.D.C.; De-Carvalho, C.B.; de Freitas, E.B.; Franco, S.D.C.; Batista, C.D.C.; Coelho, W.A.; Faria, R.G. Diet of Rhinella Schneideri (Werner, 1894) (Anura: Bufonidae) in the Cerrado, Central Brazil. Herpetol. Notes 2011, 4, 17–21. [Google Scholar]
- Díaz-Ricaurte, J.C.; Villegas, N.C.A.; Coronado, J.D.L.; Garzón, G.X.M.; Fiorillo, B.F. Effects of Agricultural Systems on the Anuran Diversity in the Colombian Amazon. Stud. Neotrop. Fauna Environ. 2022, 57, 18–28. [Google Scholar] [CrossRef]
- Da Rocha, M.; Ribeiro, C.; Garcez, R. Riqueza e Abundância de Anuros (Amphibia) Em Áreas de Pastagem e de Floresta Secundária Próxima a Porto Velho (Rondnia, Brasil). Rev. Colomb. Cienc. Anim. 2016, 8, 8. [Google Scholar]
- Moravec, J.; Lehr, E.; Cusi, J.C.; Córdova, J.H.; Gvoždík, V. A New Species of the Rhinella Margaritifera Species Group (Anura, Bufonidae) from the Montane Forest of the Selva Central, Peru. Zookeys 2014, 371, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.P.; Magnusson, W.E.; Menin, M.; Erdtmann, L.K.; Rodrigues, D.D.J.; Keller, C.; Hödl, W. Guia de Sapos Da Reserva Adolpho Ducke, Amazônia Central; Áttema Design Editorial: Manaus, Brazil, 2005; ISBN 85-99387-01-4. [Google Scholar]
- Halverson, M.A.; Skelly, D.K.; Kiesecker, J.M.; Freidenburg, L.K. Forest Mediated Light Regime Linked to Amphibian Distribution and Performance. Oecologia 2003, 134, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Navas, C.A.; Antoniazzi, M.M.; Carvalho, J.E.; Suzuki, H.; Jared, C. Physiological Basis for Diurnal Activity in Dispersing Juvenile Bufo Granulosus in the Caatinga, a Brazilian Semi-Arid Environment. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Zug, G.R.; Zug, P.B. The Marine Toad, Bufo Marinus: A Natural History Resume of Native Populations. Smithson Inst. 1979, 1–68. [Google Scholar]
- Cassemiro, F.A.S.; Gouveia, S.F.; Diniz-Filho, J.A.F. Distribuição de Rhinella Granulosa: Integrando Envelopes Bioclimáticos e Respostas Ecofisiológicas. Rev. Biol. 2012, 8, 38–44. [Google Scholar] [CrossRef]
- Lima, L.L.C.; Oliveira, J.P.S.; Silva, L.E.B.; Santos, C.B.; dos Santos, C.B. Características Gerais Dos Anfíbios Anuros e Sua Biodiversidade. Divers. J. 2019, 4, 774–789. [Google Scholar] [CrossRef]
- Junior, B.T.; Gomes, F.R. Balanço Hídrico e a Distribuição Geográfica Dos Anfíbios Water Balance and Geographical Distribution of Amphibians. Rev. Biol. 2012, 8, 49–57. [Google Scholar] [CrossRef]
- Portik, D.M.; Blackburn, D.C. The Evolution of Reproductive Diversity in Afrobatrachia: A Phylogenetic Comparative Analysis of an Extensive Radiation of African Frogs. Evolution 2016, 70, 2017–2032. [Google Scholar] [CrossRef]
- Maciel, N.M.; Collevatti, R.G.; Colli, G.R.; Schwartz, E.F. Late Miocene Diversification and Phylogenetic Relationships of the Huge Toads in the Rhinella Marina (Linnaeus, 1758) Species Group (Anura: Bufonidae). Mol. Phylogenet. Evol. 2010, 57, 787–797. [Google Scholar] [CrossRef]
- Moreno-Barbosa, S.E.; Hoyos-Hoyos, J.M. Ontogeny of the Diet in Anurans (Amphibia) Collected at La Vieja River Basin in the Deparmento of Quindio (Colombia). Caldasia 2014, 36, 365–372. [Google Scholar] [CrossRef]
- Toft, C.A. Society for the Study of Amphibians and Reptiles Feeding Ecology of Panamanian Litter Anurans: Patterns in Diet and Foraging Mode. J. Herpetol. 1981, 15, 139–144. [Google Scholar] [CrossRef]
- Maria, L.; González, J.; Señaris, C.; Rodríguez-Contreras, A. Dieta Del Sapito Rayado Atelopus Cruciger (Amphibia: Anura: Bufonidae) En El Tramo Central de La Cordillera de La Costa, Venezuela. Mem. Fund. Salle Cienc. Nat. 2012, 2012, 173–174. [Google Scholar]
- Toledo, L.F.; Zina, J.; Haddad, C.F.B. Distribuição Espacial e Temporal de Uma Comunidade de Anfíbios Anuros Do Município de Rio Claro, São Paulo, Brasil. Holos Environ. 2003, 3, 136–149. [Google Scholar] [CrossRef]
- Gascon, C. Population-and Community-Level Analyses of Species Occurrences of Central Amazonian Rainforest Tadpoles. Ecology 1991, 72, 1731–1746. [Google Scholar] [CrossRef]
- Duellman, W.E. Society for the Study of Amphibians and Reptiles Temporal Fluctuations in Abundances of Anuran Amphibians in a Seasonal Amazonian Rainforest Temporal Fluctuations in Abundances of Anuran Amphibians in a Seasonal Amazonian Rainforest. Society 2011, 29, 13–21. [Google Scholar]
- Young, B.E.; Lips, K.R.; Reaser, J.K.; Ibanez, R.; Salas, A.W.; Cedeno, J.R.; Coloma, L.A.; Ron, S.; La Marca, E.; Meyer, J.R.; et al. Population Declines and Priorities for Amphibian Conservation in Latin America. Conserv. Biol. 2001, 15, 1213–1223. [Google Scholar] [CrossRef]
- Becker, C.G.; Fonseca, C.R.; Haddad, C.F.B.; Batista, R.F.; Prado, P.I. Habitat Split and the Global Decline of Amphibians. Science 2007, 318, 1775–1777. [Google Scholar] [CrossRef]
| Ecological Variable | Acronym | Description |
|---|---|---|
| Type of vegetation where the species is found | ||
| Primary Forest | PF | Undisturbed forest, or with little disturbance |
| Secondary Forest | SF | Forest in regeneration |
| Anthropized Area | AA | Areas whose original features (in soil, vegetation, relief) have been changed |
| Type of environment | ||
| Terra Firme Forest | TF | Unflooded/non-flooded forest/dry forest |
| Flooded Forest | FF | Flooded areas and wetlands |
| Next to the Water | NW | Margins of rivers, lagoons, and dams |
| Open Area | AO | Pasture areas, plantations, gardens, or urban areas |
| Litter | LT | Aboveground layer covered by decomposing organic remains |
| Understory | US | Subshrub vegetation found within the forest |
| Spawning site | ||
| Temporary Pond | TP | Formed in the rainy season |
| Plant Structure | OS | Nut capsules, hollow trunks and roots, etc. |
| Stream | S | Streams |
| Activity period | ||
| Diurnal | D | Active during the day |
| Nocturnal | N | Active during the night |
| Variable | Combinations | Group |
|---|---|---|
| FvA | PF | Vegetation Type |
| Fobs | SF | |
| FvC | AA | |
| FvD | PF × SF | |
| FvE | PF × AA | |
| FvF | SF × AA | |
| FvG | PF × SF × AA | |
| DnA | D | Activity Period |
| DnB | N | |
| HbA | FF × NW × LT | Environment where the species occurs |
| HbB | TF × NW × LT | |
| HbC | TF × FF × OA | |
| HbD | TF × FF × LT | |
| HbE | TF × FF × NW × US | |
| HbF | TF × FF × NW × LT | |
| HbG | TF × FF × NW × LT × US | |
| HbH | TF × FF × NW × OA | |
| HbI | TF × FF × NW × OA × US | |
| DeA | TP | Spawning site |
| DeB | OS | |
| DeC | S | |
| DeD | TP × PS | |
| DeE | TP × S | |
| DeF | PS × S | |
| DeG | TP × PS × S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.S.Q.A.; Alexandre, R.J.R.; Pena, S.A.; Correia, L.L.; Souza, T.S.; Dias, S.V.; Vieira, T.B.; Gomes, F.B.R. Can the Morphological Variation of Amazonian Bufonidae (Amphibia, Anura) Be Predicted by Their Habits and Habitats? J. Zool. Bot. Gard. 2025, 6, 50. https://doi.org/10.3390/jzbg6040050
Oliveira ASQA, Alexandre RJR, Pena SA, Correia LL, Souza TS, Dias SV, Vieira TB, Gomes FBR. Can the Morphological Variation of Amazonian Bufonidae (Amphibia, Anura) Be Predicted by Their Habits and Habitats? Journal of Zoological and Botanical Gardens. 2025; 6(4):50. https://doi.org/10.3390/jzbg6040050
Chicago/Turabian StyleOliveira, Andressa Sasha Quevedo Alves, Rafaela Jemely Rodrigues Alexandre, Simone Almeida Pena, Letícia Lima Correia, Thais Santos Souza, Samantha Valente Dias, Thiago Bernardi Vieira, and Felipe Bittioli R. Gomes. 2025. "Can the Morphological Variation of Amazonian Bufonidae (Amphibia, Anura) Be Predicted by Their Habits and Habitats?" Journal of Zoological and Botanical Gardens 6, no. 4: 50. https://doi.org/10.3390/jzbg6040050
APA StyleOliveira, A. S. Q. A., Alexandre, R. J. R., Pena, S. A., Correia, L. L., Souza, T. S., Dias, S. V., Vieira, T. B., & Gomes, F. B. R. (2025). Can the Morphological Variation of Amazonian Bufonidae (Amphibia, Anura) Be Predicted by Their Habits and Habitats? Journal of Zoological and Botanical Gardens, 6(4), 50. https://doi.org/10.3390/jzbg6040050








