Why Measuring and Building Resilience Is Applicable to Zoo and Aquarium Animal Welfare
Abstract
1. Introduction
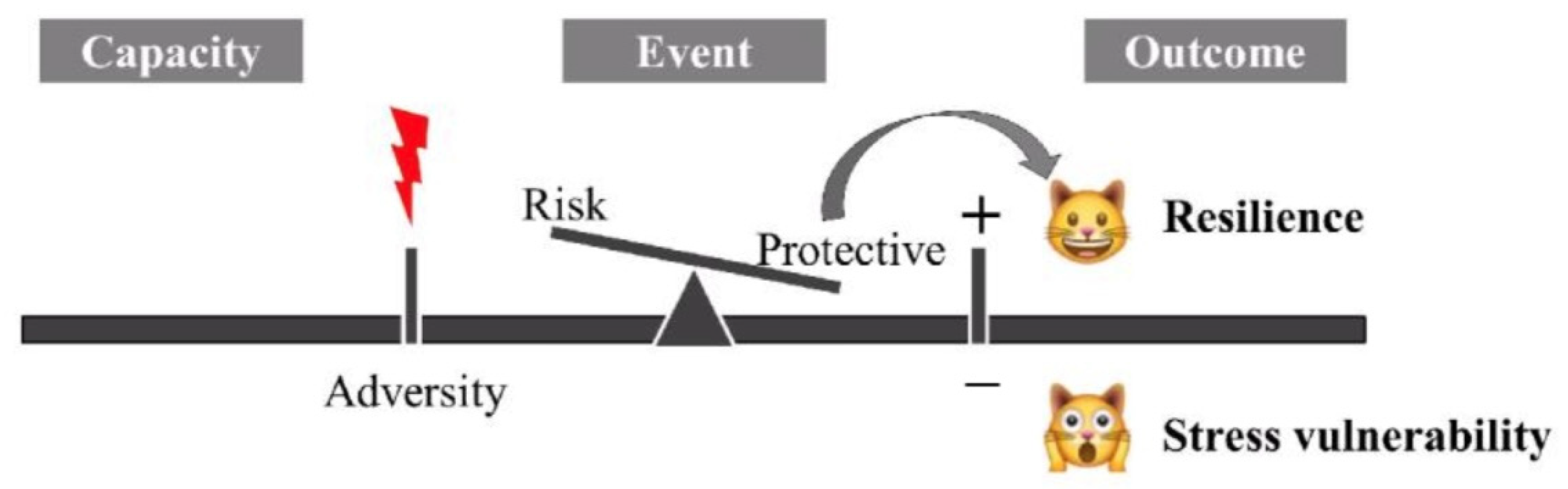
1.1. What Is Resilience?
1.2. How to Measure Resilience in Professionally Managed Animals
1.3. How to Build Resilience in Professionally Managed Animals
2. Discussion
3. Future Directions
What Next Steps Should Welfare Researchers and Animal Care Specialists Be Taking?
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colditz, I.G.; Hine, B.C. Resilience in farm animals: Biology, management, breeding and implications for animal welfare. Anim. Prod. Sci. 2016, 56, 1961–1983. [Google Scholar] [CrossRef]
- Basile, F.; Capaccia, C.; Zampini, D.; Biagetti, T.; Diverio, S.; Guelfi, G. Omics Insights into Animal Resilience and Stress Factors. Animals 2021, 11, 47. [Google Scholar] [CrossRef]
- Colditz, I.G. Competence to thrive: Resilience as an indicator of positive health and positive welfare in animals. Anim. Prod. Sci. 2022, 62, 1439–1458. [Google Scholar] [CrossRef]
- American Psychological Association. Available online: https://www.apa.org/topics/resilience (accessed on 1 June 2025).[Green Version]
- Wechsler, B.; Lea, S.E. Adaptation by learning: Its significance for farm animal husbandry. Appl. Anim. Behav. Sci. 2007, 108, 197–214. [Google Scholar] [CrossRef]
- Špinka, M.; Wemelsfelder, F. Environmental challenge and animal agency. In Animal Welfare, 2nd ed.; Appleby, M.C., Mench, J.A., Olsson, I.A.S., Hughes, B.O., Eds.; CABI Publishing: Wallingford, UK, 2011; pp. 27–43. [Google Scholar]
- Boissy, A.; Lee, C. How assessing relationships between emotions and cognition can improve farm animal welfare. Rev. Sci. Tech. 2014, 33, 103–110. [Google Scholar] [CrossRef]
- Jensen, P.; Toates, F.M. Stress as a state of motivational systems. Appl. Anim. Behav. Sci. 1997, 53, 145–156. [Google Scholar] [CrossRef]
- Lee, C.; Colditz, I.G.; Campbell, D.L.M. A framework to assess the impact of new animal management technologies on welfare: A case study of virtual fencing. Front. Vet. Sci. 2018, 5, 187. [Google Scholar] [CrossRef]
- Broom, D.M. Animal welfare defined in terms of attempts to cope with the environment. Acta Agric. Scand. Sect. A Anim. Sci. 1996, 27, 22–28. [Google Scholar]
- Franklin, T.B.; Saab, B.J.; Mansuy, I.M. Neural mechanisms of stress resilience and vulnerability. Neuron 2012, 75, 747–761. [Google Scholar] [CrossRef]
- Beery, A.K.; Kaufer, D. Stress, social behavior, and resilience: Insights from rodents. Neurobiol. Stress 2015, 1, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, G.A.; Van de Kar, L.D. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003, 463, 235–272. [Google Scholar] [CrossRef]
- Bergström, A.; Jayatissa, M.N.; Mørk, A.; Wiborg, O. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Res. 2008, 1196, 41–52. [Google Scholar] [CrossRef]
- Taliaz, D.; Stall, N.; Dar, D.E.; Zangen, A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol. Psychiatry 2010, 15, 80–92. [Google Scholar] [CrossRef]
- Schmidt, M.V.; Trümbach, D.; Weber, P.; Wagner, K.; Scharf, S.H.; Liebl, C.; Datson, N.; Namendorf, C.; Gerlach, T.; Kühne, C.; et al. Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. J. Neurosci. 2010, 30, 16949–16958. [Google Scholar] [CrossRef]
- Russo, S.J.; Murrough, J.W.; Han, M.H.; Charney, D.S.; Nestler, E.J. Neurobiology of resilience. Nat. Neurosci. 2012, 15, 1475–1484. [Google Scholar] [CrossRef]
- Wu, G.; Feder, A.; Cohen, H.; Kim, J.J.; Calderon, S.; Charney, D.S.; Mathe, A.A. Understanding resilience. Front. Behav. Neurosci. 2013, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.A., 3rd; Wang, S.; Southwick, S.M.; Rasmusson, A.; Hazlett, G.; Hauger, R.L.; Charney, D.S. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol. Psychiatry 2000, 47, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.A., 3rd; Rasmusson, A.M.; Wang, S.; Hoyt, G.; Hauger, R.L.; Hazlett, G. Neuropeptide-Y, cortisol, and subjective distress in humans exposed to acute stress: Replication and extension of previous report. Biol. Psychiatry 2002, 52, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Clunies Ross, I. Observations on the resistance of sheep to infestation by the stomach worm (Haemonchus contortus). J. Sci. Ind. Res. 1932, 5, 73–80. [Google Scholar]
- Albers, G.A.A.; Gray, G.D.; Piper, L.R.; Barker, J.S.F.; Le Jambre, L.F.; Barger, I.A. The genetics of resistance and resilience to Haemonchus contortus infection in young Merino sheep. Int. J. Parasitol. 1987, 17, 1355–1363. [Google Scholar] [CrossRef]
- Mengistu, U.L.; Puchala, R.; Sahlu, T.; Gipson, T.A.; Dawson, L.J.; Goetsch, A.L. Conditions to evaluate differences among individual sheep and goats in resilience to high heat load index. Small Rumin. Res. 2017, 147, 89–95. [Google Scholar] [CrossRef]
- Sánchez-Molano, E.; Kapsona, V.V.; Oikonomou, S.; McLaren, A.; Lambe, N.; Conington, J.; Banos, G. Breeding strategies for animal resilience to weather variation in meat sheep. BMC Genet. 2020, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Tsartsianidou, V.; Kapsona, V.V.; Sánchez-Molano, E.; Basdagianni, Z.; Carabaño, M.J.; Chatziplis, D.; Arsenos, G.; Triantafyllidis, A.; Banos, G. Understanding the seasonality of performance resilience to climate volatility in Mediterranean dairy sheep. Sci. Rep. 2021, 11, 1889. [Google Scholar] [CrossRef]
- Hine, B.C.; Bell, A.M.; Niemeyer, D.D.O.; Duff, C.J.; Butcher, N.M.; Dominik, S.; Ingham, A.B.; Colditz, I.G. Immune competence traits assessed during the stress of weaning are heritable and favorably genetically correlated with temperament traits in Angus cattle. J. Anim. Sci. 2019, 97, 4053–4065. [Google Scholar] [CrossRef]
- Revilla, M.; Friggens, N.C.; Broudiscou, L.P.; Lemonnier, G.; Blanc, F.; Ravon, L.; Mercat, M.J.; Billon, Y.; Rogel-Gaillard, C.; Le Floch, N.; et al. Towards the quantitative characterisation of piglets’ robustness to weaning: A modelling approach. Animal 2019, 13, 2536–2546. [Google Scholar] [CrossRef]
- Elgersma, G.G.; de Jong, G.; van der Linde, R.; Mulder, H.A. Fluctuations in milk yield are heritable and can be used as a resilience indicator to breed healthy cows. J. Dairy Sci. 2018, 101, 1240–1250. [Google Scholar] [CrossRef]
- Nunes Marsiglio Sarout, B.; Waterhouse, A.; Duthie, C.-A.; Candal Poli, C.H.E.; Haskell, M.J.; Berger, A.; Umstatter, C. Assessment of circadian rhythm of activity combined with random regression model as a novel approach to monitoring sheep in an extensive system. Appl. Anim. Behav. Sci. 2018, 207, 26–38. [Google Scholar] [CrossRef]
- Nguyen-Ba, H.; van Milgen, J.; Taghipoor, M. A procedure to quantify the feed intake response of growing pigs to perturbations. Animal 2020, 14, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.; Veerkamp, R.F.; van Pelt, M.L.; Mulder, H.A. Exploration of variance, autocorrelation, and skewness of deviations from lactation curves as resilience indicators for breeding. J. Dairy Sci. 2020, 103, 1667–1684. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.; Mulder, H.A.; Veerkamp, R.F. Validation of resilience indicators by estimating genetic correlations among daughter groups and with yield responses to a heat wave and disturbances at herd level. J. Dairy Sci. 2021, 104, 8094–8106. [Google Scholar] [CrossRef]
- Sun, D.; Webb, L.; van der Tol, P.P.J.; van Reenen, K. A systematic review of automatic health monitoring in calves: Glimpsing the future from current practice. Front. Vet. Sci. 2021, 8, 761468. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Plastow, G.S. Breeding for disease resilience: Opportunities to manage polymicrobial challenge and improve commercial performance in the pig industry. CABI Agric. Biosci. 2022, 3, 6. [Google Scholar] [CrossRef]
- María, G.A.; Escós, J.; Alados, C.L. Complexity of behavioural sequences and their relation to stress conditions in chickens (Gallus gallus domesticus): A non-invasive technique to evaluate animal welfare. Appl. Anim. Behav. Sci. 2004, 86, 93–104. [Google Scholar] [CrossRef]
- Steel, J.W. Effects of protein supplementation of young sheep on resistance development and resilience to parasitic nematodes. Aust. J. Exp. Agric. 2003, 43, 1469–1476. [Google Scholar] [CrossRef]
- Douhard, F.; Douhard, M.; Gilbert, H.; Monget, P.; Gaillard, J.-M.; Lemaître, J.-F. How much energetic trade-offs limit selection? Insights from livestock and related laboratory model species. Evol. Appl. 2021, 14, 2726–2749. [Google Scholar] [CrossRef]
- Garland, T., Jr.; Downs, C.J.; Ives, A.R. Trade-offs (and constraints) in organismal biology. Physiol. Biochem. Zool. 2022, 95, 82–112. [Google Scholar] [CrossRef]
- Animal Behavior Management Alliance. Available online: https://www.theabma.org/glossary (accessed on 1 June 2025).
- Garcia-Baccino, C.A.; Marie-Etancelin, C.; Tortereau, F.; Marcon, D.; Weisbecker, J.-L.; Legarra, A. Detection of unrecorded environmental challenges in high-frequency recorded traits, and genetic determinism of resilience to challenge, with an application on feed intake in lambs. Genet. Sel. Evol. 2021, 53, 4. [Google Scholar] [CrossRef]
- Buller, H.; Blokhuis, H.; Lokhorst, K.; Silberberg, M.; Veissier, I. Animal welfare management in a digital world. Animals 2020, 10, 1779. [Google Scholar] [CrossRef]
- Frodl, T.; O’Keane, V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol. Dis. 2012, 52, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Markopoulou, K.; Papadopoulos, A.; Juruena, M.F.; Poon, L.; Pariante, C.M.; Cleare, A.J. The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology 2009, 34, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Rasmusson, A.; Pietrzak, R. Relationships among plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate, cortisol, symptoms of dissociation, and objective performance in humans. Biol. Psychiat. 2009, 66, 334–340. [Google Scholar] [CrossRef]
- Yehuda, R.; Brand, S.R.; Golier, J.A.; Yang, R.K. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr. Scand. 2006, 114, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Peric, T.; Corazzin, M.; Romanzin, A.; Bovolenta, S.; Prandi, A.; Montillo, M.; Comin, A. Cortisol and DHEA concentrations in the hair of dairy cows managed indoor or on pasture. Livest. Sci. 2017, 202, 39–43. [Google Scholar] [CrossRef]
- Trevisan, C.; Montillo, M.; Prandi, A.; Mkupasi, E.M.; Ngowi, H.A.; Johansen, M.V. Hair cortisol and dehydroepiandrosterone concentrations in naturally Taenia solium infected pigs in Tanzania. Gen. Comp. Endocr. 2017, 246, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Whitham, J.C.; Bryant, J.L.; Miller, L.J. Beyond glucocorticoids: Integrating dehydroepiandrosterone (DHEA) into animal welfare research. Animals 2020, 10, 1381. [Google Scholar] [CrossRef]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Stevenson, M.A.; Fisher, A.D. Associations between immune competence, stress responsiveness, and production in Holstein-Friesian and Holstein-Friesian × Jersey heifers reared in a pasture-based production system in Australia. J. Dairy Sci. 2019, 102, 3282–3294. [Google Scholar] [CrossRef]
- Hine, B.C.; Acton, G.A.; Elks, D.J.; Niemeyer, D.D.O.; Bell, A.M.; Colditz, I.G.; Ingham, A.B.; Smith, J.L. Targeting improved resilience in Merino sheep: Correlations between immune competence and health and fitness trait. Animal 2022, 16, 100544. [Google Scholar] [CrossRef]
- Mallard, B.A.; Emam, M.; Paibomesai, M.; Thompson-Crispi, K.; Wagter-Lesperance, L. Genetic selection of cattle for improved immunity and health. Jpn. J. Vet. Res. 2015, 63, S37–S44. [Google Scholar] [CrossRef]
- König, S.; May, K. Invited review: Phenotyping strategies and quantitative-genetic background of resistance, tolerance and resilience associated traits in dairy cattle. Animal 2019, 13, 897–908. [Google Scholar] [CrossRef]
- Shilja, S.; Sejian, V.; Bagath, M.; Mech, A.; David, C.G.; Kurien, E.K.; Varma, G.; Bhatta, R. Adaptive capability as indicated by behavioral and physiological responses, plasma HSP70 level, and PBMC HSP70 mRNA expression in Osmanabadi goats subjected to combined (heat and nutritional) stressors. Int. J. Biometeorol. 2016, 60, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Aleena, J.; Sejian, V.; Bagath, M.; Krishnan, G.; Beena, V.; Bhatta, R. Resilience of three indigenous goat breeds to heat stress based on phenotypic traits and PBMC HSP70 expression. Int. J. Biometeorol. 2018, 62, 1995–2005. [Google Scholar] [CrossRef]
- Asher, L.; Collins, L.M.; Ortiz-Pelaez, A.; Drewe, J.A.; Nicol, C.J.; Pfeiffer, D.U. Recent advances in the analysis of behavioural organization and interpretation as indicators of animal welfare. J. R. Soc. Interface 2009, 6, 1103–1119. [Google Scholar] [CrossRef]
- MacIntosh, A.J.J.; Alados, C.L.; Huffman, M.A. Fractal analysis of behaviour in a wild primate: Behavioural complexity in health and disease. J. R. Soc. Interface 2011, 8, 1497–1509. [Google Scholar] [CrossRef]
- Freund, J.; Brandmaier, A.M.; Lewejohann, L.; Kirste, I.; Kritzler, M.; Krüger, A.; Sachser, N.; Lindenberger, U.; Kempermann, G. Emergence of individuality in genetically identical mice. Science 2013, 340, 756–759. [Google Scholar] [CrossRef]
- Briefer, E.F. Vocal expression of emotions in mammals: Mechanisms of production and evidence. J. Zool. 2012, 288, 1–20. [Google Scholar] [CrossRef]
- Whitham, J.C.; Miller, L.J. Utilizing vocalizations to gain insight into the affective states of non-human mammals. Front. Vet. Sci. 2024, 11, 1366933. [Google Scholar] [CrossRef]
- Špinka, M.; Newberry, R.C.; Bekoff, M. Mammalian play: Training for the unexpected. Q. Rev. Biol. 2001, 76, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Boissy, A.; Manteuffel, G.; Bak Jensen, M.; Oppermann Moe, R.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Yeates, J.W.; Main, D.C.J. Assessment of positive welfare: A review. Vet. J. 2008, 175, 293–300. [Google Scholar] [CrossRef]
- Miller, L.J.; Vicino, G.A.; Sheftel, J.; Lauderdale, L.K. Behavioral diversity as a potential indicator of positive animal welfare. Animals 2020, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.; Mulder, H.A.; Kamphuis, C.; Veerkamp, R.F. Between-herd variation in resilience and relations to herd performance. J. Dairy Sci. 2021, 104, 616–627. [Google Scholar] [CrossRef]
- Whitham, J.C.; Wielebnowski, N. New directions for zoo animal welfare science. Appl. Anim. Behav. Sci. 2013, 147, 247–260. [Google Scholar] [CrossRef]
- Rodrigues, G.R.; Valente, J.P.; Rezende, V.T.; Araújo, L.F.; Silva Neto, J.B.; Mota, L.F.; Santana, M.L.; Canesin, R.C.; Bonilha, S.F.M.; Albuquerque, L.G.; et al. Genetic parameters of resilience indicators across growth in beef heifers and their associations with weight, reproduction, calf performance and pre-weaning survival. J. Anim. Breed Genet. 2025, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chapillon, P.; Manneché, C.; Belzung, C.; Caston, J. Rearing environmental enrichment in two inbred strains of mice: 1. Effects on emotional reactivity. Behav. Genet. 1999, 1, 41–46. [Google Scholar] [CrossRef]
- Shepherdson, D.J.; Carlstead, K.; Mellen, J.D.; Seidensticker, J. The influence of food presentation on the behavior of small cats in confined environments. Zoo Biol. 1993, 12, 203–216. [Google Scholar] [CrossRef]
- Swaisgood, R.R.; White, A.M.; Zhou, X.; Zhang, H.; Zhang, G.; Wei, R.; Hare, V.J.; Tepper, E.M.; Lindburg, D.G. A quantitative assessment of the efficacy of an environmental enrichment programme for giant pandas. Anim. Behav. 2001, 61, 447–457. [Google Scholar] [CrossRef]
- Clark, F.E. Cognitive enrichment and welfare: Current approaches and future directions. Anim. Behav. Cogn. 2017, 4, 52–71. [Google Scholar] [CrossRef]
- Bassett, L.; Buchanan-Smith, H.M. Effects of predictability on the welfare of captive animals. Appl. Anim. Behav. Sci. 2007, 102, 223–245. [Google Scholar] [CrossRef]
- Carlstead, K.; Shepherdson, D. Alleviating stress in zoo animals with environmental enrichment. In The Biology of Animal Stress; Moberg, G.P., Mench, J.A., Eds.; CABI Publishing: New York, NY, USA, 2000; pp. 337–354. [Google Scholar]
- Meehan, C.L.; Mench, J.A. The challenge of challenge: Can problem solving opportunities enhance animal welfare? Appl. Anim. Behav. Sci. 2007, 102, 246–261. [Google Scholar] [CrossRef]
- Mellen, J.; MacPhee, M.S. Philosophy of environmental enrichment: Past, present, and future. Zoo Biol. 2001, 20, 211–226. [Google Scholar] [CrossRef]
- Levine, S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science 1962, 135, 795–796. [Google Scholar] [CrossRef]
- Taylor, G.T. Fear and affiliation in domesticated male rats. J. Comp. Physiol. Psychol. 1981, 95, 685–693. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Alberts, S.C.; Altmann, J. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch. Gen. Psychiatry 1997, 54, 1137–1143. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; and Hawkley, L.C. Social isolation and health, with an emphasis on underlying mechanisms. Perspect. Biol. Med. 2003, 46 (Suppl. 3), S39–S52. [Google Scholar] [CrossRef]
- Engh, A.L.; Beehner, J.C.; Bergman, T.J.; Whitten, P.L.; Hoffmeier, R.R.; Seyfarth, R.M.; Cheney, D.L. Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus). Proc. Biol. Sci. 2006, 273, 707–712. [Google Scholar] [CrossRef]
- Silk, J.B.; Beehner, J.C.; Bergman, T.J.; Crockford, C.; Engh, A.L.; Moscovice, L.R.; Wittig, R.M.; Seyfarth, R.M.; Cheney, D.L. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 2010, 20, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.A.; Mann, J. Early social networks predict survival in wild bottlenose dolphins. PLoS ONE 2012, 7, e47508. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.; Wang, Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry 2014, 76, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Pellis, S.; Pellis, V. The Playful Brain; Oneworld Publications: Oxford, UK, 2009. [Google Scholar]
- Whitham, J.C.; Hall, K.; Lauderdale, L.K.; Bryant, J.L.; Miller, L.J. Integrating reference intervals into chimpanzee welfare research. Animals 2023, 13, 639. [Google Scholar] [CrossRef]
- Coe, J.; Dykstra, G. New and sustainable directions in zoo exhibit design. In Wild Mammals in Captivity: Principles and Techniques for Zoo Management, 2nd ed.; Kleiman, D.G., Thompson, K.V., Kirk Baer, C., Eds.; The University of Chicago Press: Chicago, IL, USA, 2010; pp. 202–215. [Google Scholar]
- Coe, J. Embedding environmental enrichment into zoo animal facility design. In Proceedings of the Zoo Design Conference, Wroclaw, Poland, 4–7 April 2017. [Google Scholar]
- Yamanashi, Y.; Matsunaga, M.; Shimada, K.; Kado, R.; Tanaka, M. Introducing tool-based feeders to zoo-housed chimpanzees as a cognitive challenge: Spontaneous acquisition of new types of tool use and effects on behaviours and use of space. J. Zoo Aquar. Res. 2016, 4, 147–155. [Google Scholar] [CrossRef]
- Krebs, B.L.; Watters, J.V. Simple but temporally unpredictable puzzles are cognitive enrichment. Anim. Behav. Cogn. 2017, 4, 119–134. [Google Scholar] [CrossRef]
- American Association of Zoos and Aquariums. Available online: https://www.aza.org/becoming-accredited/ (accessed on 1 June 2025).
- Kiyokawa, Y.; Kikusui, T.; Takeuchi, Y.; Mori, Y. Partner’s stress status influences social buffering effects in rats. Behav. Neurosci. 2004, 118, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, S.S.; Shepherdson, D.; Lewis, K.; Prado, N.; Brown, J.L.; Lee, B.; Wielebnowski, N. Supporting zoo Asian elephant (Elephas maximus) welfare and herd dynamics with a more complex and expanded habitat. Animals 2021, 11, 2566. [Google Scholar] [CrossRef] [PubMed]
- Fazio, J.M.; Freeman, E.W.; Bauer, E.; Rockwood, L.; Brown, J.L.; Hope, K.; Siegal-Willott, J.; Parsons, E.C.M. Longitudinal fecal hormone monitoring of adrenocortical function in zoo housed fishing cats (Prionailurus viverrinus) during institutional transfers and breeding introductions. PLoS ONE 2020, 15, e0230239. [Google Scholar] [CrossRef]
| Term | Definition |
|---|---|
| Adaptation | Limited to a specific type of stressor. The acclimatization of the individual to certain environmental conditions. Does not indicate flexibility in successful adaptation to all new challenges over a lifetime. |
| Allostasis | The ability for systems to maintain physiological stability as changes occur. A dynamic, proactive process of adjustments to the internal environment so the body can adapt to changing conditions. Internal variables are modified to prevent errors, with stability being achieved through change. |
| Coping | The ongoing strategy or processes an animal employs when reacting to stressors. Behavioral, cognitive, and physiological responses to stressors. These stressors may reflect internal or external demands. Coping strategies help minimize the impact of stress and determine the degree of resilience or susceptibility. |
| Desensitization | The process of decreasing an organism’s reactivity to a stimulus. |
| Habituation | A progressive decrease in the intensity or probability of an elicited response that occurs as a result of repeated exposure to the eliciting stimulus. |
| Homeostasis | An individual’s self-regulatory capacity that allows the internal environment to remain constant despite variations in the external environment. Feedback mechanisms allow for individuals to maintain the status quo in terms of physiological regulation. A reactive process aimed at restoring balance and maintaining a stable internal environment. |
| Resilience | The capacity of the animal to be minimally affected by a disturbance or to rapidly return to the physiological, behavioral, cognitive, health, affective and production states that pertained before exposure to a disturbance. Involves dynamic and complex processes that allow for active, positive adaptation to new conditions and adverse events. Not limited to a specific type of stressor and relies on the reaction of the animal to stressors. Tends to be expressed in response to environmental challenges lasting a matter of days. |
| Systematic Desensitization | The process of exposing an individual to an anxiety-provoking stimulus in a stepwise manner while a state of relaxation is maintained. The goal is to gradually increase the intensity of the stimulus until the maximum-intensity stimulus does not elicit anxiety. Throughout the process it is important to always keep the stimulus below the threshold that causes anxiety. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitham, J.C.; Miller, L.J. Why Measuring and Building Resilience Is Applicable to Zoo and Aquarium Animal Welfare. J. Zool. Bot. Gard. 2025, 6, 48. https://doi.org/10.3390/jzbg6030048
Whitham JC, Miller LJ. Why Measuring and Building Resilience Is Applicable to Zoo and Aquarium Animal Welfare. Journal of Zoological and Botanical Gardens. 2025; 6(3):48. https://doi.org/10.3390/jzbg6030048
Chicago/Turabian StyleWhitham, Jessica C., and Lance J. Miller. 2025. "Why Measuring and Building Resilience Is Applicable to Zoo and Aquarium Animal Welfare" Journal of Zoological and Botanical Gardens 6, no. 3: 48. https://doi.org/10.3390/jzbg6030048
APA StyleWhitham, J. C., & Miller, L. J. (2025). Why Measuring and Building Resilience Is Applicable to Zoo and Aquarium Animal Welfare. Journal of Zoological and Botanical Gardens, 6(3), 48. https://doi.org/10.3390/jzbg6030048







