Cell Viability of Skin Tissue Collected from Postmortem Neotropical Deer: A Novel Perspective for Conservation Biotechnology
Abstract
1. Introduction
2. Materials and Methods
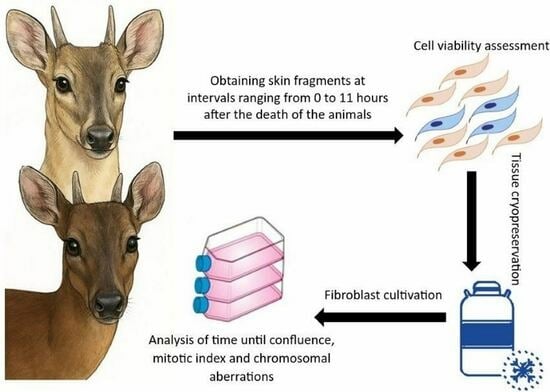
2.1. Sample Collection
2.2. Cell ViabilityCell Dissociation
Cell Dissociation
2.3. Cell Viability Counting and Calculation
2.4. Cryopreservation of Skin Fragments
2.5. Thawing and Cell Culture of Cryopreserved Fragments
2.6. Cell Harvesting
2.7. Mitotic Index Analysis
2.8. Chromosomal Alteration Analysis
2.9. Statistical Analysis
3. Results
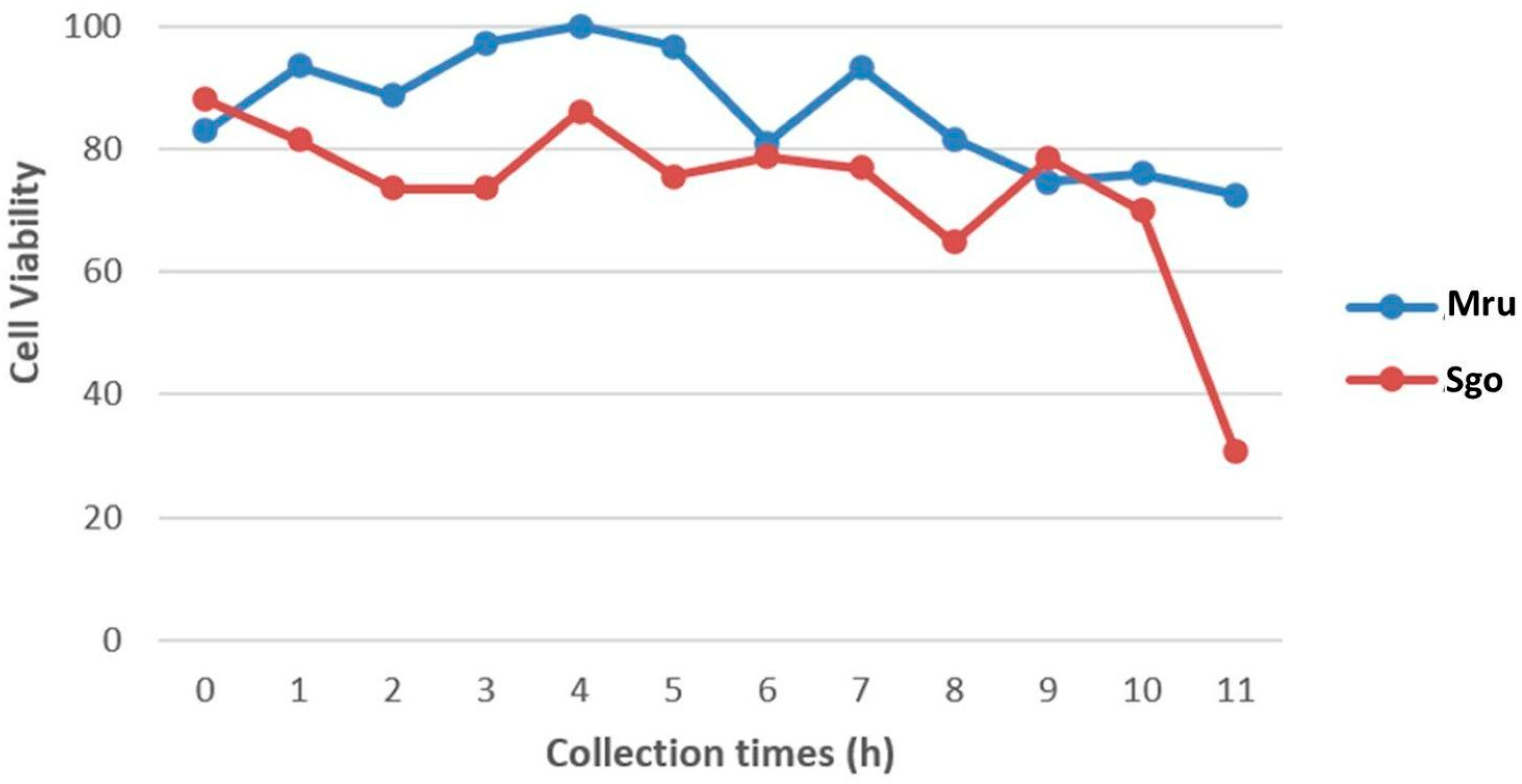
3.1. Cell Viability of Fresh Tissues
3.2. Cryopreservation Methodology
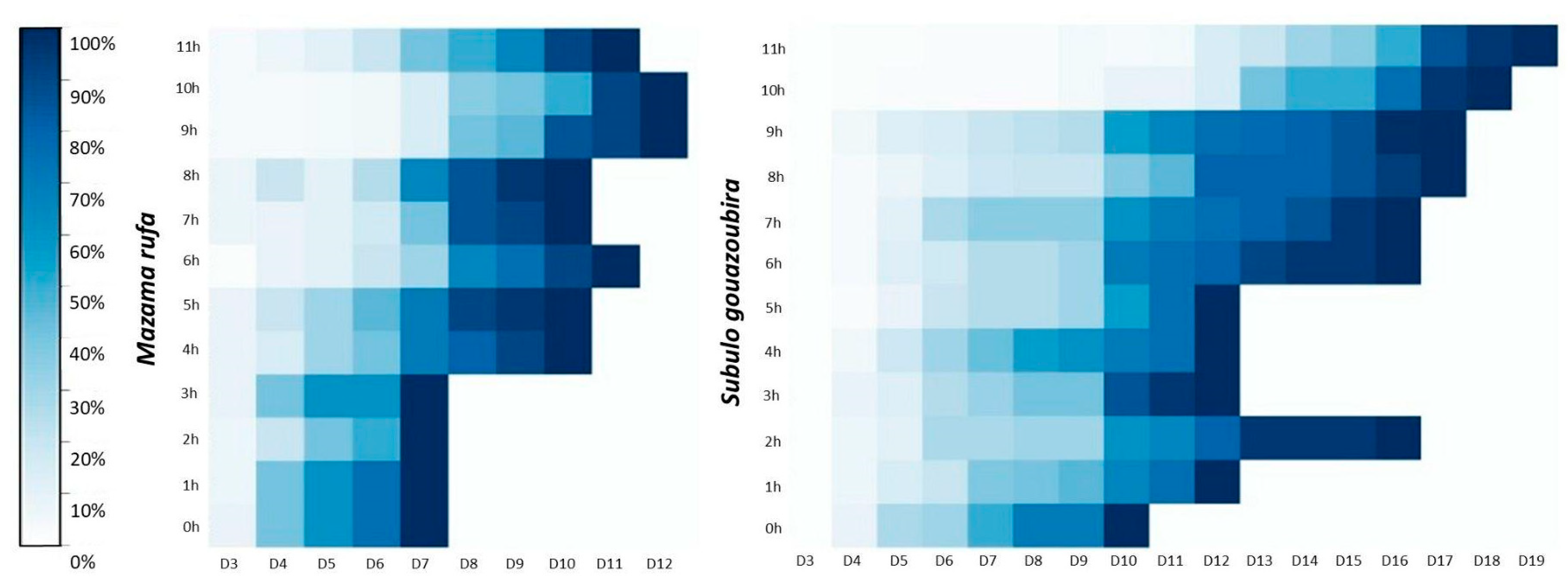
3.3. Confluence Analysis of Cell Cultures Derived from Cryopreserved Skin Fragments
3.4. Mitotic Index Analysis
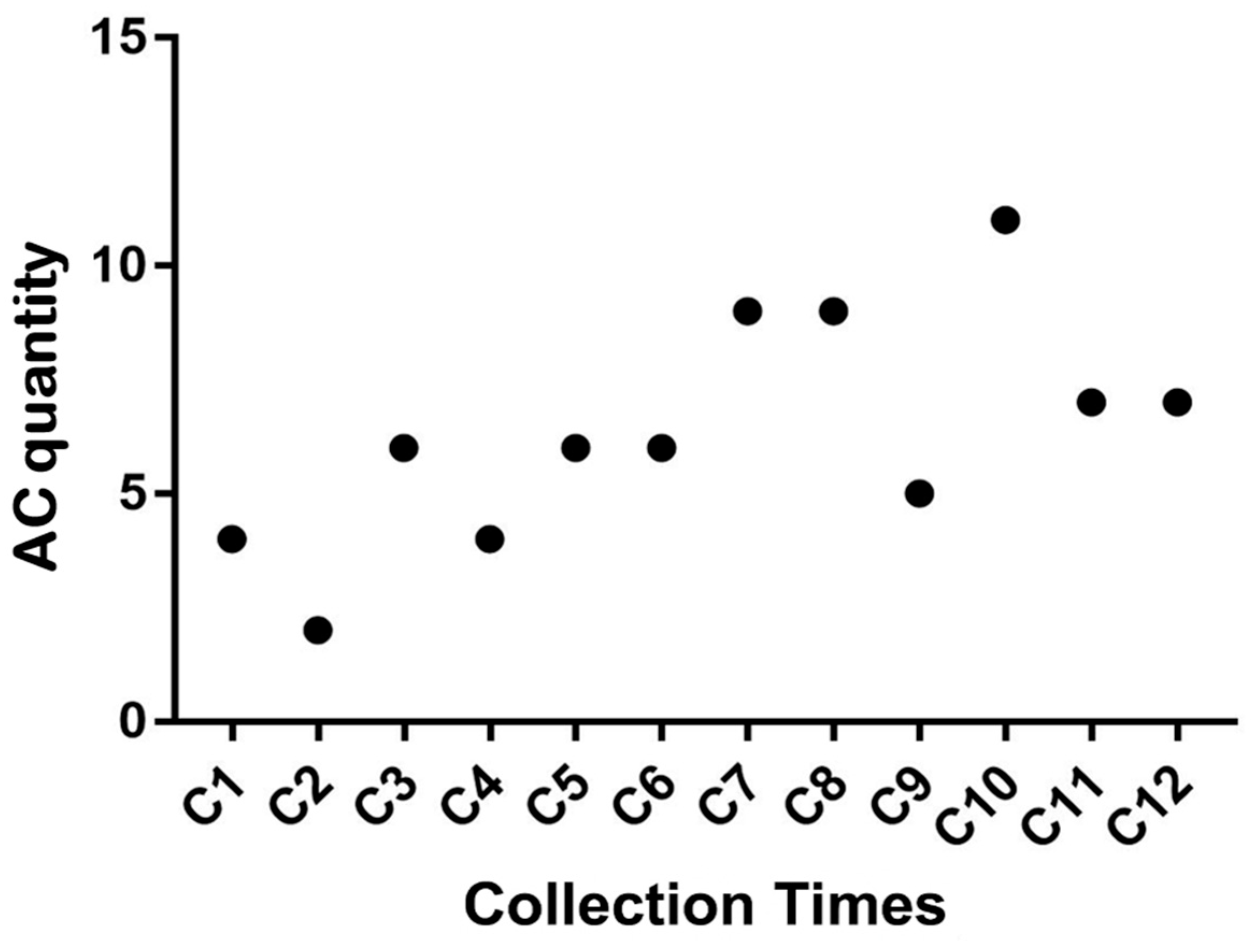
3.5. Chromosomal Alteration Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCNT | Somatic cell nuclear transfer |
| DMEM | Dulbecco’s Modified Eagle medium |
| DMSO | Dimethyl sulfoxide |
| PVP | Polyvinylpyrrolidone |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
| MI | Mitotic Index |
| R | Ring |
| Dic | Dicentric chromosome |
| Gct | Chromatid gap |
| Gcr | Chromosome gap |
| Qct | Chromatid break |
| Qcr | Chromosome break |
| Ft | Triradial figure |
| Fq | Quadriradial figure |
| Re | Rearrangement |
References
- Weber, M.; Gonzalez, S. Latin American deer diversity and conservation: A review of status and distribution. Écoscience 2003, 10, 443–454. [Google Scholar] [CrossRef]
- Schipper, J.; Chanson, J.S.; Chiozza, F.; Cox, N.A.; Hoffmann, M.; Katariya, V.; Lamoreux, J.; Rodrigues, A.S.L.; Stuart, S.N.; Temple, H.J.; et al. The status of the world’s land and marine mammals: Diversity, threat, and knowledge. Science 2008, 322, 225–230. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 31 March 2025).
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. A Primer of Conservation Genetics; Cambridge University Press: Cambridge, UK, 2004; 220p. [Google Scholar]
- Keller, L.F.; Biebach, I.; Ewing, S.R.; Hoeck, P.E.A. Then genetics of Reintroductions: Inbreding and Genetic Drift. In Reintroduction Biology, Integrating Science and Management; Ewen, J.G., Armstrong, D.P., Parker, K.A., Seddon, P.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; 499p. [Google Scholar]
- González, S.; Duarte, J.M.B. Speciation, evolutionary history and conservation trends of neotropical deer. Mastozool. Neotrop. 2020, 27, 37–47. [Google Scholar] [CrossRef]
- Maran, M.L.H. Filogenia Molecular de Mazama americana (Artiodactyla: Cervidae) como auxílio na Resolução das Incertezas Taxonômicas. Master’s Thesis, University of São Paulo, São Paulo, Brazil, 2016. Available online: https://repositorio.unesp.br/handle/11449/142902 (accessed on 11 April 2025).
- Ryder, O.A.; Onuma, M. Viable cell culture banking for biodiversity characterization and conservation. Annu. Rev. Anim. Biosci. 2018, 6, 83–97. [Google Scholar] [CrossRef]
- Comizzoli, P.; Holt, W. Breakthroughs and new horizons in reproductive biology of rare and endangered animal species. Biol. Reprod. 2019, 101, 514–525. [Google Scholar] [CrossRef]
- Rola, L.D.; Buzanskas, M.E.; Melo, L.M.; Chaves, M.S.; Freitas, V.J.F.; Duarte, J.M.B. Assisted reproductive technology in neotropical deer: A model approach to preserving genetic diversity. Animals 2021, 11, 1961. [Google Scholar] [CrossRef]
- Mastromonaco, G.F.; King, W.A. Cloning in companion animal, non-domestic and endangered species: Can the technology become a practical reality? Reprod. Fertil. Dev. 2007, 19, 748–761. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Vacari, G.Q.; Duarte, J.M.B. A method to freeze skin samples for cryobank: A test of some cryoprotectants for an endangered deer. Biopreserv. Biobank. 2023, 22, 211–216. [Google Scholar] [CrossRef]
- Singh, M.; Ma, X. In vitro culture of fibroblast-like cells from sheep ear skin stored at 25–26 °C for 10 days after animal death. Int. J. Biol. 2014, 6, 96–102. [Google Scholar] [CrossRef]
- Louis, K.S.; Siegel, A.C. Cell viability analysis using trypan blue: Manual and automated methods. In Mammalian Cell Viability: Methods and Protocols; Stoddart, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 7–12. [Google Scholar]
- Verma, R.S.; Babu, A. Human Chromosomes: Principles and Techniques, 2nd ed.; McGraw-Hill: New York, NY, USA, 1995; p. 419. [Google Scholar]
- O’Connor, P.M.; Ferris, D.K.; Pagano, M.; Draetta, G.; Pines, J.; Hunter, T.; Longo, D.L.; Kohn, K.W. G2 delay induced by nitrogen mustard in human cells affects cyclin A/cdk2 and cyclin B1/cdc2-kinase complexes differently. J. Biol. Chem. 1993, 268, 8298–8308. [Google Scholar] [CrossRef]
- Galloway, S.M.; Aardema, M.J.; Ishidate, M., Jr.; Ivett, J.L.; Kirkland, D.J.; Morita, T.; Moses, P.; Sofuni, T. Report from working group on in vitro tests for chromosomal aberrations. Mutat. Res. 1994, 312, 241–261. [Google Scholar] [CrossRef]
- Evans, H.J.; O’Riordan, M.L. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat. Res. 1975, 31, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.R.K. Classification and relationships of induced chromosomal structural changes. J. Med. Genet. 1975, 12, 103–122. [Google Scholar]
- Savage, J.R.K.; Simpson, P.J. FISH “painting” patterns resulting from complex exchanges. Mutat. Res. 1994, 312, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.M.; Silva, S.B.; Magalhães, L.C.; Cortez, J.V.; Kumar, S.; Duarte, J.M.; Rola, L.D.; Chaves, M.S.; Freitas, V.J.F. The use of somatic cell nuclear transfer to obtain interspecific cloned embryos from brown brocket deer karyoplast and bovine cytoplast: Embryo development and nuclear gene expression. Theriogenol. Wild 2022, 1, 100001. [Google Scholar] [CrossRef]
- Magalhaes, L.C.; Bhat, M.H.; Freitas, J.L.; Melo, L.M.; Teixeira, D.I.; Pinto, L.C.; Câmara, L.M.C.; Duarte, J.M.B.; Freitas, V.J.F. The effects of cryopreservation on different passages of fibroblast cell culture in brown brocket deer (Mazama gouazoubira). Biopreserv. Biobank. 2017, 15, 463–468. [Google Scholar] [CrossRef]
- Huijsmans, T.E.R.G.; Hassan, H.A.; Smits, K.; Van Soom, A. Postmortem collection of gametes for the conservation of endangered mammals: A review of the current state-of-the-art. Animals 2023, 13, 1360. [Google Scholar] [CrossRef] [PubMed]
- Centro Brasileiro de Ecologia de Estradas (CBEE). Atropelômetro. 2019. Available online: https://ecoestradas.com.br/ (accessed on 14 March 2024).
- Abra, F.D.; Huijser, M.P.; Magioli, M.; Bovo, A.A.A.; de Barros, K.M.P.M. An estimate of wild mammal roadkill in São Paulo state, Brazil. Heliyon 2021, 7, e06084. [Google Scholar] [CrossRef]
- Zdravkovic, M. Ultrastructural changes of renal epithelial cells during post-mortem autolysis—Experimental work. Med. Pregl. 2010, 63, 15–20. [Google Scholar] [CrossRef]
- Neta, L.B.Q.; Lira, G.P.O.; Borges, A.A.; de Oliveira Santos, M.V.; Silva, M.B.; de Oliveira, L.R.M.; Silva, A.R.; de Oliveira, M.F.; Pereira, A.F. Influence of storage time and nutrient medium on recovery of fibroblast like cells from refrigerated collared peccary (Pecari Tajacu Linnaeus, 1758) skin. In Vitro Cell Dev. Biol. Anim. 2018, 54, 486–495. [Google Scholar] [CrossRef]
- Pegg, D.E. Viability assays for preserved cells, tissues and organs. Cryobiology 1989, 26, 212–231. [Google Scholar] [CrossRef] [PubMed]
- Hartford, J.B. Cell culture. In Current Protocols in Cell Biology; Bonifacino, J.S., Hartford, J.B., Lippincott-Schwartz, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1998; pp. 101–102. [Google Scholar]
- Stoddart, M.J. (Ed.) Cell viability assays: Introduction. In Mammalian Cell Viability: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2011; pp. 1–6. [Google Scholar]
- Silvestre, M.A.; Saeed, A.M.; Cervera, R.P.; Escriba, M.J.; Garcia-Ximenez, F. Rabbit and pig ear skin sample cryobanking: Effects of storage time and temperature of the whole ear extirpated immediately after death. Theriogenology 2003, 59, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, M.A.; Sanchez, J.P.; Gomez, E.A. Vitrification of goat, sheep, and cattle skin samples from whole ear extirpated after death and maintained at different storage times and temperatures. Cryobiology 2004, 49, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Ma, X.; Sharma, A. Effect of postmortem time interval on in vitro culture potential of goat skin tissues stored at room temperature. Vitr. Cell. Dev. Biol.—Anim. 2012, 48, 478–482. [Google Scholar] [CrossRef]
- Jackman, J.; O’Connor, P.M. Methods for synchronizing cells at specific stages of the cell cycle. In Current Protocols in Cell Biology; Bonifacino, J.S., Hartford, J.B., Lippincott-Schwartz, J., Yamada, K.M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1998; pp. 8.3.1–8.3.20. [Google Scholar]
- Vargas-Munar, D.S.F. Relação entre Fragilidade Cromossômica e Trocas entre Cromátides Irmãs com a Variabilidade Cariotípica de Cervídeos Brasileiros. Master’s Dissertation, Paulista State University, São Paulo, Brazil, 2003. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rola, L.D.; Tomazella, I.M.; Sandoval, E.D.P.; Morales-Donoso, J.A.; Borges, C.H.d.S.; Duarte, J.M.B. Cell Viability of Skin Tissue Collected from Postmortem Neotropical Deer: A Novel Perspective for Conservation Biotechnology. J. Zool. Bot. Gard. 2025, 6, 31. https://doi.org/10.3390/jzbg6020031
Rola LD, Tomazella IM, Sandoval EDP, Morales-Donoso JA, Borges CHdS, Duarte JMB. Cell Viability of Skin Tissue Collected from Postmortem Neotropical Deer: A Novel Perspective for Conservation Biotechnology. Journal of Zoological and Botanical Gardens. 2025; 6(2):31. https://doi.org/10.3390/jzbg6020031
Chicago/Turabian StyleRola, Luciana Diniz, Iara Maluf Tomazella, Eluzai Dinai Pinto Sandoval, Jorge Alfonso Morales-Donoso, Carolina Heloisa de Souza Borges, and José Maurício Barbanti Duarte. 2025. "Cell Viability of Skin Tissue Collected from Postmortem Neotropical Deer: A Novel Perspective for Conservation Biotechnology" Journal of Zoological and Botanical Gardens 6, no. 2: 31. https://doi.org/10.3390/jzbg6020031
APA StyleRola, L. D., Tomazella, I. M., Sandoval, E. D. P., Morales-Donoso, J. A., Borges, C. H. d. S., & Duarte, J. M. B. (2025). Cell Viability of Skin Tissue Collected from Postmortem Neotropical Deer: A Novel Perspective for Conservation Biotechnology. Journal of Zoological and Botanical Gardens, 6(2), 31. https://doi.org/10.3390/jzbg6020031