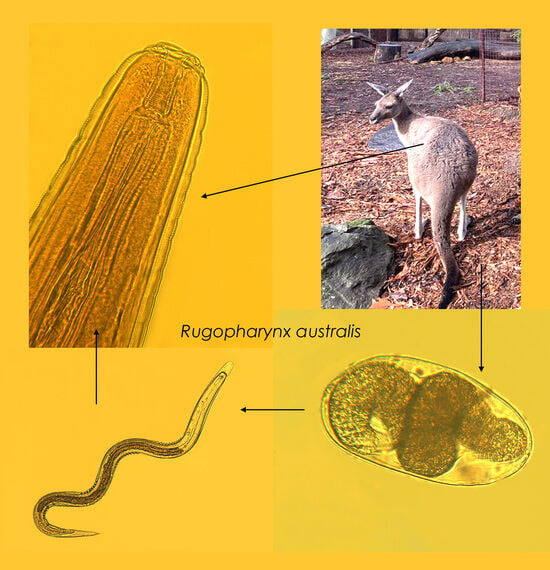
Rugopharynx australis (Nematoda: Strongyloidea) Infection in Captive Red Kangaroos (Osphranter rufus) in Bulgaria: A Case Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Origin and Coprological Study
2.2. Animals Deworming and Nematodes Collection
2.3. Morphological and Morphometric Study
2.4. DNA Extraction and PCR Amplification
2.5. Phylogenetic Analysis
3. Results
3.1. Coprological Findings
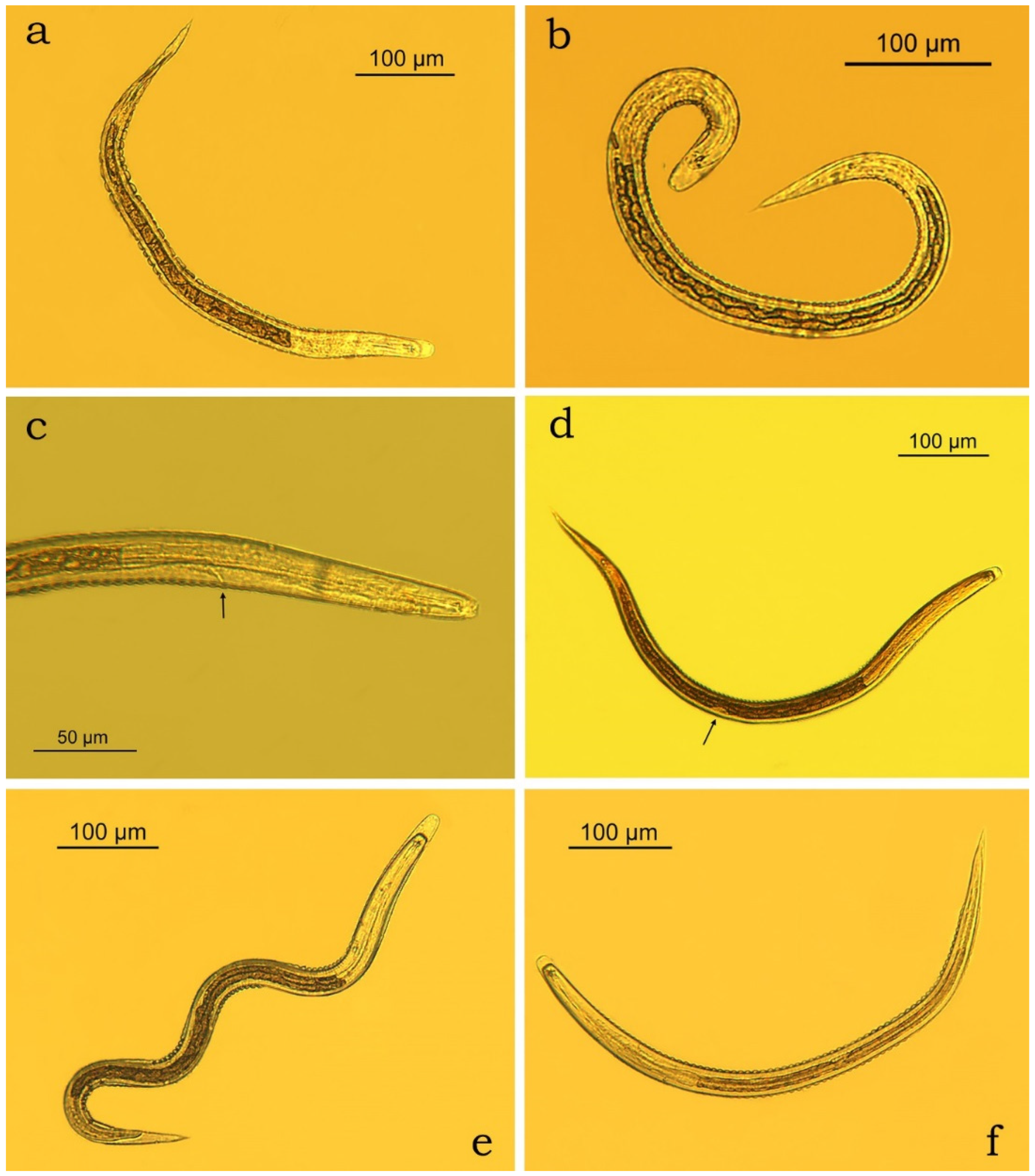
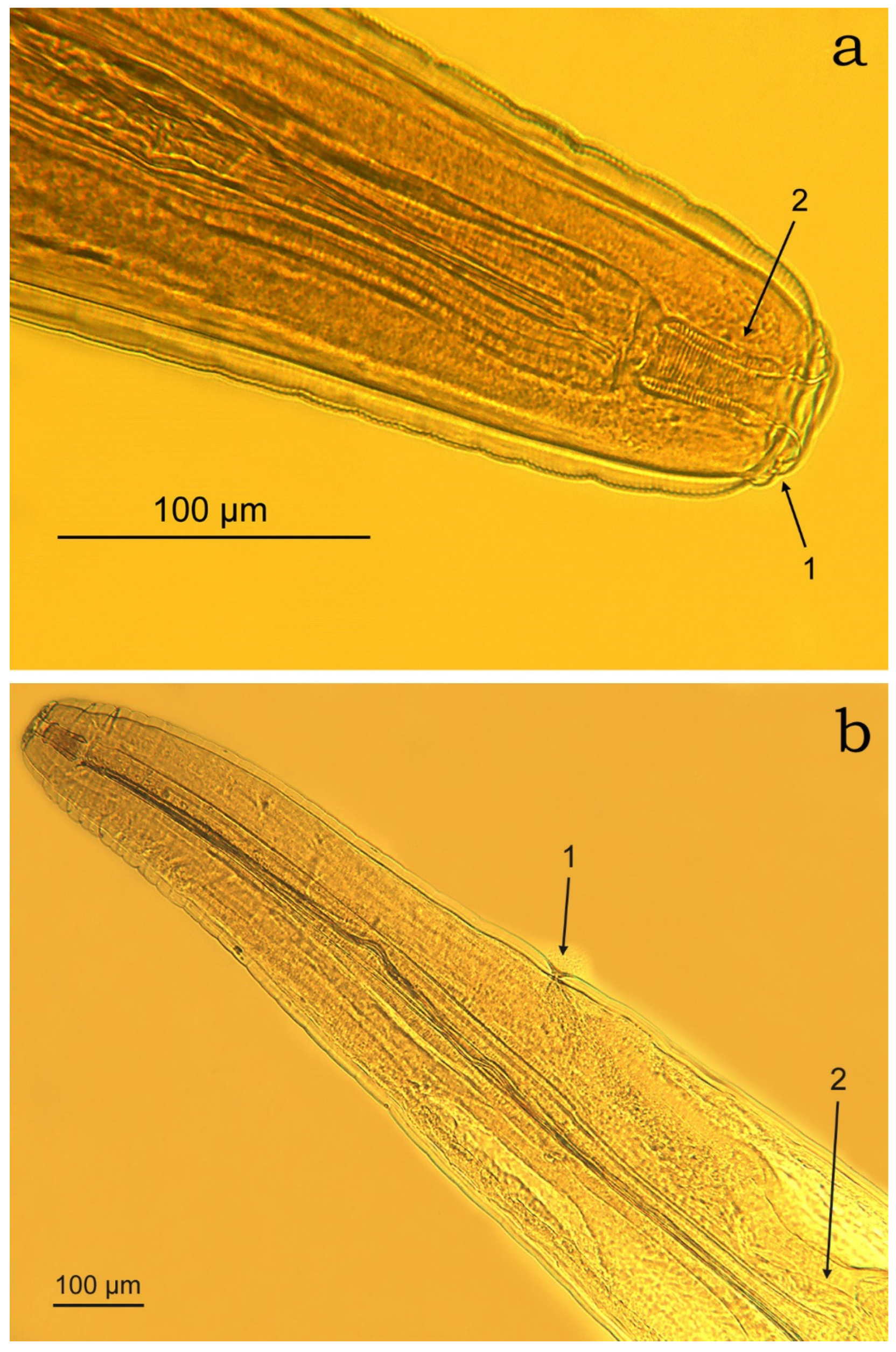
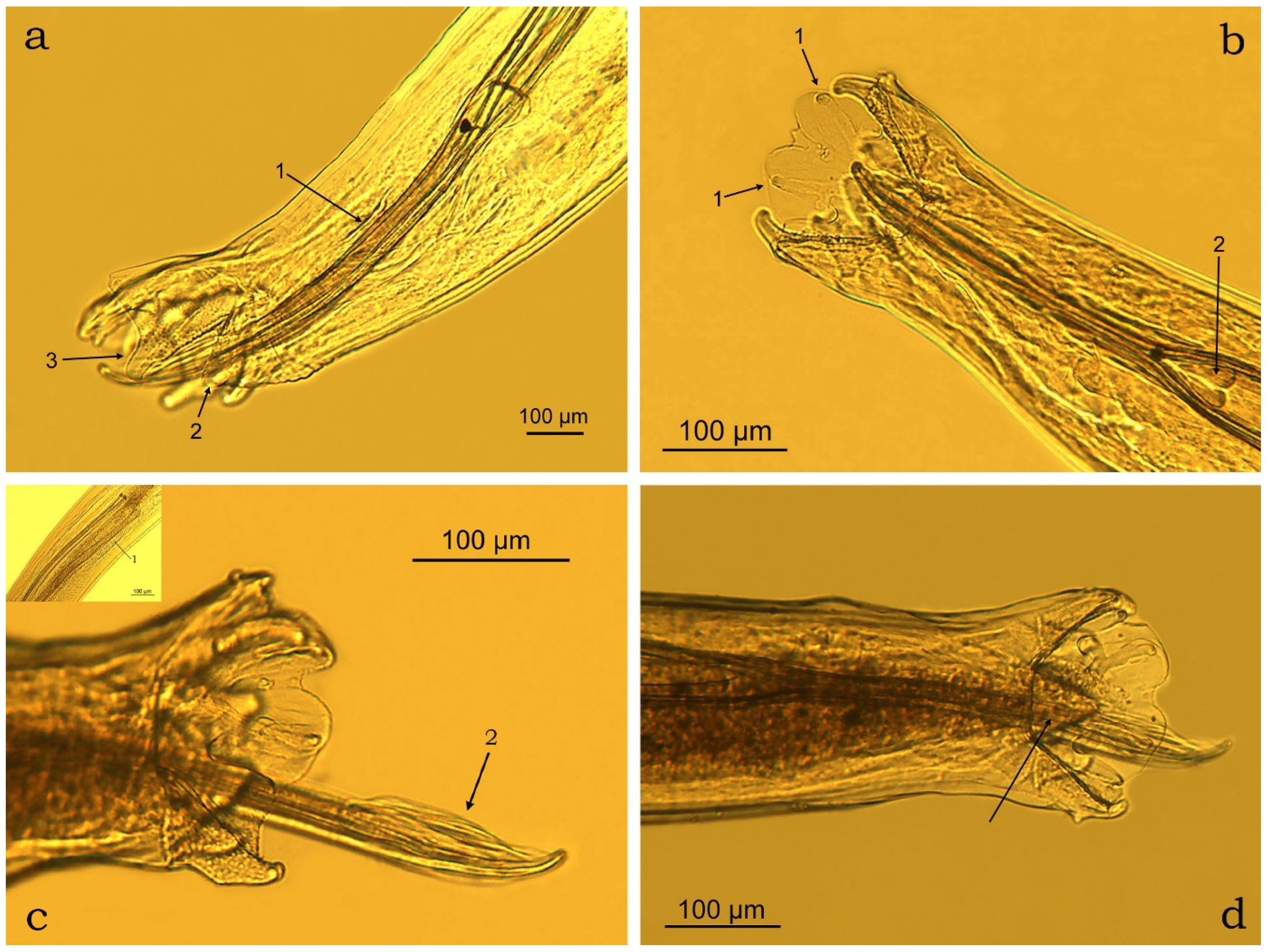
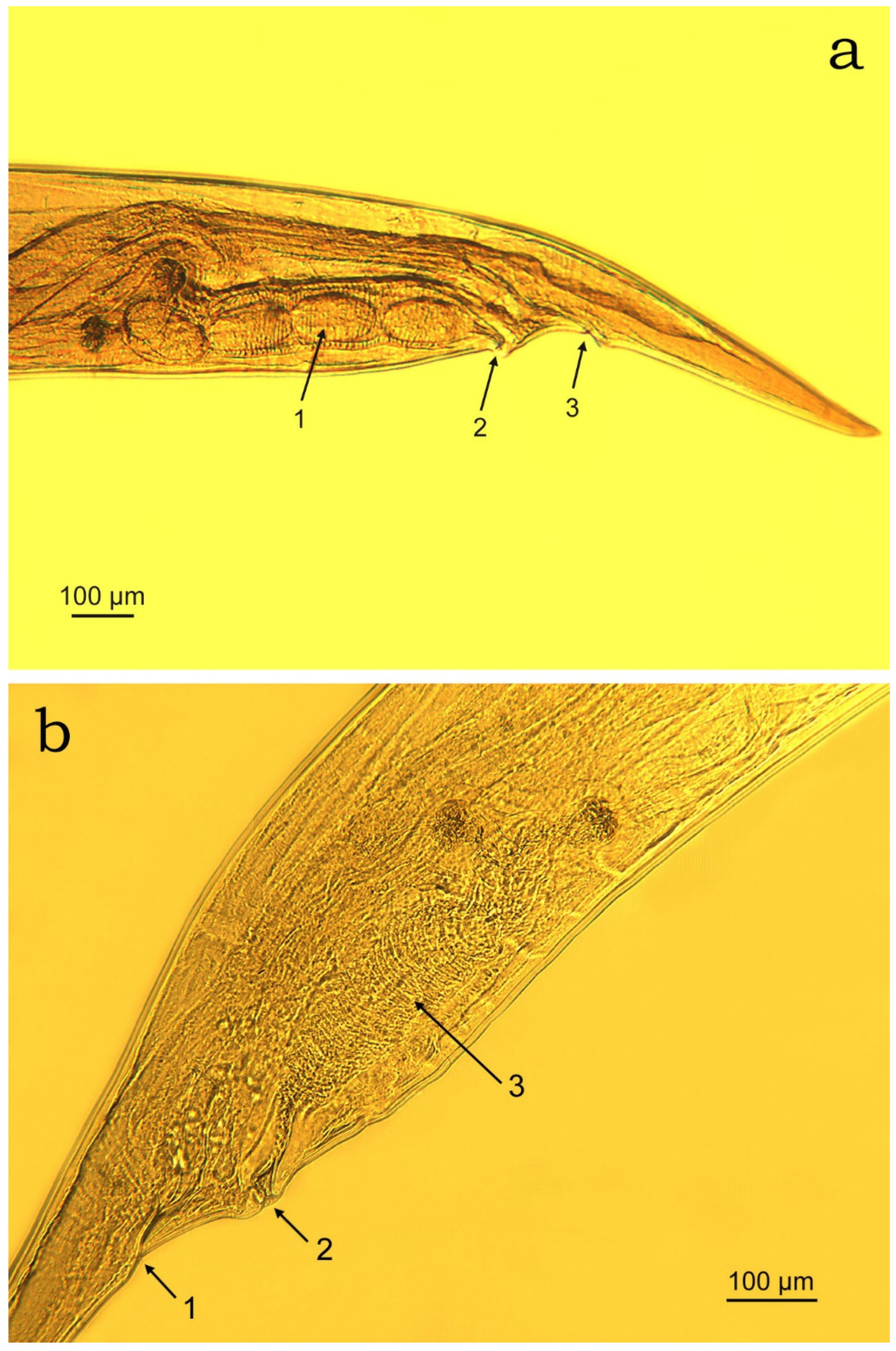
3.2. Morphometric Characterisation
3.3. Nucleotide Seuences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawson, T.J. Kangaroos: Biology of the Largest Marsupials; Cornell University Press: Ithaca, NY, USA, 1995; p. 162. [Google Scholar]
- Finlayson, G.; Tschirner, K.; McCann, J.; Appleby, M. Kangaroo management in the South Australian rangelands: Impacts and challenges for conservation management. Ecol. Manag. Restor. 2021, 22, 24–34. [Google Scholar] [CrossRef]
- Colgan, S.A.; Perkins, N.R.; Green, L.A. The large-scale capture of eastern grey kangaroos (Macropus giganteus) and red kangaroos (Osphranter rufus) and its application to a population management project. Austr. Vet. J. 2019, 97, 515–523. [Google Scholar]
- Lott, M.J.; Hose, G.C.; Power, M.L. Parasitic nematode communities of the red kangaroo, Macropus rufus: Richness and structuring in captive systems. Parasitol. Res. 2015, 114, 3181. [Google Scholar] [PubMed]
- Rampacci, E.; Diaferia, M.; Lucentini, L.; Brustenga, L.; Capasso, M.; Girardi, S.; Gizzi, I.; Primavilla, S.; Veronesi, F.; Passamonti, F. Detection of zoonotic enteropathogens in captive large felids in Italy. Zoonoses Public Health 2024, 71, 200–209. [Google Scholar] [PubMed]
- Kamburov, P.; Georgieva, D.; Ivanov, Y.; Koynarski, V. Guide for Exercise in Veterinary Parasitology; Zemizdat: Sofia, Bulgaria, 1989; p. 184. (In Bulgarian) [Google Scholar]
- Popova, K.I. Strongyloidei Jivotnih i Cheloveka. Trichonematidi. Osnovy Nematodologii; Izdatelstvo Akademii Nauk SSSR: Moscow, Russia, 1958; Volume 7, p. 424. (In Russian) [Google Scholar]
- Beveridge, I.; Sukee, T.; Jabbar, A. Redescription of Rugopharynx australis (Mönnig, 1926) and the description of R. moennigi n. sp. (Nematoda: Strongyloidea) from kangaroos (Marsupialia: Macropodidae) in Australia. Syst. Parasitol. 2021, 98, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.B.; Huby-Chilton, F.; Koehler, A.V.; Gasser, R.B. Detection of cryptic species of Rugopharynx (Nematod: Strongylida) from the stomachs of Australian macropodid marsupials. Intern. J. Parasitol.: Paras. Wildl. 2016, 5, 124–133. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2003, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 27, 1253–1256. [Google Scholar] [CrossRef]
- Beveridge, I. Gastrointestinal helminth parasites of the grey kangaroos, Macropus fuliginosus and M. giganteus. Austr. J. Zool. 2023, 71, ZO23038. [Google Scholar] [CrossRef]
- Chilton, N.B.; Andrews, R.H.; Beveridge, I. Genetic evidence for a species complex within Rugopharynx australis (Mönnig, 1926) (Nematoda: Strongyloidea) from macropodid marsupials. Syst. Parasitol. 1996, 34, 125–133. [Google Scholar] [CrossRef]
- McKenna, P.B. Checklist of helminth parasites of terrestrial mammals in New Zealand. New Zealand J. Zool. 1997, 24, 277–290. [Google Scholar]
- Sotohira, Y.; Ito, Y.; Sano, T. Parasitic nematodes obtained from marsupials reared at a semi-free ranging facility in a Japanese zoological park. Res. One Health 2016, 2016, 1–5. [Google Scholar]
- Mönnig, H.O. Three new helminths. Trans. R. Soc. South Afr. 1926, 13, 291–298. [Google Scholar]
- Smales, L.R. The life history of Labiostrongylus eugenii, a nematode parasite of the Kangaroo Island Wallaby (Macropus eugenii): Development and hatching of the egg and the free living stages. Int. J. Parasitol. 1977, 7, 449–456. [Google Scholar] [CrossRef] [PubMed]
- WWF Official Website: Issues with no End in Sight. Available online: https://wwf.panda.org/wwf_offices/australia/environmental_problems_in_australia/ (accessed on 10 February 2025).


| Species | GenBank no. | Species | GenBank no. |
|---|---|---|---|
| Rugopharynx australis | PQ526355 | R. zeta | KJ776397 |
| PQ526356 | KJ776398 | ||
| PQ526357 | KJ776399 | ||
| PQ526358 | KJ776401 | ||
| LN906947 | KJ776402 | ||
| LN906948 | LN906992 | ||
| LN906949 | LN906993 | ||
| LN906950 | LN906994 | ||
| LN906951 | R. rho | LN906975 | |
| LN906952 | LN906976 | ||
| LN906953 | LN906977 | ||
| R. sigma | LN906985 | LN906978 | |
| LN906986 | LN906979 | ||
| LN906987 | R. alpha | LN906946 | |
| R. setonicus | LN906985 |
| Species | GenBank no. | Host | Country | Similarity (%) |
|---|---|---|---|---|
| Rugopharynx australis | LN906947 | Macropus rufus | Australia | 98.94–99.60 |
| LN906948 | Macropus rufus | Australia | 94.29–94.55 | |
| LN906949 | Macropus giganteus | Australia | 99.47 | |
| LN906950 | Macropus giganteus | Australia | 98.94–99.07 | |
| LN906951 | Macropus dorsalis | Australia | 98.94–99.60 | |
| LN906952 | Macropus robustus | Australia | 98.94–99.07 | |
| LN906953 | Macropus fuliginosus | Australia | 94.03–94.29 | |
| Rugopharynx zeta | KJ776397 | Not provided | Australia | 94.88–95.14 |
| KJ776398 | Not provided | Australia | 95.01–95.26 | |
| KJ776399 | Not provided | Australia | 95.01–95.26 | |
| KJ776401 | Not provided | Australia | 95.49–95.75 | |
| KJ776402 | Not provided | Australia | 94.41–95.63 | |
| LN906992 | Petrogale assimilis | Australia | 93.80–93.93 | |
| LN906993 | Petrogale inornata | Australia | 94.46–94.59 | |
| LN906994 | Petrogaleherberti | Australia | 94.06–94.20 | |
| Rugopharynx rho | LN906975 | Macropus eugenii | Australia | 94.87–95.13 |
| LN906976 | Macropus eugenii | Australia | 94.87–95.13 | |
| LN906977 | Macropus fuliginosus | Australia | 95.92–96.05 | |
| LN906978 | Macropus fuliginosus | Australia | 92.26–95.13 | |
| LN906979 | Macropus irma | Australia | 95.65–95.78 | |
| Rugopharynx sigma | LN906985 | Thylogale thetis | Australia | 92.63–92.76 |
| LN906986 | Thylogale stigmatica | Australia | 94.73–94.99 | |
| LN906987 | Thylogale stigmatica | Australia | 94.60–94.86 | |
| Rugopharynx setonicis | LN906984 | Setonix brachyurus | Australia | 95.52–95.65 |
| Parameter | Origin/Reference | |||
|---|---|---|---|---|
| Osphranter rufus, Sofia Zoo, Bulgaria [Present Data] | Osphranter rufus, Australia Beveridge et al. [8] | Osphranter rufus, Mönnig [17] | ||
| Range (Mean ± SD) | ||||
| Adult male nematodes (n = 12) | Body length (mm) | 7–12 (9 ± 0.14) | 7.3–10.5 (9.4) | 9.2–10 |
| Body width at the end of the oesophagus (µm) | 254–370 (282 ± 32.47) | 290–330 (310) * | 380 * | |
| Length of pharynx (µm) | 39–50 (45 ± 3.45) | 40–45 (44) | - | |
| Width of pharynx (µm) | 24–31 (27 ± 2.19) | 25–30 (28) | - | |
| Oesophagus length (µm) | 830–1143 (1007 ± 84.87) | 940–1080 (1010) | - | |
| Maximum esophagus width (µm) | 75–138 (110 ± 16.57) | - | - | |
| Excretory pore—ant. end | 617–797 (699 ± 57.59) | 500–770 (620) | - | |
| Spicule length (µm) | 1702–2400 (1953 ± 229.88) | 1750–2050 (1860) | 2020 | |
| Gubernaculum length (µm) | 32–39 (35 ± 2.45) | 30 (30) | - | |
| - | ||||
| Adult female nematodes (n = 14) | Body length (mm) | 8–13 (11 ± 0.12) | 7.6–9.4 (8.7) | 12.5 |
| Body width at the end of the oesophagus (µm) | 264–363 (332 ± 34.11) | 360–420 (390) * | 500 * | |
| Length of pharynx (µm) | 41–53 (47 ± 4.57) | 40–50 (47) | 76 | |
| Width of pharynx (µm) | 24–33 (29 ± 2.67) | 30–35 (33) | - | |
| Oesophagus length | 1084–1268 (1168 ± 61.90) | 980–1180 (1070) | 1170 | |
| Maximum esophagus width (µm) | 111–155 (129 ± 12.01) | - | 130 | |
| Excretory pore—ant. end | 681–1003 (784 ± 118.58) | 600–710 (660) | 840 | |
| Ovijector/vagina total length (µm) | 572–805 (672 ± 85.66) | 650–800 (740) | 520 | |
| Vulva-tail tip distance (µm) | 539–715 (640 ± 53.46) | 550–760 (650) | - | |
| Anus-tail tip distance (µm) | 436–560 (513 ± 43.06) | - | - | |
| Tail length (µm) | 410–677 (553 ± 85.90) | 400–600 (490) | 560 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panayotova-Pencheva, M.; Banasiewicz, J.; Pyziel, A.M. Rugopharynx australis (Nematoda: Strongyloidea) Infection in Captive Red Kangaroos (Osphranter rufus) in Bulgaria: A Case Report. J. Zool. Bot. Gard. 2025, 6, 20. https://doi.org/10.3390/jzbg6020020
Panayotova-Pencheva M, Banasiewicz J, Pyziel AM. Rugopharynx australis (Nematoda: Strongyloidea) Infection in Captive Red Kangaroos (Osphranter rufus) in Bulgaria: A Case Report. Journal of Zoological and Botanical Gardens. 2025; 6(2):20. https://doi.org/10.3390/jzbg6020020
Chicago/Turabian StylePanayotova-Pencheva, Mariana, Joanna Banasiewicz, and Anna Maria Pyziel. 2025. "Rugopharynx australis (Nematoda: Strongyloidea) Infection in Captive Red Kangaroos (Osphranter rufus) in Bulgaria: A Case Report" Journal of Zoological and Botanical Gardens 6, no. 2: 20. https://doi.org/10.3390/jzbg6020020
APA StylePanayotova-Pencheva, M., Banasiewicz, J., & Pyziel, A. M. (2025). Rugopharynx australis (Nematoda: Strongyloidea) Infection in Captive Red Kangaroos (Osphranter rufus) in Bulgaria: A Case Report. Journal of Zoological and Botanical Gardens, 6(2), 20. https://doi.org/10.3390/jzbg6020020