Abstract
The Alaotran gentle lemur (Hapalemur alaotrensis) is one of the world’s most endangered primates and shows low success rates in captive breeding programmes. This study tested biologically relevant scent enrichment using two synthesised mixtures likely to convey information about female fertility on the behaviour of three unsuccessful breeding pairs in captivity. Specifically, we compared the baseline and enrichment periods by combining behavioural observations (n = 240 h) with faecal endocrinology (n = 80 samples), focussing on cortisol and testosterone measurements via enzyme immunoassay techniques. Then, we tested two different mixtures to assess potential behavioural differences and evaluate the effectiveness of olfactory enrichment using scented and unscented cotton strips. Olfactory behaviours differed by sex and enrichment conditions, with both sexes exhibiting increased behaviours during enrichment compared to the baseline. Sexual behaviours increased during the enrichment period, with variations in frequency between males and females depending on the condition. No significant changes were observed in faecal cortisol levels. However, one male showed a significant increase in testosterone during the second enrichment mixture. Nevertheless, overall differences between baseline and enrichment were not significant. Our findings suggest that while the scent enrichment showed limited effectiveness, biologically meaningful scents may trigger species-specific behaviours.
1. Introduction
With nearly 60% of primate species classified as endangered or critically endangered by the International Union for Conservation of Nature (IUCN) [1], primate conservation is critical [2]. Institutions in the European Association of Zoos and Aquaria (EAZA) participate in conservation initiatives that integrate in situ and ex situ programmes, benefiting target and coexisting species [3]. However, many endangered primate species, including several lemurs [3], have low reproductive success in captivity, limiting their role as a buffer against extinction (reviewed in [4]). It is crucial for captive animals to express natural behaviours [5]; but, in zoo environments, lack of stimuli and routine can lead to boredom [6], stereotypic behaviours [7], and endocrinological dysfunction [8], which may decrease reproductive fitness [9,10,11,12]. On the other hand, captivity enhances breeding through the evidence-based facilitation of reproductive behaviours and environmental enrichments [10,13,14]. The latter, including motor, cognitive, sensory, and social stimulation, improves psychological and physiological welfare, promoting natural species-specific behaviours [14,15] and breeding success [10].
Primates are typically considered microsmatic, relying more on visual and vocal than olfactory cues [16]. However, olfactory signalling is crucial in sociosexual communication in strepsirrhine primates [17,18]. Indeed, scent-marking behaviour conveys information about age, rank, reproductive status, diet, and identity [19,20]. Furthermore, sexual pheromones may advertise female fertility and elicit male responses [21], including in primate species such as rhesus monkeys and humans [22]. Odour provides information about male qualities as potential mates [23,24]; moreover, scents have the potential to trigger olfactory and sexual behaviours in lemurs [23]. While scents can facilitate mate choice and reproductive success in mammals [25,26], studies on olfactory enrichment in primates are scarce [4]. They often focus on anthropogenic rather than biologically relevant scents [27], which may also impact reproductive success [28].
This study focusses on the Alaotran gentle lemur (Hapalemur alaotrensis), one of the world’s 25 most endangered primate species, with an estimated wild population of about 2500 individuals [29], listed in Appendix I of the CITES (Convention on International Trade in Endangered Species for Wild Fauna and Flora) and Critically Endangered on the IUCN (International Union for Conservation of Nature) Red List [1]. Despite EEP (EAZA Ex situ Programmes, i.e., population management activities endorsed by EAZA for species managed by EAZA Members aimed at maintaining healthy animal populations within EAZA or beyond) management since 1990 [30], and the international studbook through WAZA (World Association of Zoos and Aquariums), which tracks and facilitates breeding management, the EAZA population currently has 56 adults [31], with Europe being the only region that maintains Alaotran gentle lemurs. Moreover, recent declines in breeding in European zoos and only a few active breeding pairs have been highlighted by the latest Species Holdings report for Alaotran gentle lemurs in the Species360 Zoological Information Management System (ZIMS), which shows only seven births from five breeding pairs within the last year [31]. Therefore, it is crucial to better understand their reproductive biology to enhance their wellbeing and breeding success [32]. Their reproductive patterns are mainly monogamous, though polygyny occurs, with some offspring sired by extra-group males [33]. They are seasonally polyoestrous, with mating during the dry season leading to births in the wet season [34]. In captivity, they lack a clear breeding season [30,31] and show no clear behavioural indicators of female fertility [32,35]. While not extensively studied, recent work indicates that anogenital odour encodes fertility information in females [32].
In our previous work [32], we investigated the chemical profile of anogenital odour secretions from a successful breeding female and identified four compounds (2-heptanone; 3-heptanone; 3-octanone; 4-methyl, 3-hexanone) distinguishing the chemical profile of odour secretions during the lemur ovulation window, then recreated the chemical mixture in our semiochemistry laboratory. In this study, we tested two versions of the chemical mixture on three unsuccessful breeding pairs to stimulate sexual behaviours. We aimed to evaluate the effects of both scent enrichments by using behavioural observations and faecal hormone measurements considering both overall effects and sex-specific responses. Additionally, we assessed the effectiveness of the olfactory enrichment using scented and unscented cotton strips, comparing the olfactory behaviours and proximity directed towards these differently treated strips. We predicted, compared to the baseline condition (i.e., before exposure to scented strips), that stress-related behaviours (such as abnormal and aggressive actions) would decrease, and species-specific behaviours (such as olfactory activities) would increase, while stress-related hormone levels (specifically cortisol concentrations) would reduce. Furthermore, we predicted that sexual behaviours in both males and females (including male mating behaviours) and sex hormone levels (male testosterone concentrations) would increase during enrichment conditions.
2. Materials and Methods
2.1. Subjects and Housing
The study involved three non-breeding pairs (i.e., all study subjects have never bred successfully) of gentle lemurs (n = 6) housed at Parc Zoologique et Botanique de Mulhouse (Mulhouse, France), Jersey Zoo (formerly Durrell Wildlife Park, Jersey—Channel Islands), and ZSL London Zoo (London, UK) (Table 1). All pairs were housed in indoor enclosures maintained at 25 °C, with access to outdoor areas. Behavioural observations and faecal sampling were conducted from July 2022 to February 2023.

Table 1.
Sampling period and study subjects.
2.2. Study Design
Data collection at each zoo included a 10-day baseline period (PRE) followed by a 6-day enrichment period (Table 1). The enrichment phase was divided into three days using Enrichment 1 (ENR1, with Mixture 1) and three days using Enrichment 2 (ENR2, with Mixture 2). We collected behavioural data (n = 240 h) and faecal samples (n = 80) from early morning to early afternoon (5 h continuously per day). Faecal samples were collected the day after each observation to account for the previous day’s hormone levels. At London Zoo, the lemurs’ off-show enclosure time made it difficult to identify faecal samples, resulting in fewer samples than at other zoos.
2.3. Olfactory Enrichment
Our previous work [32] focussed on sampling anogenital odour secretions in female gentle lemurs via positive reinforcement training [36] and chemical investigation of anogenital odour secretions [37]. We managed to identify a portion of the chemical signature conveying information about female fertility. In that study, we included measurements of sex hormone levels (progesterone and oestradiol), using enzyme immunoassay techniques, and investigating the volatile component of odour signals using solid-phase microextraction and gas chromatography–mass spectrometry techniques [37]. Four compounds distinguished the volatile chemical profile of anogenital scent secretions during the breeding period: 2-heptanone, 3-heptanone, 3-octanone, and 4-methyl 3-hexanone. We then synthesised this chemical signature (1:1 proportion) as an olfactory enrichment for our study pairs (Mixture 1 of the present study). In addition to the four volatile compounds identified in the successfully breeding fertile female, we added decanal and benzaldehyde (Table 2) to create Mixture 2 of the present study. These compounds were identified in our recent work on ruffed lemurs [4,38] as key signals of female fertility and found in scent-marking secretions of several primate species [36,39]. Thus, we used Enrichment 1 (ENR1), from Mixture 1, for the first three days and then Enrichment 2 (ENR2), from Mixture 2.

Table 2.
Mixtures composition for Enrichment 1 and Enrichment 2.
2.4. Enrichment Protocol
For the scent enrichment preparation, we followed our established protocol [40]. Briefly, we used sterilised white cotton strips 75 cm long and 5 cm wide. During the enrichment period, every day we soaked the cotton strips in three 50 mL vials with 20 drops of the scent mixture diluted with 12 mL of cold-boiled water. We prepared the scent cotton strips and then positioned 2 unscented and 6 scented strips around both indoor and outdoor enclosures before 8 AM of each sampling day over the scent enrichment period. We tied the strips approximately 1 m from the ground around the climbing frames, as these were the most used areas of the enclosures by females when marking, and eventually removed them at the end of the observations. Every enrichment day, we randomised the placement of both scented and unscented cotton as well as the locations where they were placed inside the enclosure.
2.5. Behavioural Data Collection
We conducted in-person behavioural observations using all occurrences of some behaviours and ad libitum sampling methods [41] every study day (6 days per week) between 8 AM and 1 PM (5 h per day), excluding Sundays to avoid peak visitor hours. Thus, we collected data on olfactory, sexual, aggressive, and abnormal behaviours using an ethogram (Table 3) developed by Errington [42] and modified based on prior studies by other authors [30,32,35,43]. The same observer (A.B.C.) collected data from the three host zoos.

Table 3.
Ethogram.
We determined the frequency of each behaviour by calculating the number of behaviours performed out of the total hours of observation. Furthermore, during the enrichment period, we included additional behaviours towards the cotton strips:
- Proximity to cotton: time spent in proximity (within 12 cm) of a given cotton strip while showing no other response;
- Olfactory behaviours:
- Sniffing cotton (SC): time spent sniffing the cotton with the nose held at or within 2 cm of the placement of the odorant;
- Licking cotton (LC): time spent licking the cotton;
- Scent-marking cotton (CSM): scent-marking behaviours towards the cotton.
2.6. Faecal Hormone Sampling and Measurements
We collected faecal samples from both male and female study subjects every morning during behavioural observations, when defecation was observed, and the animal’s identity was certain. We immediately stored the samples in a −20 °C freezer at the zoo before transferring them, using a freezer box with ice packs to avoid any risk of defrosting, to the Rosalind Franklin Science Centre, University of Wolverhampton, for laboratory analyses.
For hormone extraction, we used our established protocol [32]. Briefly, we lyophilised the faecal samples for 72 h using a freeze-drying machine (Christ®, Beta 1–8 LSC plus, Osterode am Harz, Germany); then, we pulverised and sieved them to separate the faecal residue from the fibrous material. The extraction methods were based on those detailed in [32,44]. In brief, we extracted in a 15 mL plastic tube 0.05–0.1 g of pulverised faeces in 3 mL of 80% methanol. Then, after vortexing for 15 min using a multi-tube vortexer (Grant Instruments R, Multi-Vortexer V32, Cambridge, UK) and centrifugation for 20 min at 3266× g, we immediately stored the supernatant at −20 °C.
For male and female cortisol analyses, we used a commercially available ELISA kit (Enzo Life Sciences®, New York, USA). We diluted the sample 1:10 with the assay buffer and all faecal samples and standards were assayed in duplicate. For male testosterone, we used DetectX® Immunoassay Kit (ArborAssay®, Place Ann Arbor, USA). We diluted the samples 1:5 with the assay buffer and all faecal samples and standards were assayed in duplicate.
To validate the enzyme immunoassays, we conducted a parallelism test between the standard curves of each kit and the serial dilutions of two samples [45]. Then, using a microplate reader, we read the optical density, and these data were analysed using a 4-parameter logistic fitting programme (MyAssays®). Hormone concentrations were calculated in pg/mg.
2.7. Statistical Analysis
We conducted statistical analyses using R Studio software (version 4.2.2) to evaluate behavioural and hormonal outcomes from the enrichment period. To assess the impact of scent enrichment on lemur behaviours and hormones, we first categorised the behaviours into two groups: sexual behaviours and olfactory behaviours (Table 4). Only observed behaviours were included in the analysis to ensure statistical significance. Due to the limited number of data points and low frequency of abnormal and aggressive behaviours, statistical analysis could not be performed for these categories.

Table 4.
Category of behaviours considered in two-way ANOVA.
We employed the Wilcoxon test to compare olfactory and proximity behaviours towards scented and unscented cotton strips. We employed a two-way ANOVA to analyse the data, comparing the sexes within each zoo (sample size: N = 2 per zoo) across several categories. Specifically, we compared the baseline period (PRE) to the overall enrichment period (ENR1 and ENR2 combined) with regard to olfactory behaviours, sexual behaviours, and faecal cortisol concentrations. Additionally, we compared the effects of the two enrichment conditions (ENR1 and ENR2) across the same categories, again examining differences between the sexes within each zoo. Post hoc analyses were conducted using Tukey’s method for multiple comparisons, with a significance level set at 0.05.
For testosterone analysis, given that each zoo had only one male (N = 1 per zoo), we used the Welch Two Sample t-test to compare baseline levels with the combined enrichment periods (ENR1 and ENR2) and examine changes in testosterone levels between ENR1 and ENR2.
2.8. Ethics Statement
This study adhered to institutional and international guidelines for the care and use of captive animals, employing non-invasive methods to collect behavioural data and faecal samples from the gentle lemurs. Additionally, this study complied with CITES regulations and received approval from the Life Sciences Ethics Committee at the University of Wolverhampton (UK) (REC number LSEC/202021/SV/52) as well as the Ethics Committees at Parc Zoologique and Botanique de Mulhouse (Mulhouse, France), Jersey Zoo (Jersey—Channel Islands), and ZSL London Zoo (London, UK). We also confirm that our research followed the ARRIVE guidelines for the ethical treatment of non-human primates [46].
3. Results
3.1. Proximity and Olfactory Behaviours towards Scented and Unscented Cotton Strips
We analysed how proximity and olfactory behaviours towards the cotton strips (SC, LC, and CSM) varied between scented and unscented cotton strips, considering both enrichment periods together (ENR1 and ENR2). The unit of analysis was the frequency of these behavioural occurrences per day. The Wilcoxon test showed a statistically significant increase in the frequency of these behaviours towards the scented cotton strips compared to the unscented strips in every zoo (see Table 5 for details about p-values). The total number of occurrences (N) was aggregated across both enrichment periods.

Table 5.
Results by the Wilcoxon test comparing proximity and olfactory behaviours towards scented versus unscented cotton strips.
3.2. Sexual Behaviours
A two-way ANOVA was conducted to compare the baseline period (PRE) with the combined enrichment periods (ENR = ENR1 and ENR2). We found that sexual behaviours significantly increased during the enrichment period at both Jersey and London zoos (F(1) = 17.47; p-value = 0.0002 and F(1) = 14.58; p-value = 0.0006, respectively). Specifically, Tukey’s test revealed that the male at Jersey Zoo increased these behaviours significantly during enrichment. Conversely, at London Zoo, the female performed more of these behaviours during the enrichment phase (Table 6).

Table 6.
Results of the Tukey test comparing sexual and olfactory behaviours between the sexes and different types of study periods. M stands for male and F for female.
Additionally, we examined the impact of the two enrichments on the sexual behaviours of both sexes. The analysis revealed no statistically significant differences in sexual behaviours between the enrichment periods at Mulhouse Zoo (F(1) = 0.33; p = 0.58) nor between the sexes (F(1) = 0.33; p = 0.58). By contrast, at Jersey Zoo, there was a statistically significant difference in sexual behaviours between the sexes (F(1) = 8.32; p-value = 0.0204), but not among the enrichment periods (F(1) = 0.01; p-value = 0.8941). The Tukey test showed that males performed significantly more sexual behaviours than females (Table 6). Similarly, at London Zoo, we found a statistically significant difference in sexual behaviours between the sexes (F(1) = 9.53; p-value = 0.015), but no difference between the enrichment periods (F(1) = 0.07; p-value = 0.796). Tukey’s test revealed that females performed more sexual behaviours than males (Table 6). In Figure 1a star denotes a p-value < 0.05.
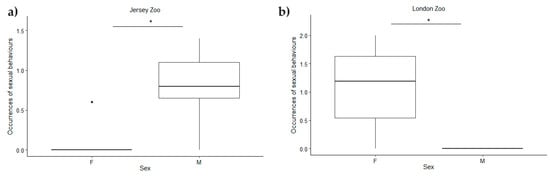
Figure 1.
Differences in sexual behaviours between the sexes at different zoos analysed using a two-way ANOVA: (a) at Jersey Zoo, males exhibited more sexual behaviours than females; (b) at London Zoo, females exhibited more sexual behaviours than males. The asterisk (*) indicates p value smaller than 0.05 (p < 0.05). The point (˙) indicates an outlier.
3.3. General Olfactory Behaviours
When comparing the baseline period to the combined enrichment periods, two-way ANOVA revealed that olfactory behaviours significantly increased during the enrichment period at London Zoos for both sexes (F(1) = 11.28; p-value = 0.002) (Figure 2). See Table 6 for the Tukey test results.
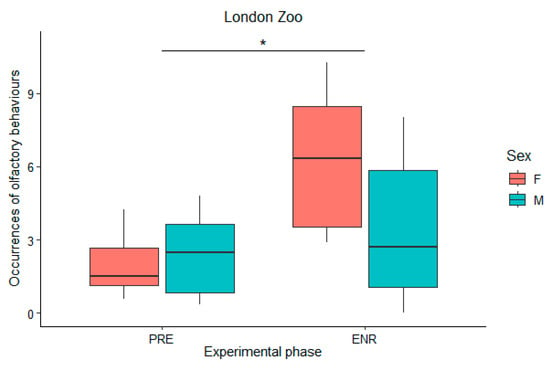
Figure 2.
Olfactory behaviours differences analysed using a two-way ANOVA between baseline (PRE) and enrichment conditions (ENR) at London Zoo. The asterisk (*) indicates p value smaller than 0.05 (p < 0.05).
Additionally, we found statistically significant differences in olfactory behaviours between the sexes at both Mulhouse Zoo (F(1) = 43.38; p-value = 0.000172) and Jersey Zoo (F(1) = 7.14; p-value = 0.0282). Specifically, males exhibited olfactory behaviours more frequently than females (Table 6). However, there were no significant differences between the two enrichment periods at either zoo (p-values = 0.582563 and 0.4406 respectively; F(1) = 0.32 and 0.65 respectively). By contrast, at London Zoo, olfactory behaviours were significantly more frequent during the Enrichment 1 period than in the Enrichment 2 period (F(1) = 6.71; p-value = 0.0321), but there were no significant differences between the sexes (F(1) = 3.42; p-value = 0.1014) (Table 6).
3.4. Cortisol
We did not find any statistically significant differences in cortisol concentrations when comparing the baseline to the combined enrichment periods in any of the zoos.
However, a two-way ANOVA was also performed to compare the effects of different enrichments on cortisol in both sexes. At Mulhouse Zoo, there was a small difference between the sexes (F(1) = 3.98; p-value = 0.081), but this was not statistically significant. Additionally, there were no significant changes between the two enrichment periods (F(1) = 0.004; p-value = 0.950). At both Jersey Zoo and London Zoo, there were no significant changes in cortisol concentrations during the two phases of the study (p-value = 0.777 and 0.649, respectively; F(1) = 0.08 and 0.22, respectively), nor between the sexes (p-value = 0.659 and 0.572, respectively; F(1) = 0.20 and 0.34, respectively).
3.5. Testosterone
None of the males showed significant changes between the baseline and enrichment phases in testosterone concentrations.
Nevertheless, the Welch Two Sample t-test analysis revealed significant changes in testosterone levels across different enrichment conditions at Mulhouse Zoo (t = −5.4439, df = 2.4778, p-value = 0.01964), with testosterone levels increasing during Enrichment 2 (Figure 3). Conversely, at both London Zoo and Jersey Zoo, the enrichment conditions did not significantly affect testosterone levels in males (p-values = 0.3269 and 0.08365, respectively).
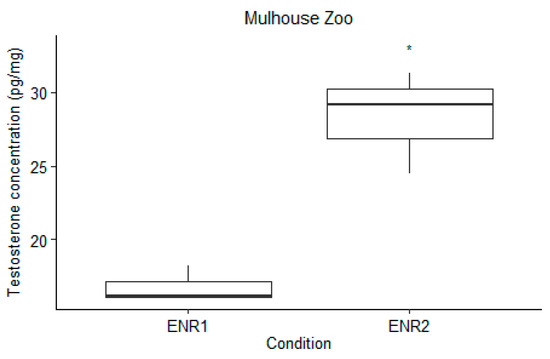
Figure 3.
Results of the t-test analysis comparing male testosterone concentrations at Mulhouse Zoo during ENR1 and ENR2. The asterisk (*) indicates p value smaller than 0.05 (p < 0.05).
4. Discussion
One of the 25 most endangered primates in the world is the Alaotran gentle lemur (Hapalemur alaotrensis) [29], which also shows a poor success rate in captive breeding programmes [31,32]. This project aimed to enhance the breeding practices of captive gentle lemurs by evaluating a novel scent enrichment based on female fertile odour signals in three unsuccessful breeding pairs housed at Mulhouse, Jersey, and ZSL London zoos. Specifically, we evaluated the effects of scent enrichment by combining behavioural observations (i.e., sexual, olfactory, abnormal, and aggressive behaviours) and faecal hormone analyses (i.e., testosterone and cortisol) and found that exposure to this treatment influenced olfactory behaviours and some sexual behaviours, but there were no significant changes in cortisol and testosterone levels (except for the increase in one male’s testosterone levels during exposure to enrichment).
We decided to test not only the mixture derived from the findings of our previous work on Alaotran gentle lemurs [32], i.e., four compounds (2-heptanone; 3-heptanone; 3-octanone; 4-methyl, 3-hexanone) distinguishing the chemical profile of odour secretions during the lemur ovulation window, but also a second mixture that contained two additional compounds: decanal and benzaldehyde. These volatile compounds were found during our studies on ruffed lemurs [4,38] and identified as potentially key compounds signalling female fertility in ruffed lemurs, crowned lemurs [39], and mandrills [47].
We did not record any abnormal behaviours. Only at Mulhouse Zoo, the female displayed some pacing, probably as an anticipatory behaviour observed before the zookeepers fed the study group. Regarding aggressive behaviours, very few were recorded (therefore, it was not possible to carry out any statistical analysis due to the small pool of data points) and all occurred at the time of feeding. As described by other authors [48], aggressive behaviours in gentle lemurs may occur in intersexual interactions, especially in the context of feeding.
Our study results show a significant increase in olfactory behaviours and proximity towards the scented cotton strips, underscoring the potential of olfactory stimuli to enhance environmental enrichment in captive settings. These results align with previous research highlighting the role of olfaction in various animal behaviours, including foraging (i.e., Megachiroptera fruit bats [49]), reproduction (i.e., dogs, rats [50]), and social communication (i.e., ring-tailed lemurs [51], crowned lemurs [39]).
Both sexes showed an increase in olfactory behaviours during the enrichment period compared to the baseline. Additionally, we found that olfactory behaviours varied by sex and enrichment conditions. At the Mulhouse and Jersey zoos, males exhibited more olfactory behaviours than females, regardless of the enrichment condition; at London Zoo, the lemurs showed more olfactory behaviours during the first enrichment condition (ENR1) than the second (ENR2). These results suggest that olfactory stimulation is influenced by both sex and environmental contexts, which is consistent with prior findings by other scholars on the role of olfaction in animal behaviours (such as in birds [52], black-tailed deer [53], and mice [54]).
The sex-based differences found in our study, particularly the increased frequency of sexual behaviours in males at Jersey Zoo and females at London Zoo, suggest that olfactory enrichment may impact the two sexes differently. This observation could be attributed to underlying sex differences in olfactory processing or motivation, as noted in other species (i.e., mice—Mus musculus [55,56], moustached tamarins—Saguinus mystax [57], and rhesus monkeys—Macaco rhesus [58,59]). However, the lack of statistically significant changes in sexual behaviours (such as attempt mounting and copulation) and cortisol levels suggests that while olfactory enrichment can engage animals’ sensory modalities, it may not directly translate into increased reproductive success or reduced stress. Moreover, since stress-related behaviours were observed very infrequently during the study, we can assume that the study subjects had good welfare status, thereby limiting the potential impact of our scent enrichment.
Finally, the rise in testosterone levels in one male at Mulhouse Zoo during exposure to the second scent mixture highlights the potential for specific odours to influence hormonal states; however, we did not observe any copulation or mounting behaviours during the Enrichment 2 period, and this effect was not observed consistently across individuals or zoos. Moreover, no significant changes in testosterone levels were found when comparing the enrichment phase to the baseline.
Limitations
We acknowledge several major limitations that could have affected the efficacy of our scent enrichment programme. First, the small sample size (i.e., one successful breeding female and three unsuccessful breeding pairs) was a significant constraint. Additionally, verifying via objective criteria (e.g., faecal endocrinology or genital cytology) that the tested females were not ovulating during the study period would have been crucial as it is likely that, regardless of scent enrichment, males may perform more olfactory inspections and sexual behaviours if females are ovulating. Difficulties with odour sampling, including the small number of anogenital odour secretions sampled and potential swab contaminants, as well as the use of an individual female’s samples from one breeding vs. non-breeding season, may have led to an inaccurately synthesised odour mixture. In addition, the 1:1 proportion of mixed compounds did not reflect actual chemical ratios. Finally, we investigated the volatile component of female odour signals. However, the non-volatile component may also play an important role in advertising female lemur fertility to males.
5. Conclusions
In conclusion, this preliminary study aimed to use two chemical mixtures (ENR1 and ENR2), conveying a portion of Alaotran gentle lemur’s female fertile odour signature, and test their effects on three unsuccessful breeding pairs of Alaotran gentle lemurs housed in other zoos. Based on the behavioural observations and faecal hormone measurements, we can conclude that scent enrichment was not fully successful.
Overall, our findings highlight the complex and context-dependent nature of how olfactory enrichment may affect the behaviour and physiology of zoo-housed lemurs. While the scent mixtures did not produce the anticipated widespread effects, the targeted responses observed, such as for olfactory behaviours, suggest that biologically meaningful scents have the potential to enhance both the welfare and captive breeding practices of endangered lemurs.
Author Contributions
Conceptualization, A.B.C., G.G., S.F. and S.V.; formal analysis, A.B.C.; funding acquisition, S.F. and S.V.; investigation: A.B.C.; methodology: G.G., S.F. and S.V.; project administration: S.F. and S.V.; resources: S.V.; supervision: G.G. and S.F.; visualization: A.B.C.; writing—original draft preparation, A.B.C.; writing—review and editing, S.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement [no. 890341 to S.F. and S.V.]. Lab work and publication fees were funded by the University of Wolverhampton’s Research Investment Fund scheme—Phase 4 [to S.V.].
Institutional Review Board Statement
The animal study protocol was approved by the Life Sciences Ethics Committee of the University of Wolverhampton (UK) (REC number LSEC/202021/SV/52, 10 February 2021).
Data Availability Statement
The datasets are available on the Open Science Framework (OFS) repository with following persistent identifier—DOI: 10.17605/OSF.IO/PFT7C. We also confirm that the datasets have a CC0 Public Domain Dedication license applied.
Acknowledgments
We thank Sara Epis for her assistance, supervision, and guidance to A.B.C. We are also grateful to Stefano Kaburu for his kind assistance during the statistical analysis. Regarding the Mulhouse, Jersey, and ZSL London zoos, we thank Rachel Cowen, Brice Lefaux, and Lewis Rowden for granting us the opportunity to conduct this study at their respective facilities. Moreover, we appreciate the primate keeper staff’s willingness to take time out of their busy schedules to assist A.B.C. with her observations and their enthusiasm for our research work. Finally, we thank the Animal Behaviour and Wildlife Conservation group at the University of Wolverhampton for advising us over the course of this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 10 July 2024).
- Estrada, A.; Garber, P.A.; Rylands, A.B.; Roos, C.; Fernandez-Duque, E.; Di Fiore, A.; Nekaris, K.A.-I.; Nijman, V.; Heymann, E.W.; Lambert, J.E.; et al. Impending Extinction Crisis of the World’s Primates: Why Primates Matter. Sci. Adv. 2017, 3, e1600946. [Google Scholar] [CrossRef] [PubMed]
- Spiezio, C.; Regaiolli, B.; Savonitto, M.; Bruslund, S.; Vaglio, S. Zoo-Led Initiatives and Their Role in Lemur Conservation In Situ. Animals 2022, 12, 2772. [Google Scholar] [CrossRef] [PubMed]
- Elwell, E.J.; Vaglio, S. The Scent Enriched Primate. Animals 2023, 13, 1617. [Google Scholar] [CrossRef] [PubMed]
- Mcphee, M.; Carlstead, K. The Importance of Maintaining Natural Behaviors in Captive Mammals. Wild Mamm. Captiv. Princ. Tech. Zoo. Manag. 2010, 2, 303–313. [Google Scholar]
- Mcphee, M.E. Intact Carcasses as Enrichment for Large Felids: Effects on On- and Off-exhibit Behaviors. Zoo. Biol. 2002, 21, 37–47. [Google Scholar] [CrossRef]
- Swaisgood, R.R.; Ellis, S.; Forthman, D.L.; Shepherdson, D.J. Commentary: Improving Well-being for Captive Giant Pandas: Theoretical and Practical Issues. Zoo. Biol. 2003, 22, 347–354. [Google Scholar] [CrossRef]
- Jacobs, B.; Rally, H.; Doyle, C.; O’Brien, L.; Tennison, M.; Marino, L. Putative Neural Consequences of Captivity for Elephants and Cetaceans. Rev. Neurosci. 2022, 33, 439–465. [Google Scholar] [CrossRef]
- Fritz, J.; Nash, L.T.; Alford, P.L.; Bowen, J.A. Abnormal Behaviors, with a Special Focus on Rocking, and Reproductive Competence in a Large Sample of Captive Chimpanzees (Pan troglodytes). Am. J. Primatol. 1992, 27, 161–176. [Google Scholar] [CrossRef]
- Carlstead, K.; Shepherdson, D. Effects of Environmental Enrichment on Reproduction. Zoo. Biol. 1994, 13, 447–458. [Google Scholar] [CrossRef]
- Vaz, J.; Narayan, E.J.; Dileep Kumar, R.; Thenmozhi, K.; Thiyagesan, K.; Baskaran, N. Prevalence and Determinants of Stereotypic Behaviours and Physiological Stress among Tigers and Leopards in Indian Zoos. PLoS ONE 2017, 12, e0174711. [Google Scholar] [CrossRef]
- Mallapur, A. Animal Welfare research and its implications to non-human primate breeding programs: A Case Study of the Lion-tailed macaque conservation breeding program from India. REDVET Rev. Electrónica Vet. 2008, IX, 1–21. [Google Scholar]
- Moreira, N.; Brown, J.L.; Moraes, W.; Swanson, W.F.; Monteiro-Filho, E.L.A. Effect of Housing and Environmental Enrichment on Adrenocortical Activity, Behavior and Reproductive Cyclicity in the Female Tigrina (Leopardus tigrinus) and Margay (Leopardus wiedii). Zoo Biol. 2007, 26, 441–460. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, E.T. What’s New at the Zoo? BioScience 2001, 51, 172. [Google Scholar] [CrossRef][Green Version]
- Cummins, R.A.; Livesey, P.J.; Evans, J.G.M.; Walsh, R.N. A Developmental Theory of Environmental Enrichment. Science 1977, 197, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Heymann, E.W. The Neglected Sense–Olfaction in Primate Behavior, Ecology, and Evolution. Am. J. Primatol. 2006, 68, 519–524. [Google Scholar] [CrossRef]
- Colquhoun, I.C. A Review and Interspecific Comparison of Nocturnal and Cathemeral Strepsirhine Primate Olfactory Behavioural Ecology. Int. J. Zool. 2011, 2011, 362976. [Google Scholar] [CrossRef][Green Version]
- Drea, C.M. D’scent of Man: A Comparative Survey of Primate Chemosignaling in Relation to Sex. Horm. Behav. 2015, 68, 117–133. [Google Scholar] [CrossRef]
- Brahmachary, R.L.; Poddar-Sarkar, M. Fifty Years of Tiger Pheromone Research. Curr. Sci. 2015, 108, 2178–2185. [Google Scholar]
- Soso, S.B.; Koziel, J.A. Characterizing the Scent and Chemical Composition of Panthera Leo Marking Fluid Using Solid-Phase Microextraction and Multidimensional Gas Chromatography–Mass Spectrometry-Olfactometry. Sci. Rep. 2017, 7, 5137. [Google Scholar] [CrossRef]
- Coombes, H.A.; Stockley, P.; Hurst, J.L. Female Chemical Signalling Underlying Reproduction in Mammals. J. Chem. Ecol. 2018, 44, 851–873. [Google Scholar] [CrossRef]
- Grammer, K.; Fink, B.; Neave, N. Human Pheromones and Sexual Attraction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 118, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Palmer, R.; Rosell, F. The Importance of Chemical Communication Studies to Mammalian Conservation Biology: A Review. Biol. Conserv. 2011, 144, 1919–1930. [Google Scholar] [CrossRef]
- Tommasi, A.; Tredoux, A.G.J.; Koziel, J.A.; Esposito, G. The Effect of a Synthetic Scent on Cheetah Behaviour. Animals 2023, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Parrott, M.L.; Nation, A.; Selwood, L. Female Mate Choice Significantly Increases Captive Breeding Success, and Scents Can Be Frozen to Determine Choice, in the Stripe-Faced Dunnart. Appl. Anim. Behav. Sci. 2019, 214, 95–101. [Google Scholar] [CrossRef]
- Roberts, S.C.; Gosling, L.M. Manipulation of Olfactory Signaling and Mate Choice for Conservation Breeding: A Case Study of Harvest Mice. Conserv. Biol. 2004, 18, 548–556. [Google Scholar] [CrossRef]
- Wells, D.L.; Hepper, P.G.; Coleman, D.; Challis, M.G. A Note on the Effect of Olfactory Stimulation on the Behaviour and Welfare of Zoo-Housed Gorillas. Appl. Anim. Behav. Sci. 2007, 106, 155–160. [Google Scholar] [CrossRef]
- Rafacz, M.L.; Santymire, R.M. Using Odor Cues to Elicit a Behavioral and Hormonal Response in Zoo-housed African Wild Dogs. Zoo Biol. 2014, 33, 144–149. [Google Scholar] [CrossRef]
- Schwitzer, C.; Mittermeier, R.; Rylands, A.; Chiozza, F.; Williamson, L.; Byler, D.; Wich, S.; Humle, T.; Johnson, C.; Mynott, H.; et al. Primates in Peril: The World’s Most. Endangered Primates 2018–2020; IUCN SSC Primate Specialist Group: Gland, Switzerland, 2019; ISBN 978-1-5272-4806-9. [Google Scholar]
- Beattie, J.C.; Feistner, A.T.C. Husbandry and Breeding of the Alaotran Gentle Lemur: Hapalemur Griseus Alaotrensis at Jersey Wildlife Preservation Trust. Int. Zoo Yearb. 1998, 36, 11–19. [Google Scholar] [CrossRef]
- ZIMS Species Holdings, 23rd August 2024. Species360 Zoological Information Management System. Available online: http://zims.species360.org (accessed on 27 August 2024).
- Fontani, S.; Kaburu, S.S.K.; Marliani, G.; Accorsi, P.A.; Vaglio, S. Anogenital Scent-Marking Signals Fertility in a Captive Female Alaotran Gentle Lemur. Front. Vet. Sci. 2022, 9, 940707. [Google Scholar] [CrossRef]
- Nievergelt, C.M.; Mutschler, T.; Feistner, A.T.C.; Woodruff, D.S. Social System of the Alaotran Gentle Lemur (Hapalemur griseus Alaotrensis): Genetic Characterization of Group Composition and Mating System. Am. J. Primatol. 2002, 57, 157–176. [Google Scholar] [CrossRef]
- Mutschler, T.; Nievergelt, C.M.; Feistner, A.T.C. Social Organization of the Alaotran Gentle Lemur (Hapalemur Griseus Alaotrensis). Am. J. Primatol. 2000, 50, 9–24. [Google Scholar] [CrossRef]
- Haring, D.; Davis, K. Management of the Grey Gentle or Eastern Lesser Bamboo Lemur Hapaletnur Griseus Griseus at Duke University Primate Center, Durham. Int. Zoo. Yearb. 1998, 36, 20–34. [Google Scholar] [CrossRef]
- Spiezio, C.; Piva, F.; Regaiolli, B.; Vaglio, S. Positive Reinforcement Training: A Tool for Care and Management of Captive Vervet Monkeys (Chlorocebus aethiops). Anim. Welf. 2015, 24, 283–290. [Google Scholar] [CrossRef]
- Walker, D.; Vaglio, S. Sampling and Analysis of Animal Scent Signals. J. Vis. Exp. 2021, 168, e60902. [Google Scholar] [CrossRef]
- Elwell, E.; Fontani, S.; Vaglio, S. Design and Test of Novel Scent Enrichments to Enhance Breeding of Zoo-Housed Lemurs. F1000Research 2024, 13, 123. [Google Scholar] [CrossRef]
- Elwell, E.J.; Walker, D.; Vaglio, S. Sexual Dimorphism in Crowned Lemur Scent-Marking. Animals 2021, 11, 2091. [Google Scholar] [CrossRef]
- Vaglio, S.; Kaburu, S.S.K.; Pearce, R.; Bryant, L.; McAuley, A.; Lott, A.; Sheppard, D.J.; Smith, S.; Tompkins, B.E.; Elwell, E.J.; et al. Effects of Scent Enrichment on Behavioral and Physiological Indicators of Stress in Zoo Primates. Am. J. Primatol. 2021, 83, e23247. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Errington, M. An Investigation into the Effect of Olfactory Enrichment on the Behaviour of Lac Alaotra Gentle Lemurs (Hapalemur alaotrensis), at Chessington Zoo, CWOAR. Master’s Thesis, Bournemouth University, Poole, UK, 2017. [Google Scholar]
- Shideler, S.E.; Lindburg, D.G.; Lasley, B.L. Estrogen-Behavior Correlates in the Reproductive Physiology and Behavior of the Ruffed Lemur (Lemur variegatus). Horm. Behav. 1983, 17, 249–263. [Google Scholar] [CrossRef]
- Maréchal, L.; Semple, S.; Majolo, B.; Qarro, M.; Heistermann, M.; MacLarnon, A. Impacts of Tourism on Anxiety and Physiological Stress Levels in Wild Male Barbary Macaques. Biol. Conserv. 2011, 144, 2188–2193. [Google Scholar] [CrossRef]
- Gholib, G.; Wahyuni, S.; Kadar, O.H.; Adam, M.; Lubis, T.M.; Azhar, A.; Akmal, M.; Siregar, T.N.; Armansyah, T.; Nugraha, T.P. Measurement of Serum Testosterone in Kacang Goat Byusing Enzyme-Linked Immunosorbent Assay (ELISA) Technique: The Importance of Kit Validation. J. Kedokt. Hewan Indones. J. Vet. Sci. 2016, 10, 32–36. [Google Scholar] [CrossRef]
- ARRIVE Guidelines. Available online: https://arriveguidelines.org/ (accessed on 15 July 2024).
- Vaglio, S.; Minicozzi, P.; Romoli, R.; Boscaro, F.; Pieraccini, G.; Moneti, G.; Moggi-Cecchi, J. Sternal Gland Scent-Marking Signals Sex, Age, Rank, and Group Identity in Captive Mandrills. Chem. Senses 2015, 41, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Waeber, P.O.; Hemelrijk, C.K. Female Dominance and Social Structure in Alaotran Gentle Lemurs. Behaviour 2003, 140, 1235–1246. Available online: https://www.jstor.org/stable/4536089 (accessed on 21 June 2024). [CrossRef]
- Raghuram, H.; Thangadurai, C.; Gopukumar, N.; Nathar, K.; Sripathi, K. The Role of Olfaction and Vision in the Foraging Behaviour of an Echolocating Megachiropteran Fruit Bat, Rousettus leschenaulti (Pteropodidae). Mamm. Biol. 2009, 74, 9–14. [Google Scholar] [CrossRef]
- Eisenberg, J.F.; Kleiman, D.G. Olfactory Communication in Mammals. Annu. Rev. Ecol. Syst. 1972, 3, 1–32. [Google Scholar] [CrossRef]
- Scordato, E.S.; Drea, C.M. Scents and Sensibility: Information Content of Olfactory Signals in the Ringtailed Lemur, Lemur Catta. Anim. Behav. 2007, 73, 301–314. [Google Scholar] [CrossRef]
- Caro, S.P.; Balthazart, J.; Bonadonna, F. The Perfume of Reproduction in Birds: Chemosignaling in Avian Social Life. Horm. Behav. 2015, 68, 25–42. [Google Scholar] [CrossRef]
- Müller-Schwarze, D. Pheromones in Black-Tailed Deer (Odocoileus hemionus columbianus). Anim. Behav. 1971, 19, 141–152. [Google Scholar] [CrossRef]
- Bruce, H.M. A Block to Pregnancy in the Mouse Caused by Proximity of Strange Males. Reproduction 1960, 1, 96–103. [Google Scholar] [CrossRef]
- Hurst, J.L.; Payne, C.E.; Nevison, C.M.; Marie, A.D.; Humphries, R.E.; Robertson, D.H.L.; Cavaggioni, A.; Beynon, R.J. Individual Recognition in Mice Mediated by Major Urinary Proteins. Nature 2001, 414, 631–634. [Google Scholar] [CrossRef]
- Van Der Linden, C.; Jakob, S.; Gupta, P.; Dulac, C.; Santoro, S.W. Sex Separation Induces Differences in the Olfactory Sensory Receptor Repertoires of Male and Female Mice. Nat. Commun. 2018, 9, 5081. [Google Scholar] [CrossRef] [PubMed]
- Heymann, E.W. Sex Differences in Olfactory Communication in a Primate, the Moustached Tamarin, Saguinus mystax (Callitrichinae). Behav. Ecol. Sociobiol. 1998, 43, 37–45. [Google Scholar] [CrossRef]
- Bakker, J. Sexual Differentiation of the Neuroendocrine Mechanisms Regulating Mate Recognition in Mammals. J. Neuroendocrinol. 2003, 15, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Michael, R.P.; Keverne, E.B. Primate Sex Pheromones of Vaginal Origin. Nature 1970, 225, 84–85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).