Abstract
Unusual or extraordinary circumstances can cause change to normal husbandry regimes and daily care of managed animals. Increased biosecurity due to disease risk, for example, results in animals experiencing restrictions. Outbreaks of Highly Pathogenic Avian Influenza (HPAI) have caused zoos to remove birds from their regular exhibits and manage them indoors or in covered enclosures to reduce the likelihood of an HPAI outbreak on site. To date, there has been little research on the impacts of such husbandry change on bird behaviour and welfare. This paper examines the effect of an HPAI-induced enforced housing order (HO) on the behaviour and physical condition of a flock of Chilean flamingos in a UK zoo. Using ZooMonitor to record flock-wide behaviour patterns and scoring plumage condition, we collected data on flamingos during a housing order, immediately after lifting of the HO, and after a period of acclimation to their regular routine. Results showed that flamingos were very inactive under a HO and after release, that abnormal, redirected foraging actions occurred during the HO and after release, and that flamingos were more alert under the HO. An increase in records of good plumage condition correlated with social behaviour, inactivity, higher temperatures, and rain. This research highlights the multifactorial influences on zoo animal behaviour and shows why information on the animals, their inputs, the behavioural outputs they present, and their physical attributes should all be gathered and evaluated together to best understand the influences of husbandry and management changes on behaviour and welfare.
1. Introduction
Avian Influenza (AI) outbreaks have become a globally recognised challenge for the management of zoo-housed birds [1] to ensure such populations remain healthy and disease free [2]. Both low-pathogenic and highly pathogenic AI strains are recorded [3], varying in their ability to persist in the environment and cause disease outbreaks [4]. AI can mutate into new strains across different seasons, making prophylactic treatment currently challenging [5,6]. Transmission of AI by migratory wild birds sees the disease spread quickly and easily between nation states [7]. For review of the symptoms, morbidity, and mortality of AI and impacts on bird health, please see Jensen and Kuiken [1]. In the UK, AI outbreaks since 2016 [8] have regularly caused enforced indoor housing orders (HO) and the implementation of increased biosecurity measures for all captive birds (domestic and exotic species) [9]. In 2021, the UK (to date) recorded its largest and most widespread AI outbreak [10], resulting in the enforced housing of many bird species that would normally have access to large, naturalistic outdoor enclosures. Spatial restriction is known to cause welfare challenges for a range of managed species, including farmed livestock [11] and poultry [12]. In zoo-housed animals, reduced choice and control that the animal has over its immediate environment [13] and associated limited opportunities for behavioural performance [14], as well as enforced proximity to conspecifics and to human caregivers that may be perceived as threatening [15], all negatively impact animal welfare.
Welfare can be defined as the state of the individual as it attempts to cope with its environment [16] and comprises behavioural, psychological, and physical components [17]. Welfare assessment (i.e., consistent and repeated data collection to record how managed animals are faring under their current, prevailing environmental and social conditions) methods use these components as indicators, whereby behavioural-based approaches represent a widely researched and implemented animal-focused technique [18]. Behavioural assessment of welfare is one method available to understand the impacts of management, housing, and husbandry change on the responses of captive animals to their environment and care [19,20]. When applied in a scientifically rigorous and repeatable manner, behaviour offers a useful, non-invasive means of understanding an animal’s perception of its wellbeing within their immediate environment. Such methods of determining animal welfare should consider resource-based assessments (i.e., inputs) that review the appropriateness of housing and husbandry provided to an individual’s ability at attaining positive emotional states, i.e., outputs [21]. Observing and measuring behaviour can provide inferences of underlying welfare states [22] and therefore identify situations that animals may find stressful and beyond their (behavioural, physiological, or psychological) control [23], for example via the performance of abnormal or aberrant behaviour patterns [24]. Such abnormal repetitive behaviours (ARBs), whereby an animal repeatedly expresses behaviours without outward purpose [25], can be categorised into ‘compulsive’ behaviours, that are repeated and goal oriented, and ‘stereotypic’, with a repeated motor function [26,27]. Observation of ARBs and correlating the intensity or duration of their performance with changes to the animal’s housing and husbandry provides information on the quality and relevance of their environment and animal management regime.
AI is damaging to commercial poultry farming [28] and has zoonotic potential [29]. Extra biosecurity and quarantine measures, as well as enforced HOs resulting from AI outbreaks, are implemented to protect birds and humans from further AI infection. Such enhanced biosecurity and HOs result in drastic changes to bird housing and husbandry specifically [30] and zoo operations generally [31], with some establishments forced to cull infected birds and test and quarantine others. Waterbirds are some of the most susceptible species to AI infection [32,33,34], and amongst the most commonly housed of zoo-housed birds are the flamingos, Phoenicopteriformes [35]. Flamingos may be disproportionately exposed to the presence of wild bird vectors of AI as zoo flamingos are commonly exhibited in open-topped zoo exhibits. Wild flamingos are known to succumb to AI [36], and as a result, captive flamingos are frequently housed indoors or under cover during AI outbreaks to reduce the likelihood of AI transmission and infection. Such a change to housing restricts space availability for a flock and can limit time spent in pools or other important wetland areas that promote positive behavioural diversity; such spatial restrictions and limited opportunities to diversify behaviour patterns may cause negative welfare impacts.
The impact of AI-related confinement on bird (generally) and flamingo (specifically) behaviour and welfare is poorly understood, but research recognises the importance of specific standards of flamingo housing and husbandry to the promotion of good bird health and wellbeing [35,37,38]. The availability, distribution, and size of biologically relevant features within an enclosure can impact time-activity budgets and behavioural diversity [39], but this has yet to be measured when overall space and resource access is restricted under enforced HO conditions.
In the winter of 2022–2023, compulsory HOs due to incidences of AI in managed bird flocks were in force from 7 November 2022 to 18 April 2023 across the UK, affecting numerous zoological establishments. To understand any potential impacts on flamingo behaviour and welfare from an enforced HO due to AI, we investigated the behavioural responses of a group of Chilean flamingos (Phoenicopterus chilensis) at Banham Zoological Gardens (BZG), Norfolk, UK, to changes in their housing. Zoo staff noticed occurrences of redirected foraging behaviour (mock filter feeding whereby birds attempted to feed but their bill was held near the ground or in the air, and the birds were not in water or using a feeding bowl), and therefore a research project was implemented to understand the impact of the HO on performance of time activity budgets, presence of abnormal behaviour, and ultimately bird welfare. This research project aimed to identify and examine the potential causal factors of such behaviour change as well as to evaluate how the flamingo’s physical condition may be affected by restrictions on space and on behavioural performance (due to AI-enforced housing measures).
We hypothesised that an increase in such redirected foraging/abnormal behaviour would be observed as the HO continued, compared to behaviour patterns displayed when flamingos had full access to their enclosure. Ultimately, we hoped to identify potential behavioural indicators of flamingo welfare that keepers could use to regularly monitor their birds when they experience a change in their typical housing and husbandry regime due to AI. Such evidence can then be passed to other zoological institutions to help improve flamingo management under similar situations as caregivers would be able to identify potential outputs of poorer welfare and intervene when needed. Physical condition can also be an indicator of avian welfare state [40] and thus impact on behavioural outputs. Therefore, we aimed to quantify any relationship between flamingo behaviour and plumage condition.
Given that plumage condition and feather colour are indicators of flamingo health status [41], social preferences [42], and reproductive quality [43], we considered measurement of bird plumage condition as a potentially easy-to-score welfare output for busy keepers to record. We assumed that if flamingos were struggling with spatial restriction and change to housing and husbandry, plumage condition and feather colouration may decline and birds would become paler. Therefore, we wished to investigate any correlation between performance of social behaviour, as well as maintenance behaviour and time spent inactive, on the proportion of birds expressing good plumage condition. Captive flamingos can show a complicated relationship with climate and weather variables [44,45], and therefore local weather data were collected to further define any effect on flamingo time activity budget (at the flock level) of husbandry and housing change due to the HO.
2. Materials and Methods
2.1. Study Population and Husbandry
Data were collected on the BZG’s Chilean flamingo flock (n = 36) between 5th December 2022 and 24th July 2023. This group of 17 male and 19 female adult birds are all flight restrained and reside in an open-topped enclosure. Under regular housing and husbandry, the flamingos have access to an open-topped paddock with a lake (c2400 m2) plus access to their indoor housing (Figure 1). The flamingos would usually share this enclosure with 13 red-breasted (Branta ruficollis) and two bar-headed (Answer indicus) geese. Under the HO, flamingos were confined to their already existing indoor house and attached yard (c130 m2) that was subsequently covered over. When the HO was lifted on 21 April 2023, the flamingos once again had access to their open-topped paddock and lake and were once again mixed with the geese. Husbandry routines by keepers were similar for both the HO and regular husbandry conditions (termed “R” for regular access). The flamingos’ morning husbandry routine under HO conditions involved cleaning the indoor pool and food troughs and then replenishing troughs with species-specific pelleted feed. In the outdoor covered yard, rubber matting was hosed down and the sand raked and turned. When the HO was lifted, food troughs were cleaned and replenished and placed in the covered yard instead of inside the house. Most husbandry duties took place between 09:00 and 10:30, for around 30 min. One further check would take place at the end of the working day.

Figure 1.
Photographs of the main flamingo enclosure (full access under non-AI conditions) showing loafing area, pool and indoor housing, (left), and smaller enclosure showing the indoor house and attached covered aviary used during the enforced period of housing (HO), (right).
2.2. Data Collection
The observational methods for this project were reviewed by conservation, animal management, and veterinary staff at Banham Zoo. No changes to husbandry and no direct manipulation of birds occurred; therefore, no ethical infringements to animal welfare would be likely from data collection. Flamingos were observed for a total of 57 days, with 33 days (149 observations) during the housing order (5 December 2022–17 April 2023) and 24 days (98 observations) when regular husbandry had returned (22 April 2023–24 July 2023). Of the period once the housing order had ended, 10 days (46 observations) were considered immediately after release (22 April–4 May) and 14 days (52 records) were considered as when the birds had settled back into their regular husbandry routine and enclosure access (5 June–24 July).
An ethogram of flamingo state behaviours, in part based on previous literature [44,46], was used to describe specific behaviours for data collection (Table 1). All data were recorded using the ZooMonitor app version 4.1 [47] available from https://zoomonitor.org/ (accessed on 1 December 2022) in person at the enclosure by the same person (TC). State behaviours, of the whole flock, were recorded using instantaneous scan sampling at 60 s time intervals. All birds could be observed in the main enclosure, or house and smaller enclosure, for all times of observation. Up to six 20 min observations could be conducted each day, which was divided into AM (09:30–11:00), noon (12:00–13:30), and PM (14:30–16:00) timeslots. Two observations could be conducted within each timeslot, but a full six daily observations were not consistently conducted due to data collection being completed around the regular work duties of the person collecting data (TC).

Table 1.
Ethogram of flamingo state behaviours used for behavioural categorisation during data collection of the whole flock.
Overall flamingo plumage condition was recorded before the start of each day’s first session of data collection. Using information from zookeepers and zoo records, as well as from experience of the authors in flamingo care, poor plumage was described as “dried or frayed feathers; wet, stained and/or dishevelled plumage overall”. Good plumage condition was described as “smooth, clean feathers that had been oiled and waterproofed; consistent and regular coverage of head, neck, and body”. Birds showing any patches of poorer plumage condition scored a 0. Birds displaying good plumage would each be scored 1. The total number of 0 and 1 scores were recorded for this first daily observation. Figure 2 provides an example of good quality (left) and poor quality (right) plumage condition. Normally, one photo was taken of the whole flock; however, if a smaller group of flamingos were in a different area of the enclosure, then another photo would be taken. All photographs were taken by the same observer (TC) from public areas of the enclosure at the boundary closest to where the flock was located (as can be seen in Figure 2).

Figure 2.
Examples of good (left) and poor (right) flamingo plumage condition and feather colour. Good plumage condition is well-maintained and oiled, with good feather integrity that enables feathers to be zipped together when preened. Poorer plumage condition is wet or matted, lacking in waterproofing, and dishevelled in appearance.
Photographic records of the birds were reviewed for plumage quality post-live-observation (for behavioural data collection) to ensure that the activity was recorded accurately and the observer could focus on one specific aspect of data collection at a time. Supplemental data on weather and temperature were collected from https://www.bbc.co.uk/ weather at the start of each session (accessed on 1 December 2022).
2.3. Data Analyses
All data were analysed in RStudio version 2023.06.0 [49] using R version 4.1.0 [50]. For the purposes of some inferential analyses, data were split into two basic conditions (HO for housing order and R for regular access). However, overall activity of the flock under the HO, immediately after release, and during regular enclosure access was compared descriptively on a month-by-month basis to illustrate any sudden alterations to the flamingos’ time budget after use of the entire enclosure was re-instated.
2.3.1. Behavioural Analysis
An average time-activity budget of the flock under HO and release conditions was constructed in Excel version 2405 to provide visual interpretation of the flock’s allocation of time and energy to specific behavioural states. Based on these descriptive statistics, the very low levels of feeding observation (an artefact of bird management and how birds were provided with their food) were not included in any further inferential analyses. The proportion of birds performing each behaviour during each day’s 20 min observation session was calculated for further analysis (i.e., the total number of occurrences of that behaviour in the flock divided by all occurrences).
To determine if the flock’s behaviour was influenced by the prevailing management style (housing order or regular husbandry), individual mixed-effect models were fitted using the “lmerTest” package [51] to each date and time slot’s proportion of behaviour seen being performed as the outcome variable. Management style, plumage condition (which may be influenced by the housing condition), season, environmental (temperature), and weather (cloud, rain, sun, or wind as defined from the BBC weather service) predictors were all included as fixed factors. Observation date acted as a random factor as repeated observations occurred on the same population of animals. Observation sessions were assigned to a time slot (A, B, C, D, E, or F) based on the morning, noon, or afternoon period they occurred in (e.g., slots A and B were in the morning timeslot). If only one observation took place in a timeslot, it was assigned to the first slot (A) if it started in the first half of the timeslot period (i.e., from 09:30) or the second slot (B) if it started in the second half (i.e., from 10:15). Likewise, time slot C and D related to the period between 12:00 and 13:30 and time slots E and F to the period of observation from 14:30 to 16:00.
Significant predictors for each behaviour were identified from Satterthwaite’s Type III analysis of variance, using the “anova(model name)” function, and from the summary of each model. Temperature was not identified as a significant predictor of behaviour and so was not included in any of the models. Multicollinearity between predictors was checked via calculated variance inflation factors (VIF) using the “VIF” function from the “car” package [52], and model fit was determined using the r2 value, calculated using the “MuMIn” package [53]. Any predictor with VIF > 2 would have been excluded from the model, but this was unnecessary as none met this criterion. The final model run was as follows: Behaviour ~ Season + Time of day + Management (i.e., housing order or released) + Proportion of birds with good plumage condition + Weather + (1|Date).
Relevant post hoc testing was conducted using the “lsmeans” package [54] to allow the identification of significant differences in the prevalence of specific behaviours between different levels of the predictors (e.g., HO vs Regular Access).
2.3.2. Plumage Condition Analysis
To understand any potential relationship between flock-wide performance of social behaviour, maintenance, and inactivity, and attainment of a good plumage condition, scatterplots where drawn in Minitab v21.4 [55]. These behaviours were selected based on their likely influence over plumage condition—either a direct impact (preening activity for the maintenance of feather quality), social behaviour that will be instigated by good plumage condition, or inactivity (that may suggest apathy or lethargy due to spatial restriction caused by a change in housing). We took the maximum occurrence of these behaviours per day, and we combined this with the average temperature and weather for each date that plumage condition was scored. This approach was chosen to capture the daily climatic conditions experienced by the flock overall, and maximum occurrence of behaviour was used to give those managing flamingos an indication of what the effect on plumage condition may be if birds were most often seen being social, being inactive, or performing maintenance activity. This descriptive analysis was then followed by a Poisson regression run in RStudio. For the first observation conducted for each date of data collection (i.e., at either 09:30 or at 10:15 depending on the availability of the data collector), the calculated proportion of birds performing maintenance, being inactive, and engaging in social behaviour were used to see if there was any effect on the number of birds recorded with good plumage condition in the flock. Season, the housing condition of the birds (HO or R), overall weather condition for that day, and the average temperature for that day were also included in the model. The overall weather condition for each day was removed from the final model due to high VIFs (>2). McFadden’s r2 value was calculated using the “pscl” package [56].
3. Results
A time-activity budget (Figure 3) shows that, under both housing conditions, inactivity is the commonest behavioural state observed in this flock of flamingos. Time spent feeding is very low in both conditions and likely reflects the husbandry of the birds (i.e., their feeding times occurring outside of the data collection period).
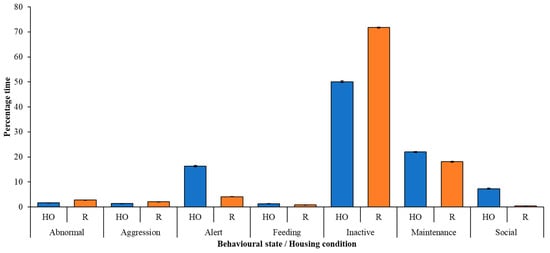
Figure 3.
Time-activity budget (mean ± standard deviation) of flamingos under restricted (HO) and regular access (R) conditions. Inactivity is the commonest behavioural state performed by these flamingos under each housing condition.
Figure 3 shows that inactivity increased in the flock post-release. Flamingos spent more time being alert and more time being social during the HO compared to R. The performance of abnormal behaviour is similar between the two conditions.
Figure 4 shows that flamingo behaviour varies across month and there is marked variation in the number of birds seen performing each behaviour by month, irrespective of the housing conditions the flamingos were in. Despite such variation, Figure 4 illustrates that feeding behaviour was performed at very low incidences during the HO and release conditions and that abnormal behaviours were highest in the post release period but remained higher during regular access when compared to the housing order. Similarly, maintenance behaviours and inactivity remain high each month, although inactivity shows more variation in the months during the HO compared to release and regular husbandry conditions. The months when flamingos were outside under regular enclosure access evidence much reduced occurrence of alert behaviour compared to the months of the HO.
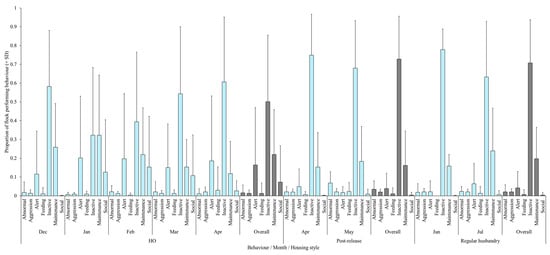
Figure 4.
Proportion of flock (+ standard deviation) displaying state behaviours during the months of housing order (HO), immediately upon release (post-release), and then after a period of normality (regular husbandry). Overall proportions of behavioural performance for the period of observation are also presented in grey.
Table 2 shows that abnormal behaviour was not predicted by any of the factors recorded during this study. Aggression was also not predicted by any of the factors that were recorded. Significant differences in the observation of flamingos being alert is noted for different housing conditions and at different times of day. Flamingos also show significant changes in inactivity and performance of maintenance behaviour according to weather, time of day, and season. The model estimate revealed an increase in the proportion of birds displaying social behaviour with good plumage condition (estimate = 0.087; SE = 0.04; df = 44.66; t value = 2.24; p < 0.030). Post hoc testing for categorical predictors identified as significant in Table 2 is outlined Table 3.

Table 2.
Model output for predictors of flock-wide behavioural states, with significant predictors indicated with an asterisk.

Table 3.
Significant outputs from post hoc testing from significant categorical predictors of state behaviours. Comparisons show the difference between the first categorial predictor against the second. A positive estimate indicates an increase in occurrence of the behaviour.
Table 3 shows that flamingos were significantly more alert in the first morning observation (time period A) period compared to all other time slots. Flamingos were significantly more alert during the HO compared to R conditions. Birds were more inactive in spring compared to winter, and generally less inactive (more active) in the morning. Rain significantly decreased inactivity. Flamingos performed significantly less maintenance behaviour in spring compared to winter and more maintenance in the later morning compared to other times of the day. Rain significantly increased maintenance behaviour.
Correlation between Behaviour and Plumage Condition
Plumage condition showed a relationship with performance of social behaviour, and flamingos spent most of their time preening and inactive (Figure 4); therefore, we further investigated such behavioural influences on attainment of good plumage quality as a potential welfare indicator for future use.
Figure 5 suggests that the number of flamingos displaying good plumage condition may increase when the performance of social behaviour increases. Inactive behaviour may also increase when the number of birds displaying good plumage increases. Maintenance behaviour seems to decrease as the number of birds with good plumage condition increases.
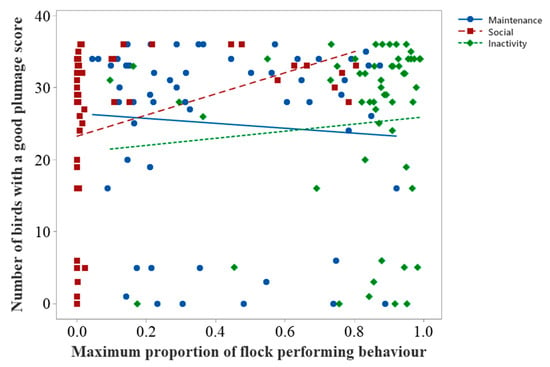
Figure 5.
Scatterplot of the number of birds counted with a good plumage score against the maximum proportion of the flock performing social, maintenance, and inactive behaviours. The trendlines are for visual aid only and are not associated with formal analysis.
Table 4 shows that inactive behaviour and social behaviour had a significant relationship with higher numbers of birds with good plumage. There was no relationship between good plumage and maintenance behaviour. More flamingos had better plumage condition when average daily temperature increased and were more often observed with good plumage in spring compared to other seasons. The AIC value for the final Poisson regression run was 723.16 and the r2 value was 8%.

Table 4.
Output from Poisson regression for variables that may impact on good plumage condition. Seasons spring and summer were compared to winter, and regular access (normal husbandry) was compared to housing order. p values for significant predictors of good plumage condition are highlighted with an asterisk.
4. Discussion
This research investigated the potential impacts of an AI-enforced change to housing and husbandry on the behaviour and plumage condition of a flock of Chilean flamingos in a UK zoo. Flamingos under HO conditions experienced an individual area of 3.6 m2 per bird compared to a potential 66.7 m2 per bird under regular husbandry and enclosure access. We identified that performance of abnormal, redirected foraging behaviour by these flamingos occurred both during HO and R husbandry conditions, and therefore the AI-enforced change to housing may not have been the proximate cause of such behaviour. Thus, further evaluation of husbandry (e.g., feeding times), housing (e.g., access to pools for foraging), and wider management regimes is required to understand the initial cause of this activity. Broader assessment of change to flock-wide plumage condition (i.e., the presence of poorer plumage condition at certain times of the year) is required to fully understand the impacts of restricted housing conditions on flamingo health and welfare. Limited access to bathing and wading water, as well as closer proximity of birds that increases competition over such resources, could result in poorer feather condition overall.
Further study of abnormal and/or redirected foraging activity in individual captive flamingos is required to understand causation and development and why it may continue even when birds are not experiencing enclosure and spatial restrictions. A persistent motivation to perform behaviour that is thwarted by barriers in the environment, e.g., a lack of access to resources or an environment that lacks suitable stimuli, can manifest in the performance of an abnormal behaviour [57]. Consequently, these flamingos may have been persistently searching for suitable habitat areas for filter feeding but, without access to them, performed the mechanical actions of this behaviour all the same. Future research could focus on enclosure use and occupancy of different “habitat” areas within an enclosure to determine where flamingos spend their time, if they avoid or are reluctant to use specific resources within the enclosure, and if this shows any relationship with performance of abnormal behaviour. Routine behavioural monitoring, and the identification of behaviours such ARBs that can be helpful welfare indicators, should be implemented as strategies to gather evidence on bird responses to HOs and to know when interventions (e.g., the provision of extra environmental enrichment or the management of the bird’s social environment) should be initiated to reduce stressors and enhance the attainment of more positive welfare states. Increased incidence of vigilance may be a more relevant behavioural measure of flamingo welfare, as an increase in the number of birds seen alert was significantly higher under HO conditions when compared to R (Figure 3, Table 2). We recommend research extensions into predictors of alarm in captive flamingos and measurement of how long birds take to relax and return to pre-alarm activities. Given that wild flamingo disturbance is measured by the degree of flock-wide vigilance and flight distance [58,59,60], future research should assess the degree to which individual flamingos respond to disturbance and how it impacts on other behaviours. Development of Qualitive Behavioural Assessment regimes that define welfare outcomes from body language and behavioural expression [61], which have been used for domestic poultry (Gallus gallus domesticus) [62], could also have potential use here.
Further research into the motivational states of the flamingos and what may be lacking from their enclosure is also required to understand why there is performance of abnormal behaviour in any area of this enclosure. Previous research on captive flamingos has identified high rates of inactivity [44] and differences between time allocated to active (higher in wild) and inactive (higher in captive) behaviours when wild and captive flocks are compared [63]. Given that this flock of birds would spend over 75% of its time inactive, alterations to husbandry (e.g., the inclusion of suitable environmental enrichment) and to the enclosure itself (e.g., by providing more wetland areas for filtering and foraging) could decrease the sedentary nature of these flamingos and improve positive behavioural diversity. Inactivity predisposes flamingos to poor foot condition [64], and therefore encouraging activity across different substrates and habitat areas is suggested.
Data on wild Chilean flamingos shows that resting is one of the more common behaviours that birds will display, but foraging and preening are the activities that most energy is expended upon [65]. Increased rates of inactivity post-release (regular access) may be explained by the flamingos experiencing reduced disturbance from animal care staff. This is evidenced by the decline in alert behaviours post-release compared to HO conditions. Flamingos may have been more comfortable, and more relaxed in their main enclosure because they have the option to remove themselves from the presence of keepers. The very low rates of feeding that we observed are likely explained by when flamingo pellet was provided and therefore when the flock chose to feed. Daily husbandry (including provision of pellets) occurred during the first observation period of data collection, and flamingos spent most of their time alert due to keeper presence. If birds were then feeding later in the day, at times when observations were not being conducted, this behaviour would not have been recorded. Crepuscular and nocturnal foraging is recorded in captive flamingos [66], and wild birds also regularly forage in the evening, overnight, and in the early morning [58,67]. Captive husbandry should consider such temporal rhythms when providing flamingo pellet to allow birds to forage when they choose. Extension of this research to record behaviour earlier in the morning and later into the evening may have provided a more complete picture of when these flamingos spent time on feeding.
Wild flamingos can spend up to 45% of their diurnal time activity budget foraging [68]; captive birds likely spend less time feeding because the bespoke pellet that zoos provide is energy-dense and is distributed in easy-to-access feeding site. Therefore, less time is required to collect enough food to be satiated. However, zoos should consider the potential health and welfare concerns of inactivity caused by a limited need to expend time on feeding and be creative with how food is distributed to enhance time spent on exploration and foraging. A wider distribution of flamingo pellet and more time to filter this food from larger feeding pools develops a more naturalistic time activity budget in captive flamingos [69]. Wild flamingos respond to rainfall as a stimulus for (amongst others) increased foraging due to more readily available food items [70]. As rain decreased inactivity (Table 3), the zoo should consider how flamingos access water bodies in their enclosure to promote natural foraging opportunities. The zoo could consider further enrichment in this enclosure, such as use of sprinklers or creating marshy areas, to reduce inactivity and promote active behaviours.
Flamingos were most alert in the morning when compared to other periods of observation, suggesting that birds were responding to the presence of animal care staff who would be provisioning the enclosure during the morning work period. In other species of captive bird, keeper presence affects individual behaviour patterns and enclosure usage [71]. And any “visitor effect” may be complicated by the animal’s responses to weather and climate [71,72], i.e., more visitors are likely to be at the zoo in better weather and the animals are more likely to be on display during better weather, which draws visitors to the enclosure. Therefore, further extension to this study should consider visitor presence around the enclosure as well as the time zookeepers spend within the enclosure and regular monitoring of climatic variables to further comprehend rates of vigilance as potential flamingo welfare indicators.
Although the average occurrence of social behaviour was higher under the HO compared to R (Figure 3), this was not significant, and closer examination of the observation of social behaviour across each month (Figure 4) reveals the variable occurrence of social behaviour across the different housing conditions. Incidence of social behaviour, particularly that associated with reproduction, may have become (more consistently) higher into the summer as captive Chilean flamingos appear to perform more successful breeding behaviour in better weather with less rainfall [73]. It is essential to interpret behavioural data (that provides inferences of welfare state) within the context of the individuals’ and population’s circumstances. For example, flamingos in June clearly would have eaten their flamingo pellet at some point during the day, yet this was not observed during the period of data collection (Figure 4). Therefore, if time spent feeding and foraging was an essential identifier of good welfare, for example, in the case of browsing herbivores where performance of stereotypic behaviours is directly linked to restricted browsing or grazing opportunities [74], any observation schedule for data collection would need to consider when the animal is going to be able to forage, when food is provided, and whether feeding behaviours can actually be observed from the observer’s position at the enclosure.
Although we have confidence in our statistical analyses, there is a limited r2 value for the abnormal behaviour model. This highlights the complexity of understanding stereotypic behaviour from observational data and suggests that other factors need to be measured to fully appreciate the causation of such abnormal behaviours in the zoo. More information on husbandry and management (e.g., lack of pool use, restrictive opportunities for foraging, range of substrates available) may provide further information on abnormal behaviour performance, including when and why it occurs.
Plumage condition may be an indicator of underlying motivational state, especially concerning social behaviour, and a potential driver of more diverse social interactions. This is an ecologically relevant assumption given that plumage colour drives social relationships and flock cohesion in flamingos [41,42,69,75]. An increased number of birds showing a good plumage condition seemed to significantly predict increased performance of social behaviour (Table 2). Although the difference in performance of social behaviour under the two housing conditions was not significant, it is evident from Figure 3 that more social behaviour was observed under HO than R, and this increase may be caused by the closer proximity of individual birds under more restrictive housing. Given that flamingos invest in plumage colour as an honest signal of individual quality via the consumption of carotenoid pigments [43], and that the brightness and intensity of this plumage colour is used for sexual selection and mate choice [41], it is probably unsurprising that good plumage condition is significantly linked to increased social behaviour performance. Thermoregulatory demands will be lessened when plumage condition is good and therefore flamingos will have more time to spend on non-maintenance activities, such as social interactions.
Flamingos that display brighter plumage colour can be more aggressive than paler conspecifics [69], and therefore may have more of an influence over social relationships or access to resources within a flock. However, it should be noted that rates of aggression within this flock of Chilean flamingos were very low and showed no difference between HO and R conditions, and aggression was not predicted by any of the variables measured. This is perhaps a comforting finding that suggests the restriction on space caused by the HO and confinement to the smaller aviary did not artificially inflate aggression between birds or competition over resources that could have resulted in more competition and antagonistic interactions. Further research should investigate plumage condition and feather colour as a welfare indicator for captive flamingos to help understand why plumage condition can become poor and how to ensure it remains consistently good. If flamingos with good plumage condition are more likely to experience positive affective states, e.g., positive arousal and valence from wider and more diverse social interactions [76], then husbandry and management should facilitate consistently good plumage condition.
Over-preening could be a response in captive birds when they are uncomfortable in their surroundings [77] and had we more time to collect data across the course of each day, we may have revealed more insight into whether plumage condition really does improve when maintenance behaviour declines (Figure 5) to strengthen the weak correlation we have noted from our observations. Therefore, future research should consider whether plumage condition is a reliable welfare indicator for captive flamingos. Potentially, when combined with data on social behaviour, on vigilance, and on inactivity (including movement around the enclosure and use of water bodies), plumage condition may reveal how well a flamingo is looking after itself under current husbandry conditions.
The limited r2 value for the Poisson regression suggests that there are other impacts on plumage condition that were not measured during this study, and they could explain the high variability in the relationship between inactivity and plumage condition. Further research should attempt to unpick and identify what these may be. Flamingos were more inactive in spring and summer, which is also when plumage improved, and consequently, there may be a spurious relationship with plumage condition and inactivity that is better explained by bird comfort and weather/climate variables improving feather condition. Perhaps there was less disturbance to flamingos post-release and therefore birds were able to maintain a better plumage condition under R conditions, as excessive preening or maintenance behaviour that could have resulted as coping mechanism when trying to calm down after disturbance experienced during HO. Consequently, this research highlights the complexities of assigning welfare states based on behavioural observations and physical attributes of non-domestic birds. Consistent data collection over a prolonged time that considers the individual animal’s responses, as well as those from the group overall, could help to clarify outputs and pinpoint important and repeatable welfare indicators for captive flamingos more clearly.
This project has shown that redirected foraging (filtering) behaviour provides a clue as to what flamingos wish to engage with in their enclosure (i.e., resources that provide foraging opportunities), even when not spatially restricted. Under a HO specifically, we show that rates of vigilance could be indication of discomfort when keepers are present and that plumage condition may correlate with important flock-wide activities such as social behaviours. From this research, we suggest that these flamingos need an environment that enhances active behaviours (e.g., feeding and foraging), reduces inactivity, and promotes positive behavioural diversity. As spatial restriction causes health problems in captive flamingos, such as pododermatitis [78], zoos should be mindful of heightened levels of inactivity and associated impacts on foot health, which may then restrict activity further. Environmental enrichment could be in the form of sanded areas for nest mound construction, saltwater pools for wading and filtering, and relevant forms of nutritional and occupational enrichment (when biosecure and suitable according to housing restrictions) to reduce time spent inactive in one location.
Finally, use of technology to collect behaviour and welfare information across a 24-h period would be useful for the interpretation of how flamingos respond to their husbandry and management under restricted housing conditions caused by AI. As observation and measurement of flamingo behaviour both indoors and outside is possible with remote cameras [79], such technology could increase the amount of data collected for further analyses. Extending research on these Chilean flamingos by using remote cameras to record activity across a wider time period of the day, and potentially overnight, would help unravel the causation of abnormal behaviour and provide more evidence for change to husbandry and enclosure layout that would enhance flamingo behavioural repertoires. Use of technology could provide greater consistency in data collection and enable flamingo behaviour to be collected during each allocated time period rather than solely relying on the availability of zoo research staff to collect data alongside of their usual required work activities. Such information could feed into guidelines for indoor spatial requirements for flamingos, as well as provide zoos with evidence-based measures on how much space is quality space for both indoor (and outdoor) housing.
5. Conclusions
This research has identified the challenges associated with interpreting abnormal repetitive behaviours and assigning causation to their performance. The apparent association between the HO and performance of abnormal (redirected foraging) activity was found to be untrue. For this flock of flamingos, time spent on social and inactive behaviours may be better indicators of welfare because of a link between them and bird plumage condition. Occurrence of alert behaviour provides a useful means of estimating how comfortable flamingos are in their immediate environment. The presence of good plumage condition links to behavioural performance and therefore provides a suitable route for further investigation to unpick the relationship between physical and behavioural indicators of bird welfare. Measurement of flamingo activity across all times of the day, and not just when a zoo is open to the public, would provide useful extra information on potential behavioural indicators of welfare state. This project provides valuable data on how zoo animals can respond to spatial restriction. In this case, mitigation should be considered to ensure that flamingos are not disturbed by human presence in a confined space. Measuring the time that flamingos take to return to a relaxed (non-vigilant) state after keeper presence would provide further information on behavioural causation. The low level of aggression observed suggests that this enclosed housing provided sufficient space for this flock of flamingos, enabling individuals to remove themselves from others. These data can be useful for other zoos moving forwards by providing evidence for minimum space per bird to promote positive social interactions and not causing undue aggression.
Author Contributions
Conceptualization, T.C. and P.R.; methodology, T.C. and P.R.; formal analysis, P.R.; resources, T.C.; data curation, T.C.; writing—original draft preparation, T.C and P.R.; writing—review and editing, T.C. and P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The observational methods for this project were reviewed by conservation, animal management and veterinary staff at Banham Zoo. No changes to husbandry and no direct manipulation of birds occurred; therefore, no ethical infringements to animal welfare occurred and permission to collect data was granted.
Data Availability Statement
Raw data are available upon reasonable request from the corresponding author.
Acknowledgments
Thanks to Sarah Lee for review and comments to the final manuscript and for facilitating the data collection for this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jensen, T.H.; Kuiken, T. Update on Avian Influenza virus. In Fowler’s Zoo and Wild Animal Medicine: Current Therapy; Miller, R.E., Calle, P.P., Lamberski, N., Eds.; Elsevier: St Louis, MI, USA, 2023; Volume 10, pp. 139–144. [Google Scholar]
- Redrobe, S.P. Avian influenza H5N1: A review of the current situation and relevance to zoos. Int. Zoo Yearb. 2007, 41, 96–109. [Google Scholar] [CrossRef]
- Swayne, D.E. Avian Influenza. Available online: https://www.msdvetmanual.com/poultry/avian-influenza/avian-influenza (accessed on 14 December 2023).
- Swayne, D.E.; Pantin-Jackwood, M. Pathogenicity of avian influenza viruses in poultry. Dev. Biol. 2006, 124, 61–67. [Google Scholar]
- Suarez, D.L. Evolution of avian influenza viruses. Vet. Microbiol. 2000, 74, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E. Impact of vaccines and vaccination on global control of avian influenza. Avian Dis. 2012, 56, 818–828. [Google Scholar] [CrossRef]
- Global Consortium for H5N8 Related Influenza Viruses. Role for migratory wild birds in the global spread of avian influenza H5N8. Science 2016, 354, 213–217. [Google Scholar] [CrossRef]
- White, K. Poultry Sector Backs Month-Long Bird Flu Prevention Zone. Available online: https://www.thegrocer.co.uk/eggs-and-poultry/poultry-sector-backs-month-long-bird-flu-prevention-zone/545830.article (accessed on 14 December 2023).
- Defra/APHA. Bird Flu (Avian Influenza): How to Prevent It and Stop It Spreading. Available online: https://www.gov.uk/guidance/bird-flu-avian-influenza-how-to-prevent-it-and-stop-it-spreading (accessed on 14 December 2023).
- Defra/APHA. Bird Flu—Latest Situation: Chief Vet Lifts Prevention Zone. Available online: https://www.gov.uk/government/news/bird-flu-latest-situation-avian-influenza-prevention-zone-declared-across-great-britain (accessed on 14 December 2023).
- East, I.J.; Roche, S.E.; Wicks, R.M.; de Witte, K.; Garner, M.G. Options for managing animal welfare on intensive pig farms confined by movement restrictions during an outbreak of foot and mouth disease. Prev. Vet. Med. 2014, 117, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Lay, D.C., Jr.; Fulton, R.M.; Hester, P.Y.; Karcher, D.M.; Kjaer, J.B.; Mench, J.A.; Mullens, B.A.; Newberry, R.C.; Nicol, C.J.; O’Sullivan, N.P. Hen welfare in different housing systems. Poult. Sci. 2011, 90, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.R. Issues of choice and control in the behaviour of a pair of captive polar bears (Ursus maritimus). Behav. Process. 2006, 73, 117–120. [Google Scholar] [CrossRef]
- Mason, G.J.; Burn, C. Behavioural restriction. In Animal Welfare; Appleby, M., Mench, J.A., Olsson, A., Hughes, B.O., Eds.; CAB International: Wallingford, UK, 2011; pp. 98–119. [Google Scholar]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Broom, D.M. Indicators of poor welfare. Br. Vet. J. 1986, 142, 524–526. [Google Scholar] [CrossRef]
- Jones, N.; Sherwen, S.L.; Robbins, R.; McLelland, D.J.; Whittaker, A.L. Welfare assessment tools in zoos: From theory to practice. Vet. Sci. 2022, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.E.; Riley, L.M. Conducting behavioural research in the zoo: A guide to ten important methods, concepts and theories. J. Zool. Bot. Gard. 2021, 2, 421–444. [Google Scholar] [CrossRef]
- Riley, L.M.; Díez-León, M.; Rose, P.E. Behavioural biology and zoo animal welfare: For the future. In The Behavioural Biology of Zoo Animals; Rose, P.E., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 285–292. [Google Scholar]
- Dawkins, M.S. Using behaviour to assess animal welfare. Anim. Welf. 2004, 13, S3–S7. [Google Scholar] [CrossRef]
- Rose, P.E. Identifying essential elements of good giraffe welfare—Can we use knowledge of a species’ fundamental needs to develop welfare-focussed husbandry? J. Zool. Bot. Gard. 2023, 4, 549–566. [Google Scholar] [CrossRef]
- Dawkins, M.S. Behaviour as a tool in the assessment of animal welfare. Zoology 2003, 106, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.M.; Kirkden, R.D. Welfare, stress, behaviour and pathophysiology. Vet. Pathophysiol. 2004, 2004, 337–369. [Google Scholar]
- Mason, G.J. Stereotypies and suffering. Behav. Process. 1991, 25, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Mellor, E.; Brilot, B.; Collins, S. Abnormal repetitive behaviours in captive birds: A Tinbergian review. Appl. Anim. Behav. Sci. 2018, 198, 109–120. [Google Scholar] [CrossRef]
- Rose, P.E.; Nash, S.M.; Riley, L.M. To pace or not to pace? A review of what abnormal repetitive behaviour tells us about zoo animal management. J. Vet. Behav. 2017, 20, 11–21. [Google Scholar] [CrossRef]
- Garner, J.P. Perseveration and stereotypy-systems-level insights from clinical psychology. In Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare; Mason, G., Rushen, J., Eds.; Cabi: Wallingford, UK, 2006; pp. 121–152. [Google Scholar]
- Levitt, T. Poultry Farmers Call for Birds to Be Kept Inside to Combat Bird Flu. Available online: https://www.theguardian.com/world/2022/oct/12/poultry-farmers-nationwide-housing-order-combat-bird-flu-uk (accessed on 14 December 2023).
- Van Reeth, K. Avian and swine influenza viruses: Our current understanding of the zoonotic risk. Vet. Res. 2007, 38, 243–260. [Google Scholar] [CrossRef]
- Marwell Zoo. Avian Infuenza: 2023 Update. Available online: https://www.marwell.org.uk/zoo-news/avian-infuenza-2023-update/ (accessed on 14 December 2023).
- Church, E. Paignton Zoo Still Shut with Birds in Quarantine as It Makes New Announcement. Available online: https://www.devonlive.com/news/devon-news/paignton-zoo-still-shut-birds-7569627 (accessed on 14 December 2023).
- Stallknecht, D.E. Ecology and epidemiology of avian influenza viruses in wild bird populations: Waterfowl, shorebirds, pelicans, cormorants, etc. Avian Dis. 2003, 47, 61–69. [Google Scholar]
- Shriner, S.A.; Root, J.J. A review of avian influenza A virus associations in synanthropic birds. Viruses 2020, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Webster, R.G. Avian influenza virus surveillance and wild birds: Past and present. Avian Dis. 2010, 54, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.E.; Croft, D.P.; Lee, R. A review of captive flamingo (Phoenicopteridae) welfare: A synthesis of current knowledge and future directions. Int. Zoo Yearb. 2014, 48, 139–155. [Google Scholar] [CrossRef]
- Buschschlüter, V. Bird Flu Kills Hundreds of Flamingos in Argentina. Available online: https://www.bbc.com/news/world-latin-america-67509697 (accessed on 14 December 2023).
- Rose, P.E.; Brereton, J.E.; Gardner, L. Developing flamingo husbandry practices through workshop communication. J. Zoo Aquar. Res. 2016, 4, 115–121. [Google Scholar] [CrossRef]
- Buckles, E.L. Chapter 28—Phoenicopteriformes. In Pathology of Wildlife and Zoo Animals; Terio, K.A., McAloose, D., St Leger, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 687–696. [Google Scholar]
- McConnell, H.; Brereton, J.; Rice, T.; Rose, P. Do Birds of a Feather Always Flock Together? Assessing Differences in Group and Individual Zoo Enclosure Usage by Comparing Commonly Available Methods. J. Zool. Bot. Gard. 2022, 3, 71–88. [Google Scholar] [CrossRef]
- Langan, J.N.; Chinnadurai, S.K. Animal welfare and birds. In Fowler’s Zoo and Wild Animal Medicine: Current Therapy; Miller, R.E., Calle, P.P., Lamberski, N., Eds.; Elsevier: St. Louis, FL, USA, 2023; pp. 279–286. [Google Scholar]
- Amat, J.A.; Garrido, A.; Rendón-Martos, M.; Portavia, F.; Rendón, M.A. Plumage coloration in greater flamingos Phoenicopterus roseus is affected by interactions between foraging site, body condition and sex. Ardeola 2022, 69, 219–229. [Google Scholar] [CrossRef]
- Freeman, H.D.; Valuska, A.J.; Taylor, R.R.; Ferrie, G.M.; Grand, A.P.; Leighty, K.A. Plumage variation and social partner choice in the greater flamingo (Phoenicopterus roseus). Zoo Biol. 2016, 35, 409–414. [Google Scholar] [CrossRef]
- Amat, J.A.; Rendón, M.A. Flamingo coloration and its significance. In Flamingos, Behavior, Biology, and Relationship with Humans; Anderson, M.J., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 77–95. [Google Scholar]
- Rose, P.E.; Brereton, J.E.; Croft, D.P. Measuring welfare in captive flamingos: Activity patterns and exhibit usage in zoo-housed birds. Appl. Anim. Behav. Sci. 2018, 205, 115–125. [Google Scholar] [CrossRef]
- Rose, P.E.; Badman-King, A.; Hurn, S.; Rice, T. Visitor presence and a changing soundscape, alongside environmental parameters, can predict enclosure usage in captive flamingos. Zoo Biol. 2021, 40, 363–375. [Google Scholar] [CrossRef]
- Kidd, P.; Ford, S.; Rose, P.E. Exploring the effect of the COVID-19 zoo closure period on flamingo behaviour and enclosure use at two institutions. Birds 2022, 3, 117–137. [Google Scholar] [CrossRef]
- Wark, J.D.; Cronin, K.A.; Niemann, T.; Shender, M.A.; Horrigan, A.; Kao, A.; Ross, M.R. Monitoring the behavior and habitat use of animals to enhance welfare using the ZooMonitor app. Anim. Behav. Cogn. 2019, 6, 158–167. [Google Scholar] [CrossRef]
- Rose, P.E.; Roper, A.; Banks, S.; Giorgio, C.; Timms, M.; Vaughan, P.; Hatch, S.; Halpin, S.; Thomas, J.; O’Brien, M. Evaluation of the time-activity budgets of captive ducks (Anatidae) compared to wild counterparts. Appl. Anim. Behav. Sci. 2022, 251, 105626. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. Available online: http://www.rstudio.com (accessed on 29 July 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Barton, K. Mu-Min-Model Interference. Available online: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (accessed on 1 July 2021).
- Lenth, R.; Lenth, M.R. Package ‘lsmeans’. Am. Stat. 2018, 34, 216–221. [Google Scholar] [CrossRef]
- Minitab 21. Minitab Statistical Software; Minitab: State College, PA, USA, 2023; Available online: www.minitab.com (accessed on 29 July 2022).
- Jackman, S. pscl: Classes and methods for R developed in the Political Science Computational Laboratory, New South Wales, Australia. R package version 1.5.5.1; United States Studies Centre, University of Sydney: Sydney, Australia, 2020; Available online: https://github.com/atahk/pscl/ (accessed on 29 July 2022).
- Greening, L. Stereotypies and other abnormal behavior in welfare assessment. In Encyclopedia of Animal Behavior, 2nd ed.; Choe, J.C., Ed.; Academic Press: London, UK, 2019; pp. 141–146. [Google Scholar]
- Beauchamp, G.; McNeil, R. Vigilance in greater flamingos foraging at night. Ethology 2003, 109, 511–520. [Google Scholar] [CrossRef]
- Beauchamp, G. Nonrandom patterns of vigilance in flocks of the greater flamingo, Phoenicopterus ruber ruber. Anim. Behav. 2006, 71, 593–598. [Google Scholar] [CrossRef]
- Yosef, R. Individual distances among greater flamingos as indicators of tourism pressure. Waterbirds 2000, 23, 26–31. [Google Scholar] [CrossRef]
- Wemelsfelder, F. How animals communicate quality of life: The qualitative assessment of behaviour. Anim. Welf. 2007, 16, 25–31. [Google Scholar] [CrossRef]
- Muri, K.; Stubsjøen, S.M.; Vasdal, G.; Moe, R.O.; Granquist, E.G. Associations between qualitative behaviour assessments and measures of leg health, fear and mortality in Norwegian broiler chicken flocks. Appl. Anim. Behav. Sci. 2019, 211, 47–53. [Google Scholar] [CrossRef]
- Brereton, J.E.; Rose, P.E. Comparing the behaviour of wild and captive flamingos: An evaluation of published data on time-activity budgets. Flamingo 2019, 2, 34–49. [Google Scholar]
- Wyss, F.S.; Wolf, P.; Wenker, C.J.; Hoby, S.; Schumacher, V.; Béchet, A.; Robert, N.; Liesegang, A. Comparison of plasma vitamin A and E, copper and zinc levels in free-ranging and captive greater flamingos (P hoenicopterus roseus) and their relation to pododermatitis. J. Anim. Physiol. Anim. Nutr. 2014, 98, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Delfino, H.C.; Carlos, C.J. Intra-annual variation in activity budgets of a wild Chilean flamingo (Phoenicopterus chilensis) population in Southern Brazil. Austral Ecol. 2022, 47, 971–982. [Google Scholar] [CrossRef]
- Rose, P.E.; Lloyd, I.; Brereton, J.E.; Croft, D.P. Patterns of nocturnal activity in captive greater flamingos. Zoo Biol. 2018, 37, 290–299. [Google Scholar] [CrossRef]
- Rendón-Martos, M.; Vargas, J.M.; Rendón, M.A.; Garrido, A.; Ramírez, J.M. Nocturnal movements of breeding greater flamingos in southern Spain. Waterbirds 2000, 23, 9–19. [Google Scholar] [CrossRef]
- Tindle, R.W.; Tupiza, A.; Blomberg, S.P.; Tindle, L.E. The biology of an isolated population of the American flamingo Phoenicopterus ruber in the Galapagos Islands. Galapagos Res. J. Sci. Conserv. Galapagos Islands 2014, 68, 15–27. [Google Scholar]
- Rose, P.E.; Soole, L. What influences aggression and foraging activity in social birds? Measuring individual, group and environmental characteristics. Ethology 2020, 126, 900–913. [Google Scholar] [CrossRef]
- Stevens, E.F. Flamingo breeding: The role of group displays. Zoo Biol. 1991, 10, 53–63. [Google Scholar] [CrossRef]
- Rose, P.E.; Scales, J.S.; Brereton, J.E. Why the “visitor effect” is complicated. Unraveling individual animal, visitor number, and climatic influences on behavior, space use and interactions with keepers- a case study on captive hornbills. Front. Vet. Sci. 2020, 7, 236. [Google Scholar] [CrossRef]
- Goodenough, A.E.; McDonald, K.; Moody, K.; Wheeler, C. Are “visitor effects” overestimated? Behaviour in captive lemurs is mainly driven by co-variation with time and weather. J. Zoo Aquar. Res. 2019, 7, 59–66. [Google Scholar] [CrossRef]
- Mooney, A.; Teare, J.A.; Staerk, J.; Smeele, S.Q.; Rose, P.E.; Edell, R.H.; King, C.E.; Conrad, L.; Buckley, Y.M. Flock size and structure influence reproductive success in four species of flamingo in 540 captive populations worldwide. Zoo Biol. 2023, 42, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.D.; Parker, M.O.; Proops, L.; McBride, S.D. Risk factors for stereotypic behaviour in captive ungulates. Proc. R. Soc. B 2022, 289, 20221311. [Google Scholar] [CrossRef] [PubMed]
- Loader, A.; Rose, P.E. Age-related change in the association choices of two species of juvenile flamingos. Animals 2023, 13, 2623. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J. Positive animal welfare states and encouraging environment-focused and animal-to-animal interactive behaviours. N. Z. Vet. J. 2015, 63, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Claydon, M.; Brereton, J.E.; Rose, P.E. Never be mute about bird welfare: Swanning around with environmental enrichment. Zoo Biol. 2023, 43, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Mooney, A.; McCall, K.; Bastow, S.; Rose, P.E. Changes in environment and management practices improve foot health in zoo-housed flamingos. Animals 2023, 13, 2483. [Google Scholar] [CrossRef]
- Rose, P.E.; Chapman, J.; Brereton, J.E.; Riley, L.M. What’s black and white and pink all over? Lesser flamingo nocturnal behaviour captured by remote cameras. J. Zool. Bot. Gard. 2022, 3, 624–640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).