Body Shape Analysis in Reticulated Giraffe, Okapi, and Black Rhinoceros Using Three-Dimensional Laser Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Instrument
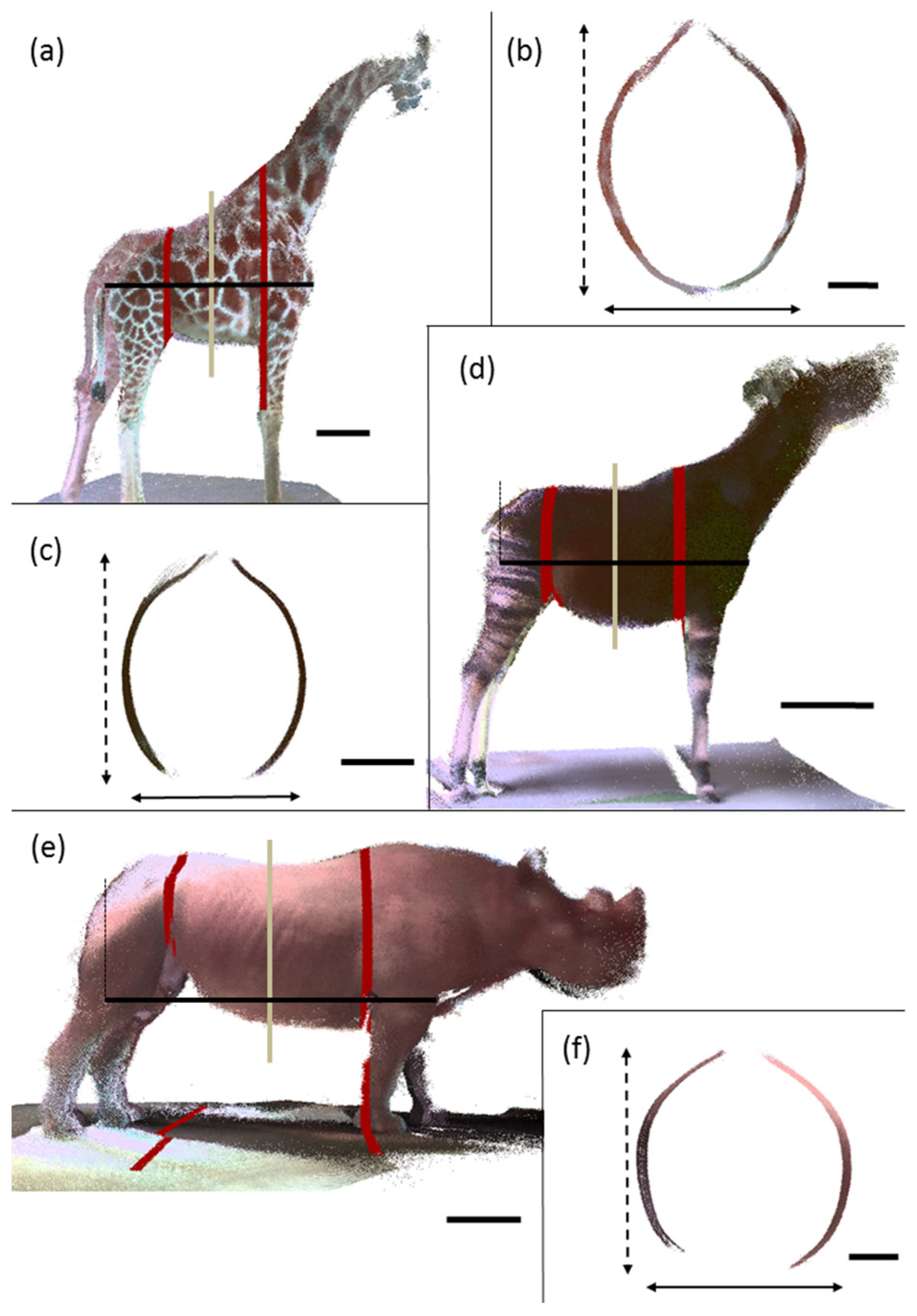
2.3. Measurement
3. Results
4. Discussion
5. Conclusions
6. Ethics
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chan-McLeod, A.C.A.; White, R.G.; Holleman, D.F. Effects of protein and energy intake, body condition, and season on nutrient partitioning and milk production in caribou and reindeer. Can. J. Zool. 1994, 72, 938–947. [Google Scholar] [CrossRef]
- Burkholder, W.J. Use of body condition scores in clinical assessment of the provision of optimal nutrition. J. Am. Vet. Med. Assoc. 2000, 217, 650–654. [Google Scholar] [CrossRef]
- Aeberhard, K.; Bruckmaier, R.M.; Kuepfer, U.; Blum, J.W. Milk Yield and Composition, Nutrition, Body Conformation Traits, Body Condition Scores, Fertility and Diseases in High-Yielding Dairy Cows—Part 1. J. Vet. Med. A 2001, 48, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Busato, A.; Faissler, D.; Küpfer, U.; Blum, J.W. Body condition scores in dairy cows: Associations with metabolic and endocrine changes in healthy dairy cows. J. Vet. Med. A 2002, 49, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.P.; Lee, J.M.; Macdonald, K.A.; Roche, J.R. Body Condition Score and Body Weight Effects on Dystocia and Stillbirths and Consequent Effects on Postcalving Performance. J. Dairy Sci. 2007, 90, 4201–4211. [Google Scholar] [CrossRef]
- Boudreau, L. Effect of Moderate Diet Restriction on Body Condition, Health, and Reproductive Performance in Female Mink. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, August 2012. [Google Scholar]
- Schiffmann, C.; Clauss, M.; Stefan Hoby, S.; Hatt, J.M. Visual body condition scoring in zoo animals—Composite, algorithm and overview approaches in captive Asian and African elephants. J. Zoo. Aquar. Res. 2017, 5, 1–10. [Google Scholar]
- Bray, R.E.; Edwards, M.S. Application of existing domestic animal condition scoring systems for captive (zoo) animals. In AZA Nutrition Advisory Group, Proceedings of the Fourth Conference on Zoo and Wildlife Nutrition, Lake Buena Vista, FL, USA, 2001; Edwards, M., Lisi, K.J., Schlegel, M.L., Bray, R.E., Eds.; AZA Nutrition Advisory Group: Gainesville, FL, USA, 2001; Available online: https://nagonline.net/wp-content/uploads/2014/02/Bray-BodyConditionScoring.pdf (accessed on 25 December 2023).
- Nutrition Advisory Group to the Association of Zoos and Aquariums. Body Condition Scoring Resource Center. Available online: https://nagonline.net/3877/body-condition-scoring/ (accessed on 23 December 2023).
- Wildman, E.E.; Jones, G.M.; Wagner, P.E.; Boman, R.L. A dairy cow body condition scoring system and its relationship to selected production characteristics. J. Dairy Sci. 1982, 65, 495–501. [Google Scholar] [CrossRef]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Ward, A.M.; Lintzenich, B.; Maslanka, M. Too much or too little of a good thing: Weight management from the zoo nutritionist’s perspective. In Proceedings of the American Association of Zoo Veterinarians; Fort Worth Zoological Park: Fort Worth, TX, USA, 1999; pp. 320–324. Available online: https://www.vin.com/apputil/content/defaultadv1.aspx?pId=26437&id=10027554 (accessed on 25 December 2023).
- Bray, R.E.; Edwards, M.S. Body condition scoring of captive (zoo) equids. In AZA Nutrition Advisory Group, Proceedings of the Third Conference on Zoo and Wildlife Nutrition, Columbus, OH, USA; AZA Nutrition Advisory Group: Columbus, OH, USA, 1999; Available online: https://nagonline.net/wp-content/uploads/2014/02/10_BRAY.pdf (accessed on 25 December 2023).
- Reuter, H.O.; Adcock, K. Standardised body condition scoring system for black rhinoceros (Diceros bicornis). Pachyderm 1998, 26, 116–121. [Google Scholar]
- Wemmer, C.; Krishnamurthy, V.; Shrestha, S.; Hayek, L.A.; Thant, M.; Nanjappa, K.A. Assessment of body condition in Asian elephants (Elephas maximus). Zoo. Biol. 2006, 25, 187–200. [Google Scholar] [CrossRef]
- Dierenfeld, E.; Fuller, L.; Meeks, K. Development of a standardized body condition score of cheetahs (Acinonyx jubatus). In AZA Nutrition Advisory Group, Proceedings of the 7th Conference on Zoo and Wildlife Nutrition, Knoxville, TN, USA; AZA Nutrition Advisory Group: Knoxville, TN, USA, 2007; Available online: https://nagonline.net/wp-content/uploads/2014/02/Dierenfeld-DEVELOPMENT-OF-A-STANDARDIZED-BODY-CONDITION-SCORE-FOR-CHEETAHS-ACINONYX-JUBATUS.pdf (accessed on 25 December 2023).
- Clauss, M.; Wilkins, T.; Hartley, A.; Hatt, J.M. Diet composition, food intake, body condition, and fecal consistency in captive tapirs (Tapirus spp.) in UK collections. Zoo. Biol. 2009, 28, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Fernando, P.; Janaka, H.K.; Ekanayaka, S.K.K.; Nishantha, H.G.; Pastorini, J. A simple method for assessing elephant body condition. Gajah 2009, 31, 29–31. [Google Scholar]
- Wright, D.J.; Omed, H.M.; Bishop, C.M.; Fidgett, A.L. Variations in Eastern bongo (Tragelaphus eurycerus isaaci) feeding practices in UK zoological collections. Zoo. Biol. 2011, 30, 149–164. [Google Scholar] [CrossRef]
- Morfeld, K.A.; Lehnhardt, J.; Alligood, C.; Bolling, J.; Brown, J.L. Development of a body condition scoring index for female African elephants validated by ultrasound measurements of subcutaneous fat. PLoS ONE 2014, 9, e93802. [Google Scholar] [CrossRef] [PubMed]
- Wijeyamohan, S.; Treiber, K.; Schmitt, D.; Santiapillai, C. A visual system for scoring body condition of Asian elephants (Elephas maximus). Zoo. Biol. 2014, 34, 53–59. [Google Scholar] [CrossRef]
- Cook, R.C.; Cook, J.G.; Murray, D.L.; Zager, P.; Johnson, B.K.; Gratson, M.W. Nutritional condition models for elk: Which are the most sensitive, accurate, and precise? J. Wildl. Manag. 2001, 65, 988–997. [Google Scholar] [CrossRef]
- Barthelmess, E.L.; Phillips, M.L.; Schuckers, M.E. The value of bioelectrical impedance analysis vs. condition indices in predicting body fat stores in North American porcupines (Erethizon dorsatum). Can. J. Zool. 2006, 84, 1712–1720. [Google Scholar] [CrossRef]
- Pitt, J.A.; Larivière, S.; Messier, F. Condition indices and bioelectrical impedance analysis to predict body condition of small carnivores. J. Mammal. 2006, 87, 717–722. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Jolles, A.E.; O’Brien, M.P. A reliable body condition scoring technique for estimating condition in African buffalo. Afr. J. Ecol. 2009, 47, 476–481. [Google Scholar] [CrossRef]
- Reppert, A.; Treiber, K.; Ward, A. Body condition scoring in cheetah (Acinonyx jubatus) advancements in methodology and visual tools for assessment. In AZA Nutrition Advisory Group, Proceedings of the 9th Conference on Zoo and Wildlife Nutrition, Kansas City, MO, USA, 2011; Ward, A., Coslik, A., Maslanka, M., Eds.; AZA Nutrition Advisory Group: Kansas City, MO, USA, 2011; Available online: https://nagonline.net/wp-content/uploads/2014/05/27_Reppert.pdf (accessed on 25 December 2023).
- Vosselman, G.; Gorte, B.G.H.; Sithole, G.; Rabbani, T. Recognising structure in laser scanning point clouds. In Proceedings of the International Society for Photogrammetry and Remote Sensing Working Group VIII/2: Laser Scanning for Forest and Landscape Assessment; Freiburg, Germany, 3–6 October 2004, Thies, M., Koch, B., Spiecker, H., Weinacher, H., Eds.; University of Freiburg: Freiburg im Breisgau, Germany, 2004; pp. 33–38. [Google Scholar]
- Kuzminsky, S.C.; Gardiner, M.S. Three-dimensional laser scanning: Potential uses for museum conservation and scientific research. J. Arch. Sci. 2012, 39, 2744–2751. [Google Scholar] [CrossRef]
- Ey-Chmielewska, H.; Chruściel-Nogalska, M.; Frączak, B. Photogrammetry and its potential application in medical science on the basis of selected literature. Adv. Clin. Exp. Med. 2015, 24, 737–741. [Google Scholar] [CrossRef]
- Klasen, M.; Steinhage, V. Wildlife 3D multi-object tracking. Ecol. Inform. 2022, 71, 101790. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kobayashi, T.; Nashimoto, M. Introducing a size measurement procedure for wildlife camera-trap by three dimensional modeling of shooting area. The report of central research institute of electric power industry. J-Global 2015, 11034, 15. (In Japanese) [Google Scholar]
- Shero, M.R.; Dale, J.; Seymour, A.C.; Hammill, M.O.; Mosnier, A.; Mongrain, S.; Johnston, D.W. Tracking wildlife energy dynamics with unoccupied aircraft systems and three-dimensional photogrammetry. Methods Ecol. Evol. 2021, 12, 2458–2472. [Google Scholar] [CrossRef]
- Bewley, J.M.; Peacock, A.M.; Lewis, O.; Boyce, R.E.; Roberts, D.J.; Coffey, M.P.; Kenyon, S.J.; Schutz, M.M. Potential for estimation of body condition scores in dairy cattle from digital images. J. Dairy Sci. 2008, 91, 3439–3453. [Google Scholar] [CrossRef]
- Azzaro, G.; Caccamo, M.; Ferguson, J.D.; Battiato, S.; Farinella, G.M.; Guarnera, G.C.; Puglisi, G.; Petriglieri, R.; Licitra, G. Objective estimation of body condition score by modelling cow body shape from digital images. J. Dairy Res. 2011, 94, 2126–2137. [Google Scholar]
- Fischer, A.; Luginbühl, T.; Delattre, L.; Delouard, J.M.; Faverdin, P. Rear shape in 3 dimensions summarized by principal component analysis is a good predictor of body condition score in Holstein dairy cows. J. Dairy Sci. 2015, 98, 4465–4476. [Google Scholar] [CrossRef]
- Gomes, R.A.; Monteiro, G.R.; Assis, G.J.F.; Busato, K.C.; Ladeira, M.M.; Chizzotti, M.L. Technical note: Estimating body weight and body composition of beef cattle trough digital image analysis. J. Anim. Sci. 2016, 94, 5414–5422. [Google Scholar] [CrossRef]
- Lynn, N.C.; Zin, T.T.; Kobayashi, I. Automatic assessing body condition score from digital images by active shape model and multiple regression technique. In Proceedings of the 2017 International Conference on Artificial Life and Robotics (ICAROB 2017), Miyazaki, Japan, 19–22 January 2017; Sugisaka, M., Ed.; ALife Robotics Corp. Ltd.: Oita, Japan, 2017; pp. 311–314. [Google Scholar]
- Kido, N.; Tanaka, S.; Omiya, T.; Wada, Y.; Shigenari, M.; Munakata, T.; Ogawa, M. Evaluation of somatotype in the reticulated giraffe (Giraffa camelopardalis reticulata) using three-dimensional laser measurement. J. Vet. Med. Sci. 2018, 80, 1528–1533. [Google Scholar] [CrossRef]
- Mantis Vision. F6 Handheld 3D Scanner. Available online: https://mantis-vision.com/handheld-3d-scanners/ (accessed on 23 December 2023).
- Kemper, K.E.; Visscher, P.M.; Goddard, M.E. Genetic architecture of body size in mammals. Genome Biol. 2012, 13, 244. [Google Scholar] [CrossRef]
- Schmidtmann, C.; Segelke, D.; Bennewitz, J.; Tetens, J.; Thaller, G. Genetic analysis of production traits and body size measurements and their relationships with metabolic diseases in German Holstein cattle. J. Dairy Sci. 2023, 106, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, H.; Jiang, Y.; Jiang, Y.; Zhang, F.; Liu, Y.; Shi, Y.; Ding, X.; Wang, C. A Single-Step Genome Wide Association Study on Body Size Traits Using Imputation-Based Whole-Genome Sequence Data in Yorkshire Pigs. Front. Genet. 2021, 12, 629049. [Google Scholar] [CrossRef]
- Fowler, M.E. Peracute mortality in captive giraffe. J. Am. Vet. Med. Assoc. 1978, 17, 1088–1093. [Google Scholar]
- Junge, R.E.; Bradley, T.A. Peracute mortality syndrome of giraffes. In Zoo and Wild Animal Medicine; Fowler, M.E., Ed.; Saunders Co.: Philadelphia, PA, USA, 1993; Volume 3, pp. 547–549. [Google Scholar]
- Potter, J.S.; Clauss, M. Mortality of captive giraffe (Giraffa camelopardalis) associated with serous fat atrophy: A review of five cases at Auckland zoo. J. Zoo. Wildl. Med. 2005, 36, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Rose, P.; Hummel, J.; Hatt, J.-M. Serous fat atrophy and other nutrition-related health problems in captive giraffe—An evaluation of 83 necropsy reports. In Proceedings of the European Association of Zoo and Wildlife Veterinarians, Budapest, Hungary, 24–28 May 2006; Volume 6, pp. 233–235. [Google Scholar]
- Bertelsen, M.F. Giraffidae. In Fowler’s Zoo and Wild Animal Medicine; Miller, R.E., Fowler, M.E., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2014; Volume 8, pp. 602–610. [Google Scholar]
- Glatston, A.R.; Smit, S. Analysis of the urine of the okapi (Okapia johnstoni). Acta Zool. Pathol. Antverp. 1980, 75, 49–58. [Google Scholar]
- Haenichen, T.; Wisser, J.; Wanke, R. Chronic tubulointerstitial nephropathy in six okapis (Okapia johnstoni). J. Zoo. Wildl. Med. 2001, 32, 459–464. [Google Scholar]
- Fleming, G.J.; Citino, S.B.; Petric, A. Glucosuria in captive okapi (Okapia johnstoni). J. Zoo. Wildl. Med. 2006, 37, 472–476. [Google Scholar] [CrossRef]
- Clauss, M. Evaluation of Okapi (Okapia johnstoni) necropsy reports and studbook data as part of the EAZWV summer school. In Proceedings of the European Association of Zoo and Wildlife Veterinarians, Leipzig, Germany, 30 April–4 May 2008; pp. 323–327. [Google Scholar]
- Vercammen, F.; Stas, L.; Bauwens, L.; De Deken, R.; Brandt, J. Long-term assessment of glucosuria in captive okapi (Okapia johnstoni) after a dietary change. J. Zoo. Wildl. Med. 2014, 45, 632–634. [Google Scholar] [CrossRef]
- Molenaar, F.M.; Sainsbury, A.W.; Waters, M.; Amin, R. High serum concentrations of iron, transferrin saturation and gamma glutamyl transferase in captive black rhinoceroses (Diceros bicornis). Vet. Rec. 2008, 162, 716–721. [Google Scholar] [CrossRef]
- Clauss, M.; Dierenfeld, E.; Goff, J.; Klasing, K.; Koutsos, L.; Lavin, S.; Livingston, S.; Nielson, B.; Schlegel, M.; Sullivan, K.; et al. IOD in rhinos—Nutrition group report: Report from the nutrition working group of the international workshop on iron overload disorder in browsing rhinoceros (February 2011). J. Zoo. Wildl. Med. 2012, 43, S108–S113. [Google Scholar] [CrossRef] [PubMed]
- Olias, P.; Mundhenk, L.; Bothe, M.; Ochs, A.; Gruber, A.D.; Klopfleisch, R. Iron overload syndrome in the black rhinoceros (Diceros bicornis): Microscopical lesions and comparison with other rhinoceros species. J. Comp. Pathol. 2012, 147, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.E.; Valdes, E.V. Update on rhinoceros nutrition. In Fowler’s Zoo and Wild Animal Medicine; Miller, R.E., Lamberski, N., Calle, P.P., Eds.; Elsevier: St. Louis, MO, USA, 2018; Volume 9, pp. 699–706. [Google Scholar]
- Dierenfeld, E.S.; Atkinson, S.; Craig, A.M.; Walker, K.C.; Streich, W.J.; Clauss, M. Mineral concentrations in serum/plasma and liver tissue of captive and free-ranging rhinoceros species. Zoo. Biol. 2005, 24, 51–72. [Google Scholar] [CrossRef]
- Clauss, M.; Dierenfeld, E.S.; Bigley, K.E.; Wang, Y.; Ghebremeskel, K.; Hatt, J.M.; Flach, E.J.; Behlert, O.; Castell, J.C.; Streich, W.J.; et al. Fatty acid status in captive and free-ranging black rhinoceros (Diceros bicornis). J. Anim. Physiol. Anim. Nutr. 2008, 92, 231–241. [Google Scholar] [CrossRef]


| Animal | No. | Sex | Age | Place | Note |
|---|---|---|---|---|---|
| Reticulated giraffe | 1 | female | 14 years | Kanazawa | |
| 2 | female | 13 years | Kanazawa | ||
| 3 | male | 3 years | Kanazawa | ||
| 4 | male | 6 years | Nogeyama | ||
| 5 | female | 4 years | Nogeyama | ||
| 6 | female | 6 years | Yokohama | Pregnant 6 months before | |
| 7 | female | 5 years | Yokohama | ||
| Okapi | 1 | male | 22 years | Kanazawa | |
| 2 | male | 17 years | Yokohama | ||
| 3 | female | 18 years | Yokohama | ||
| 4 | female | 15 years | Yokohama | ||
| 5 | female | 4 years | Yokohama | ||
| Black rhinoceros | 1 | male | 30 years | Kanazawa | |
| 2 | female | 30 years | Kanazawa | ||
| 3 | female | 6 years | Yokohama |
| Individual Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Average | S.D. |
|---|---|---|---|---|---|---|---|---|---|
| Reticulated giraffe | |||||||||
| Body length (cm) | 176.5 | 136.4 | 132.3 | 164.8 | 139.4 | 134.9 | 156.5 | 148.7 | 17.26529 |
| Approximate volume (cm3) | 396,562.2 | 287,961.2 | 145,747.4 | 355,944.6 | 149,166.2 | 248,185.5 | 251,600.9 | 262,166.9 | 95001.3 |
| Okapi | |||||||||
| Body length (cm) | 121.9 | 124.6 | 128.9 | 121.9 | 112.2 | 121.9 | 6.107367 | ||
| Approximate volume (cm3) | 125,402.5 | 130,615.7 | 141,742.4 | 136,588.1 | 116,640.3 | 130,197.8 | 9760.724 | ||
| Black rhinoceros | |||||||||
| Body length (cm) | 197.9 | 195.2 | 185.1 | 192.7 | 6.751363 | ||||
| Approximate volume (cm3) | 604,861.8 | 621,407.6 | 470,810.5 | 565,693.3 | 82586.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kido, N.; Tanaka, S.; Wada, Y.; Oura, A.; Ochiai, E.; Morita, N.; Kawaguchi, Y.; Itabashi, M.; Munakata, T. Body Shape Analysis in Reticulated Giraffe, Okapi, and Black Rhinoceros Using Three-Dimensional Laser Measurements. J. Zool. Bot. Gard. 2024, 5, 80-89. https://doi.org/10.3390/jzbg5010006
Kido N, Tanaka S, Wada Y, Oura A, Ochiai E, Morita N, Kawaguchi Y, Itabashi M, Munakata T. Body Shape Analysis in Reticulated Giraffe, Okapi, and Black Rhinoceros Using Three-Dimensional Laser Measurements. Journal of Zoological and Botanical Gardens. 2024; 5(1):80-89. https://doi.org/10.3390/jzbg5010006
Chicago/Turabian StyleKido, Nobuhide, Sohei Tanaka, Yuko Wada, Atsushi Oura, Emi Ochiai, Natsumi Morita, Yoshiya Kawaguchi, Masanori Itabashi, and Takanori Munakata. 2024. "Body Shape Analysis in Reticulated Giraffe, Okapi, and Black Rhinoceros Using Three-Dimensional Laser Measurements" Journal of Zoological and Botanical Gardens 5, no. 1: 80-89. https://doi.org/10.3390/jzbg5010006
APA StyleKido, N., Tanaka, S., Wada, Y., Oura, A., Ochiai, E., Morita, N., Kawaguchi, Y., Itabashi, M., & Munakata, T. (2024). Body Shape Analysis in Reticulated Giraffe, Okapi, and Black Rhinoceros Using Three-Dimensional Laser Measurements. Journal of Zoological and Botanical Gardens, 5(1), 80-89. https://doi.org/10.3390/jzbg5010006






