The Impacts of Evening Events in Zoos: A Christmas Event at Knowsley Safari
Abstract
1. Introduction
- (1)
- Did the study species display differences in behaviour during event hours (16:00–20:00) on days when the event was running compared to days when the event was not running?
- (2)
- Did the study species display differences in behaviour within a 12 h period (12:00–00:00) on a selection of days throughout the study period?
- (3)
- Did the study species display differences in behaviour within that 12 h period on days when the event was running compared to days when the event was not running?
2. Materials and Methods
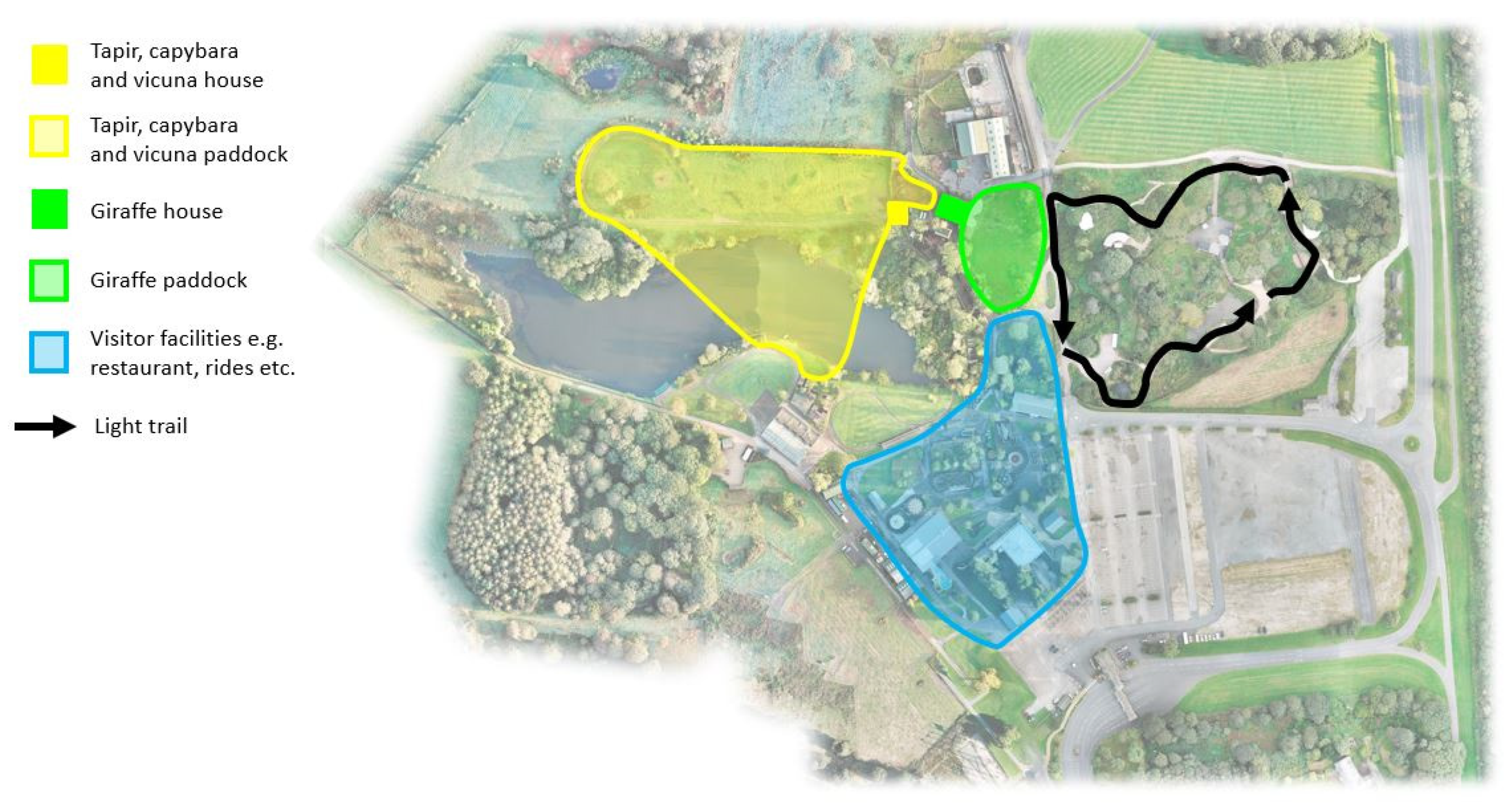
2.1. Study Site and Subjects
2.2. Data Collection
2.3. Data Analysis
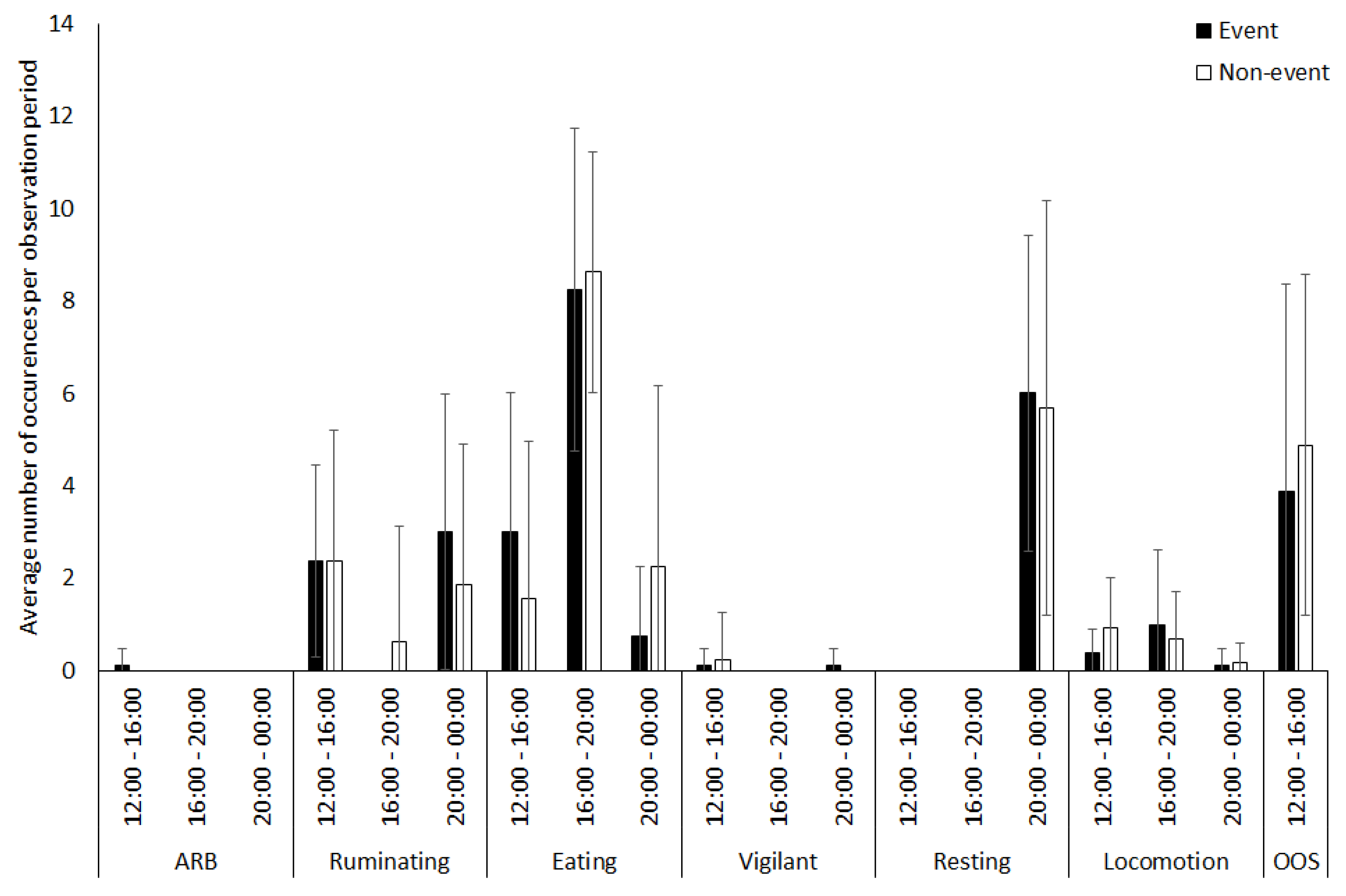
3. Results
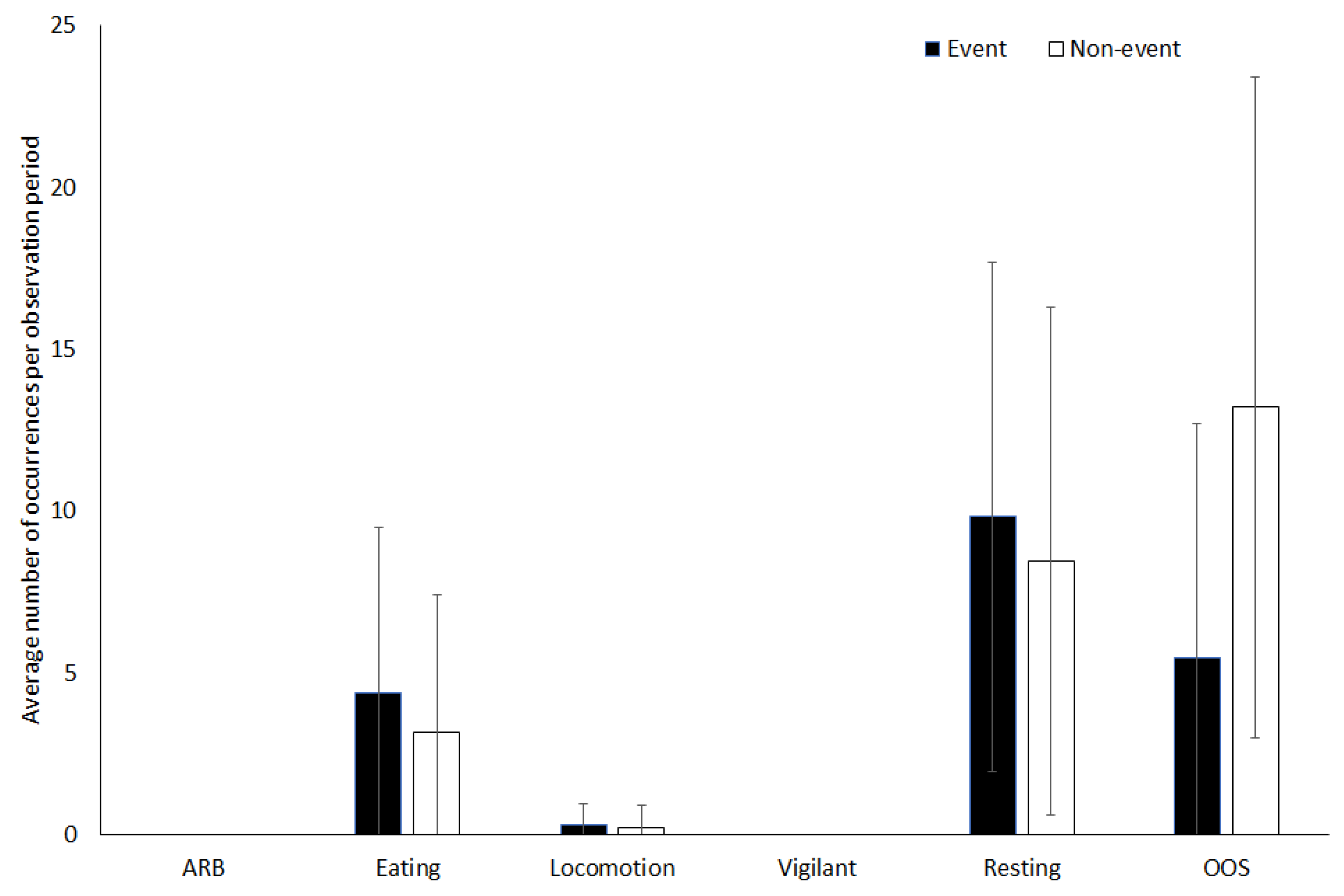
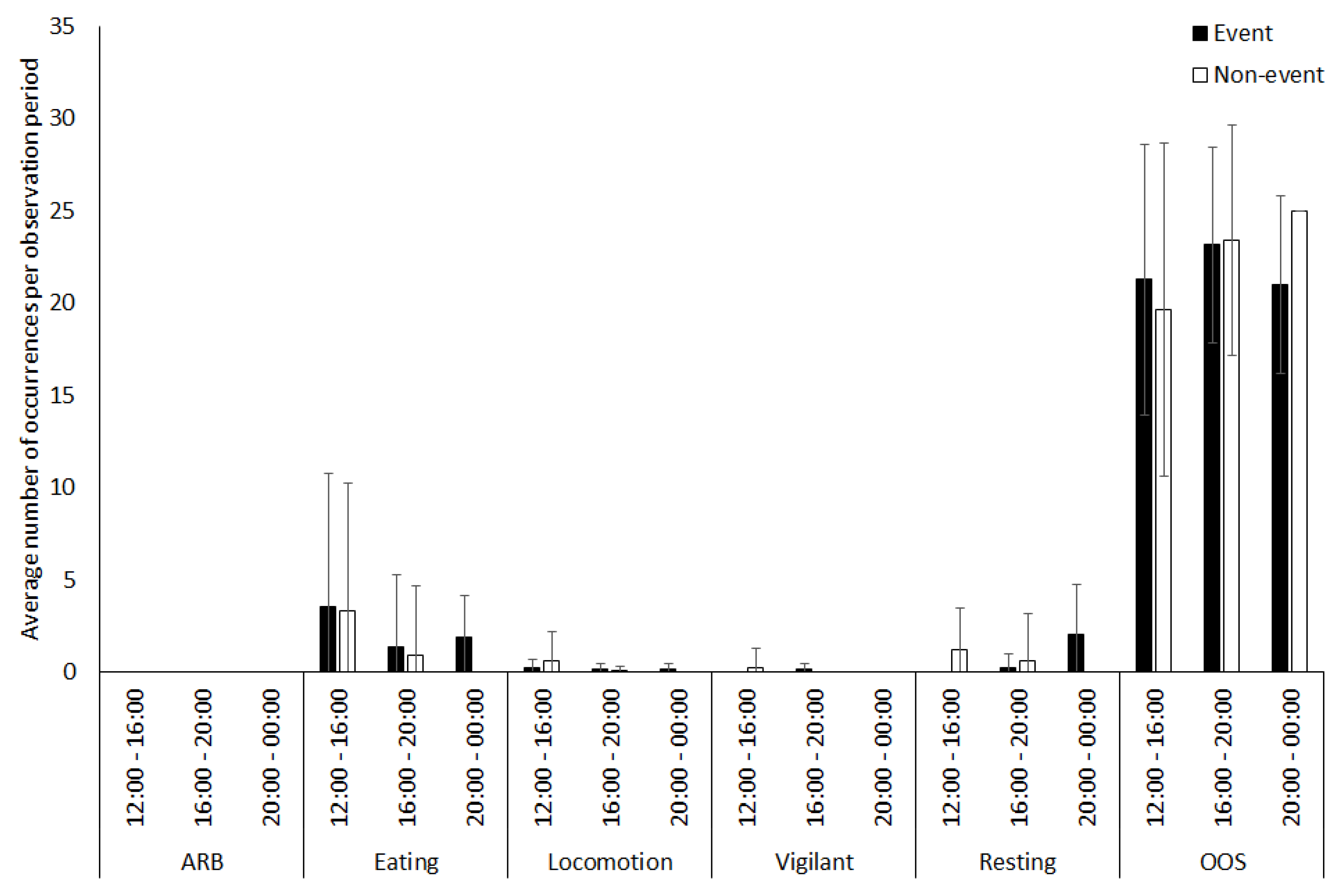
3.1. Capybara
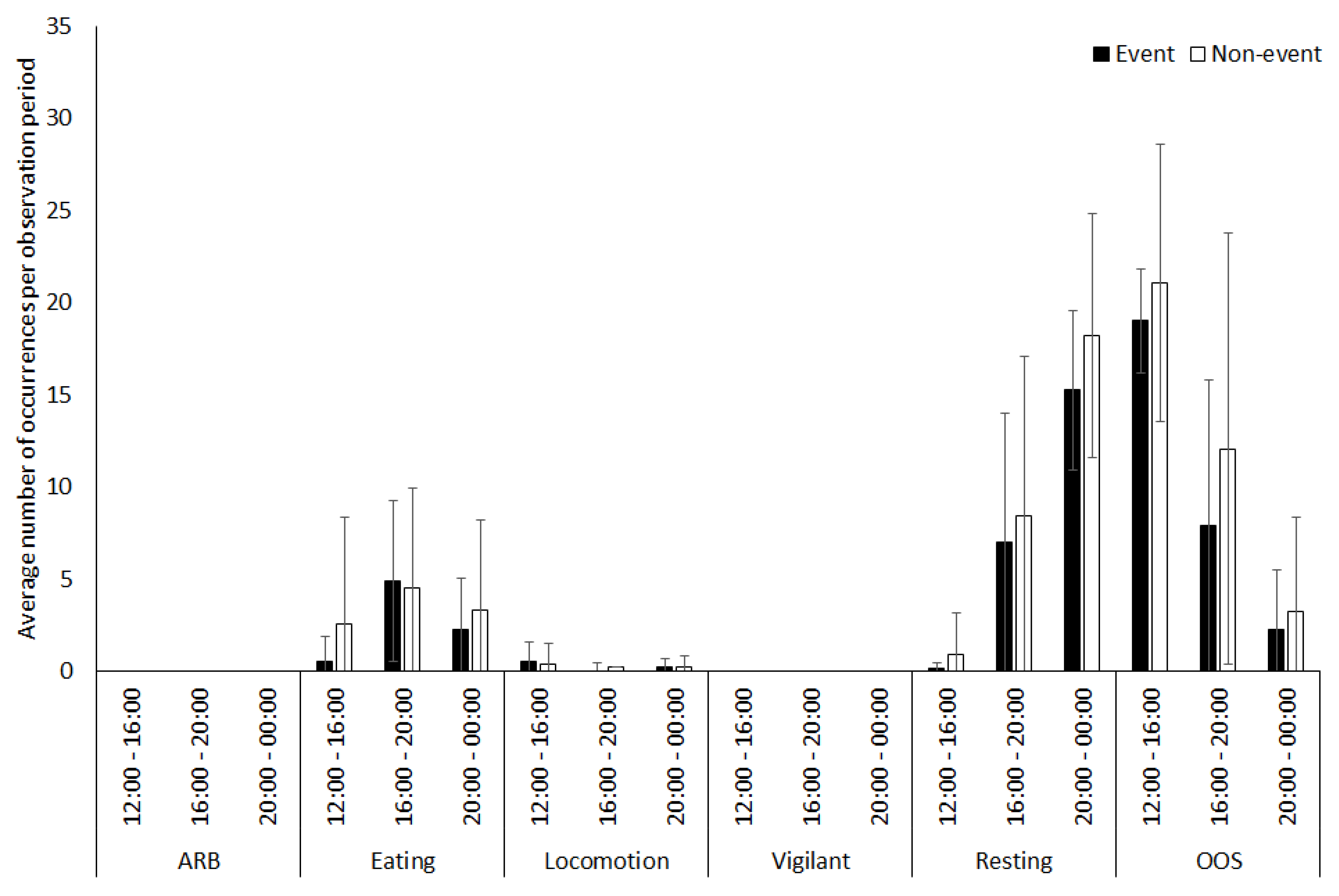
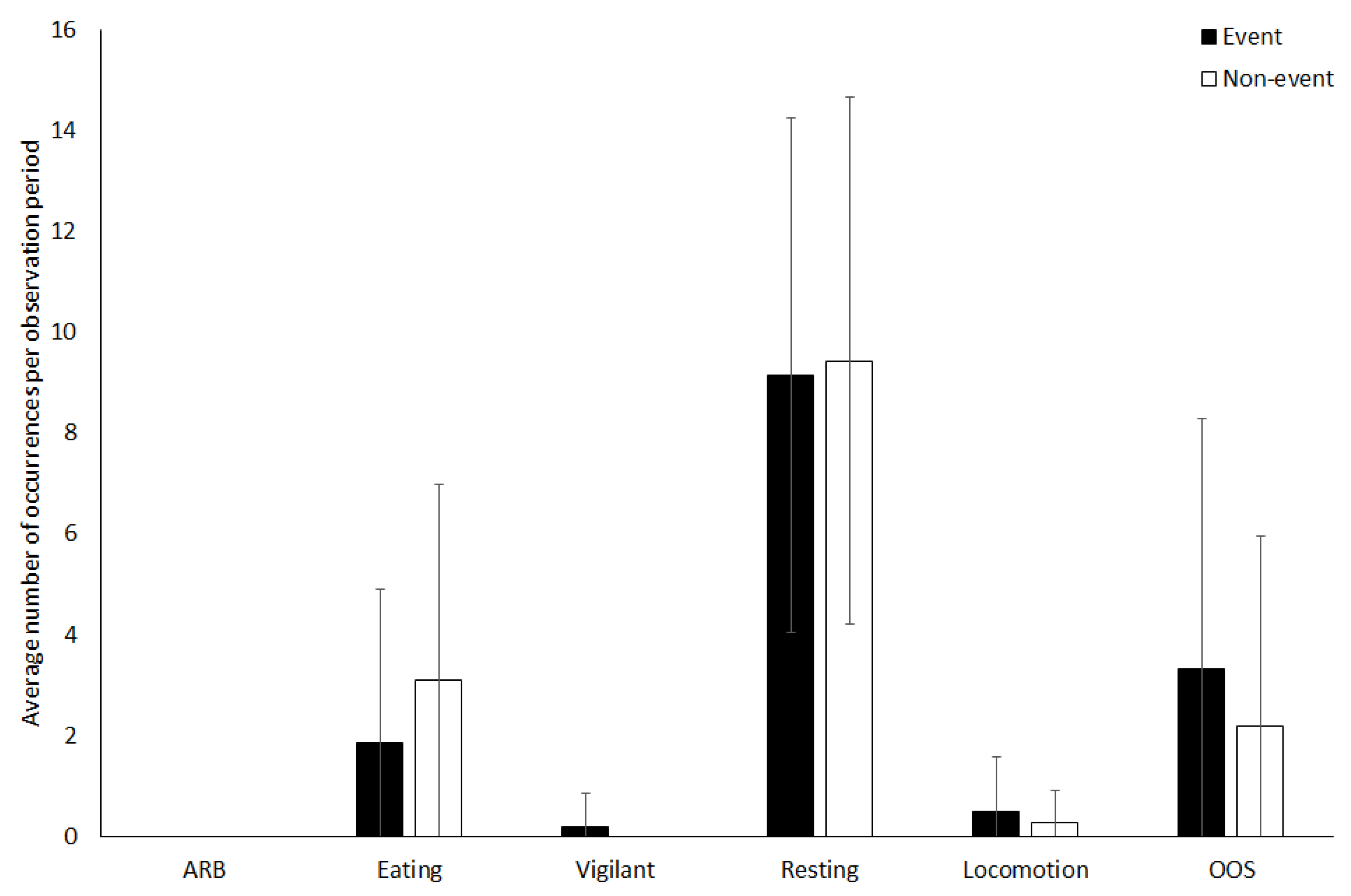
3.2. Vicuna
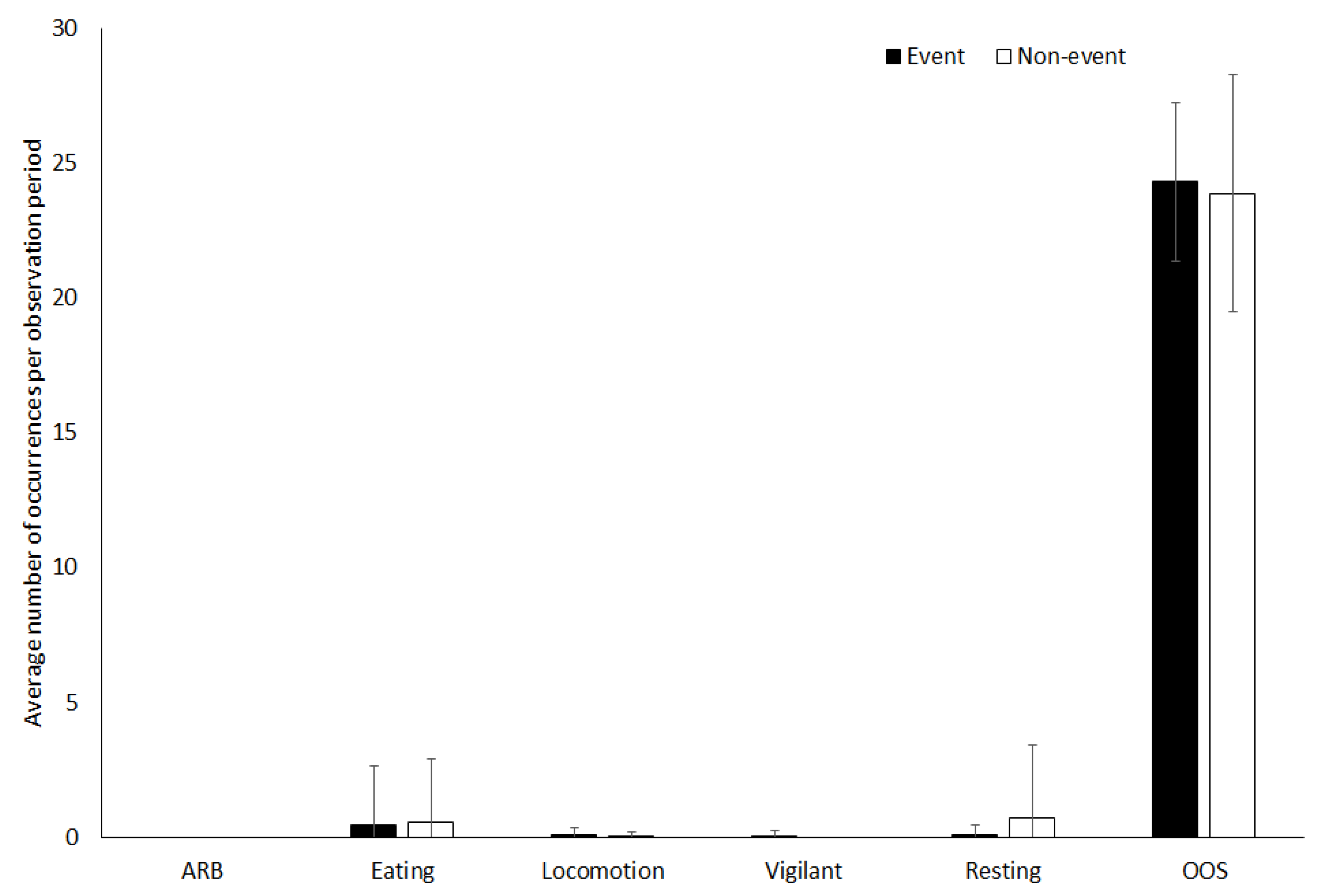
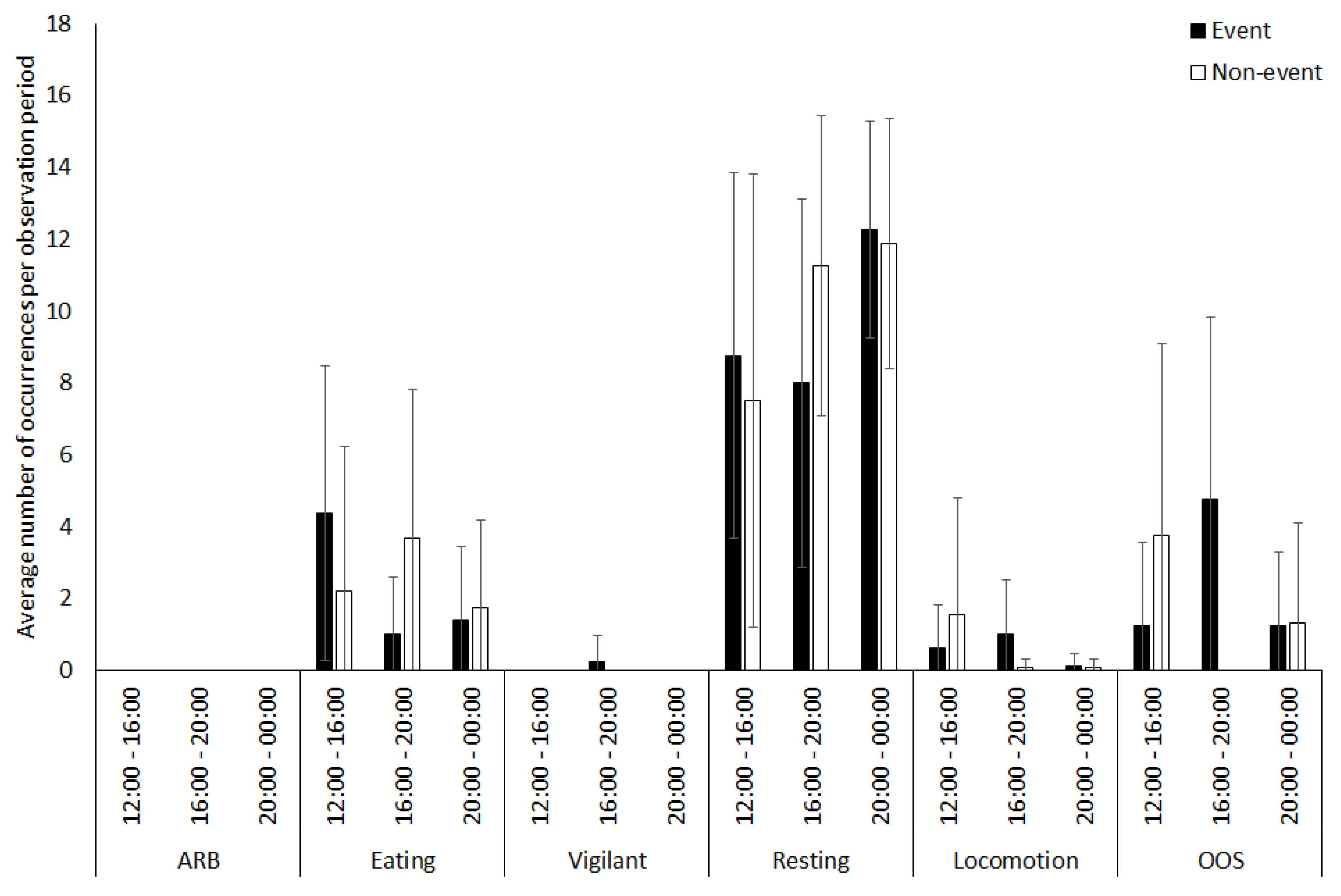
3.3. Tapir
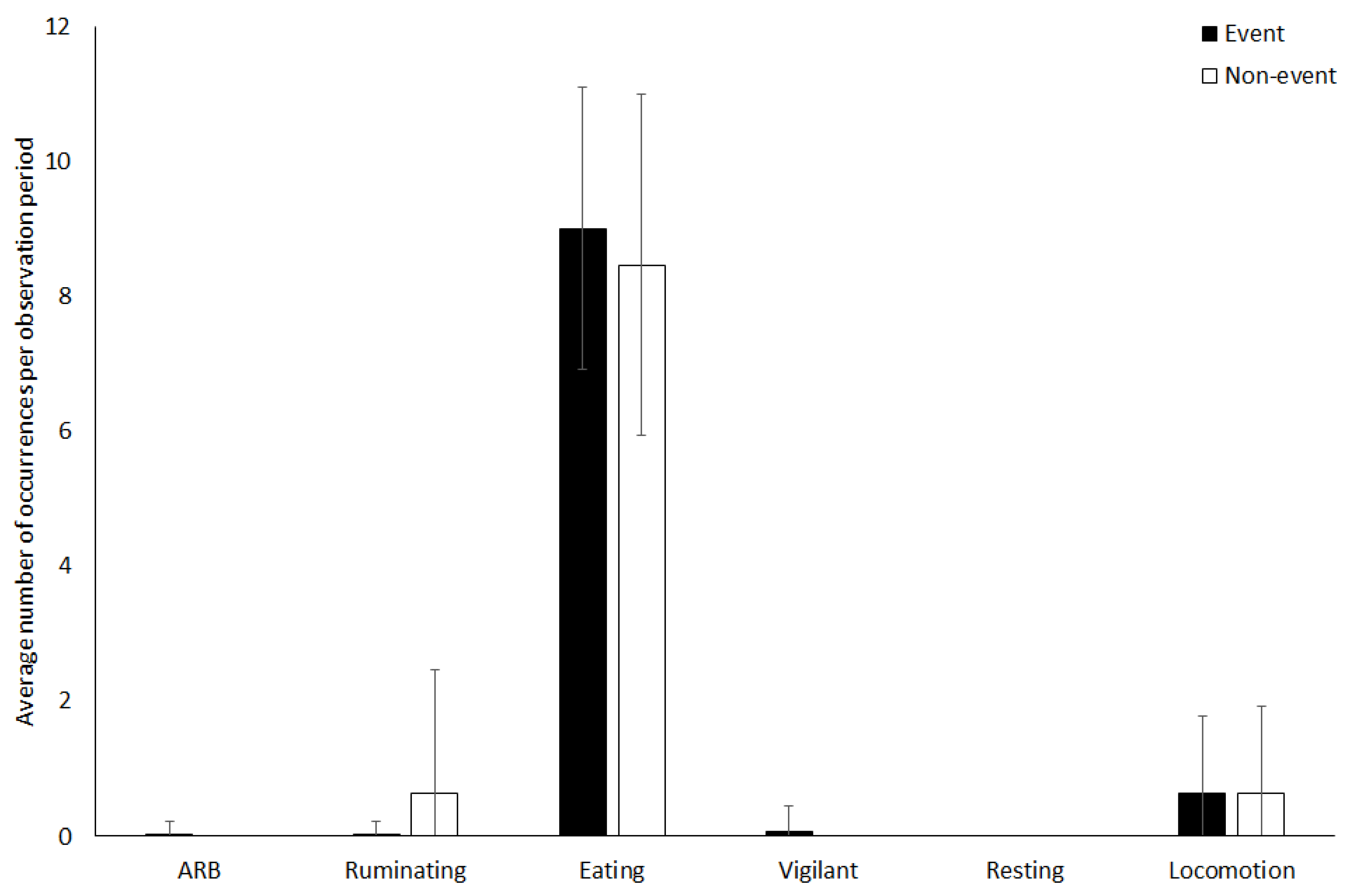
3.4. Giraffe
4. Discussion
4.1. Behavioural Changes
4.1.1. Capybara
4.1.2. Vicuna
4.1.3. Tapir
4.1.4. Giraffe
4.2. Limitations and Areas for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melfi, V. There Are Big Gaps in Our Knowledge, and Thus Approach, to Zoo Animal Welfare: A Case for Evidence-based Zoo Animal Management. Zoo Biol. 2009, 28, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Davey, G. Visitors’ Effects on the Welfare of Animals in the Zoo: A Review. J. Appl. Anim. Welf. Sci. 2007, 10, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Hosey, G.; Melfi, V.; Formella, I.; Ward, S.J.; Tokarski, M.; Brunger, D.; Brice, S.; Hill, S.P. Is Wounding Aggression in Zoo-Housed Chimpanzees and Ring-Tailed Lemurs Related to Zoo Visitor Numbers? Zoo Biol. 2016, 35, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Meade, J.; Formella, I.; Melfi, V. A Note on the Effect of Concerts on the Behaviour of Domestic Dogs (Canis lupus familiaris) at Taronga Zoo, Sydney. Int. Zoo Yearb. 2016, 51, 225–231. [Google Scholar] [CrossRef]
- Davey, G. An Hourly Variation in Zoo Visitor Interest: Measurement and Significance for Animal Welfare Research. J. Appl. Anim. Welf. Sci. 2006, 9, 249–256. [Google Scholar] [CrossRef]
- Harley, J.; Rowden, L.; Clifforde, L.; Power, A.; Stanley, C. Preliminary Investigation of the Effects of a Concert on the Behavior of Zoo Animals. Zoo Biol. 2022, 41, 308–327. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S. Assessment of Welfare in Zoo Animals: Towards Optimum Quality of Life. Animals 2018, 8, 110. [Google Scholar] [CrossRef]
- Morgan, K.N.; Tromborg, C.T. Sources of Stress in Captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Schell, C.J.; Young, J.K.; Lonsdorf, E.V.; Santymire, R.M. Anthropogenic and Physiologically Induced Stress Responses in Captive Coyotes. J. Mammal. 2013, 94, 1131–1140. [Google Scholar] [CrossRef][Green Version]
- Rodewald, A.; Ganslosser, U.; Koelpin, T. Influence of Fireworks on Zoo Animals: Studying Different Species at the Zoopark Erfurt during the Classic Nights. Int. Zoo News 2014, 61, 264–271. [Google Scholar]
- Readyhough, T.; Joseph, S.; Vyas, K.; Schreier, A. The Effects of Zoo Lights on Animal Welfare: A Case Study of Great Indian Hornbills at Denver Zoo. Zoo Biol. 2022, 41, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.B.; Young, R.J. The Different Physical and Behavioural Characteristics of Zoo Mammals That Influence Their Response to Visitors. Animals 2018, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Edes, A.N.; Liu, N.C.; Baskir, E.; Bauman, K.L.; Kozlowski, C.P.; Clawitter, H.L.; Powell, D.M. Comparing Space Use and Fecal Glucocorticoid Concentrations during and after the COVID-19 Closure to Investigate Visitor Effects in Multiple Species. J. Zool. Bot. Gard. 2022, 3, 328–348. [Google Scholar] [CrossRef]
- Boultwood, J.; O’Brien, M.; Rose, P. Bold Frogs or Shy Toads? How Did the COVID-19 Closure of Zoological Organisations Affect Amphibian Activity? Animals 2021, 11, 1982. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Rendle, J.; Fontani, S.; Walsh, N.D.; Armstrong, S.; Hickman, S.; Vaglio, S.; Ward, S.J. The Impact of COVID-19 Zoo Closures on Behavioural and Physiological Parameters of Welfare in Primates. Animals 2022, 12, 1622. [Google Scholar] [CrossRef]
- Cronin, K.A.; Bethell, E.J.; Jacobson, S.L.; Egelkamp, C.; Hopper, L.M. Evaluating Mood Changes in Response to Anthropogenic Noise with a Response-Slowing Task in Three Species of Zoo-Housed Primates. Anim. Behav. Cogn. 2018, 5, 209–221. [Google Scholar] [CrossRef]
- Hunton, V. Effects of Evening Zoo Events on the Behaviour and Faecal Consistency of Brown Spider Monkeys (Ateles Hybridus) at Bristol Zoo. Master’s Thesis, University of Bristol, Bristol, UK, 2019. [Google Scholar]
- Proctor, D.; Smurl, M.; Birkett, M. The Effect of a Nighttime Zoo Event on Spider Monkey (Ateles Geoffroyi) Behavior. Exp. Results 2020, 1, e50. [Google Scholar] [CrossRef]
- Williams, E.; Clark, F.E. Assessing the Impact of Evening Events in the Zoo: Advocating a Pro-Active Management Approach; Welsh Mountain Zoo: Colwyn Bay, UK, 2019. [Google Scholar]
- Bastian, M.L.; Glendinning, D.R.; Brown, J.L.; Boisseau, N.P.; Edwards, K.L. Effects of a Recurring Late-night Event on the Behavior and Welfare of a Population of Zoo-housed Gorillas. Zoo Biol. 2020, 39, 217–229. [Google Scholar] [CrossRef]
- Fanning, L.; Larsen, H.; Taylor, P. A Preliminary Study Investigating the Impact of Musical Concerts on the Behavior of Captive Fiordland Penguins (Eudyptes Pachyrhynchus) and Collared Peccaries (Pecari Tajacu). Animals 2020, 10, 2035. [Google Scholar] [CrossRef]
- Quintavalle Pastorino, G.; Viau, A.; Curone, G.; Pearce-Kelly, P.; Faustini, M.; Vigo, D.; Mazzola, S.M.; Preziosi, R. Role of Personality in Behavioral Responses to New Environments in Captive Asiatic Lions (Panthera Leo Persica). Vet. Med. Int. 2017, 2017, 6585380. [Google Scholar] [CrossRef]
- Shepherdson, D.; Carlstead, K.; Wielebnowski, N. Cross-Institutional Assessment of Stress Responses in Zoo Animals Using Longitudinal Monitoring of Faecal Corticoids and Behaviour. Anim. Welf. 2004, 13, 105–113. [Google Scholar]
- Clifforde, L.M.; Harley, J.J. Animal Welfare Toolkit: Zoo Events and Animal Management; BIAZA: London, UK, 2021. [Google Scholar]
- Potter, J.S.; Clauss, M. Mortality of Captive Giraffe (Giraffa Camelopardalis) Associated with Serious Atrophy. J. Zoo Wildl. Med. 2005, 36, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Margulis, S.W.; Westhus, E.J. Evaluation of Different Observational Sampling Regimes for Use in Zoological Parks. Appl. Anim. Behav. Sci. 2008, 110, 363–376. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Hemsworth, P.H. The Visitor Effect on Zoo Animals: Implications and Opportunities for Zoo Animal Welfare. Animals 2019, 9, 366. [Google Scholar] [CrossRef]
- Campos-Krauer, J.M.; Wisely, S.M.; Benitez, I.K.; Robles, V.; Golightly, R.T. Home Range and Habitat Use of Capybara in Newly Invaded Pastureland in the Dry Chaco Region of Paraguay. Therya 2014, 5, 61–79. [Google Scholar] [CrossRef]
- de Barros Ferraz, K.M.P.M.; de Barros Ferraz, S.F.; Moreira, J.R.; Couto, H.T.Z.; Verdade, L.M. Capybara (Hydrochoerus Hydrochaeris) Distribution in Agroecosystems: A Cross-Scale Habitat Analysis. J. Biogeogr. 2007, 34, 223–230. [Google Scholar] [CrossRef]
- Oliveira-Santos, L.G.R.; Machado-Filho, L.C.P.; Tortato, M.A.; Brusius, L. Influence of Extrinsic Variables on Activity and Habitat Selection of Lowland Tapirs (Tapirus Terrestris) in the Coastal Sand Plain Shrub, Southern Brazil. Mamm. Biol. 2010, 75, 219–226. [Google Scholar] [CrossRef]
- du Toit, J.T.; Yetman, C.A. Effects of Body Size on the Diurnal Activity Budgets of African Browsing Ruminants. Oecologia 2005, 143, 317–325. [Google Scholar] [CrossRef]
- Vilá, B.L.; Roig, V.G. Diurnal Movements, Family Groups and Alertness of Vicuña (Vicugna Vicugna) during the Late Dry Season in the Laguna Blanca Reserve (Catamarca, Argentina). Small Rumin. Res. 1992, 7, 289–297. [Google Scholar] [CrossRef]
- Brando, S.; Buchanan-Smith, H.M. The 24/7 Approach to Promoting Optimal Welfare for Captive Wild Animals. Behav. Process. 2018, 156, 83–95. [Google Scholar] [CrossRef]
- Kagan, R.; Carter, S.; Allard, S. A Universal Animal Welfare Framework for Zoos. J. Appl. Anim. Welf. Sci. 2015, 18, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Bacon, H.; Vigors, B.; Shaw, D.J.; Waran, N.; Dwyer, C.M.; Bell, C. Is Animal Welfare an Internationally Understood Concept in the Zoo World? Thematic Analysis of Two Regional Groups of Zoo Staff. Animals 2021, 11, 2059. [Google Scholar] [CrossRef] [PubMed]
- Wark, J.D.; Schook, M.W.; Dennis, P.M.; Lukas, K.E. Do Zoo Animals Use Off-exhibit Areas to Avoid Noise? A Case Study Exploring the Influence of Sound on the Behavior, Physiology, and Space Use of Two Pied Tamarins (Saguinus Bicolor). Am. J. Primatol. 2022, e23421. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.J.; Tamborski, M.A.; Pickens, S.R.; Timberlake, W. Animal–Visitor Interactions in the Modern Zoo: Conflicts and Interventions. Appl. Anim. Behav. Sci. 2009, 120, 1–8. [Google Scholar] [CrossRef]
- Hosey, G.R. How Does the Zoo Environment Affect the Behaviour of Captive Primates? Appl. Anim. Behav. Sci. 2005, 90, 107–129. [Google Scholar] [CrossRef]
- Fink, L.B.; Scarlata, C.D.; VanBeek, B.; Bodner, T.E.; Wielebnowski, N.C. Applying Behavioral and Physiological Measures to Assess the Relative Impact of the Prolonged COVID-19 Pandemic Closure on Two Mammal Species at the Oregon Zoo: Cheetah (A. Jubatus) and Giraffe (G. c. Reticulata and G. c. Tippelskirchii). Animals 2021, 11, 3526. [Google Scholar] [CrossRef] [PubMed]
- Finch, K.; Leary, M.; Holmes, L.; Williams, L.J. Zoo Closure Does Not Affect Behavior and Activity Patterns of Palawan Binturong (Arctictis Binturong Whitei). J. Zool. Bot. Gard. 2022, 3, 398–408. [Google Scholar] [CrossRef]
- Rose, P.E.; Brereton, J.E.; Croft, D.P. Measuring Welfare in Captive Flamingos: Activity Patterns and Exhibit Usage in Zoo-Housed Birds. Appl. Anim. Behav. Sci. 2018, 205, 115–125. [Google Scholar] [CrossRef]
- Rose, P.; Riley, L. The Use of Qualitative Behavioural Assessment to Zoo Welfare Measurement and Animal Husbandry Change. J. Zoo Aquar. Res. 2019, 7, 150–161. [Google Scholar] [CrossRef]
- Cronin, K.; Ross, S. Technical Contribution: A Cautionary Note on the Use of Behavioural Diversity (H-Index) in Animal Welfare Science. Anim. Welf. 2019, 28, 157–164. [Google Scholar] [CrossRef]
- Tobler, I.; Schwierin, B. Behavioural Sleep in the Giraffe (Giraffa Camelopardalis) in a Zoological Garden. J. Sleep Res. 1996, 5, 21–32. [Google Scholar] [CrossRef] [PubMed]
- EEPs, EAZA Giraffe. EAZA Husbandry and Management Guidelines for Giraffa Camelopardalis; European Association of Zoos and Aquaria: Arnhem, The Netherlands, 2006. [Google Scholar]
- Goodenough, A.E.; McDonald, K.; Moody, K.; Wheeler, C. Are “Visitor Effects” Overestimated? Behaviour in Captive Lemurs Is Mainly Driven by Co-Variation with Time and Weather. J. Zoo Aquar. Res. 2019, 7, 59–66. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Rendle, J.; Ward, S.J. Understanding Impacts of Zoo Visitors: Quantifying Behavioural Changes of Two Popular Zoo Species during COVID-19 Closures. Appl. Anim. Behav. Sci. 2021, 236, 105253. [Google Scholar] [CrossRef] [PubMed]
- Frost, N.; Carter, A.; Vernon, M.; Armstrong, S.; Walsh, N.D.; Colwill, M.; Turner-Jepson, L.; Ward, S.J.; Williams, E. Behavioural Changes in Zoo Animals during the COVID-19 Pandemic: A Long-Term, Multi Species Comparison. JZBG 2022, 3, 586–615. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Rendle, J.; Ward, S.J. Impacts of COVID-19 on Animals in Zoos: A Longitudinal Multi-Species Analysis. J. Zool. Bot. Gard. 2021, 2, 130–145. [Google Scholar] [CrossRef]
- Margulis, S.W.; Pruett-Jones, M. Integrating Science and Husbandry: Less Is More. In The Well-being of Animals in Zoo and Aquarium Sponsored Research: Putting Best Practices Forward; Bettinger, T., Bielitzki, J., Eds.; Scientists Centre for Animal Welfare: Bend, OR, USA, 2008. [Google Scholar]
- Freeland, L.; Ellis, C.; Michaels, C.J. Documenting Aggression, Dominance and the Impacts of Visitor Interaction on Galápagos Tortoises (Chelonoidis Nigra) in a Zoo Setting. Animals 2020, 10, 699. [Google Scholar] [CrossRef]
| Pre Event | Event | Post Event | |||
|---|---|---|---|---|---|
| Date | Time | Date | Time | Date | Time |
| 28 October 2021 | 16:00–20:00 | 11 December 2021 | 24 h | 5 January 2022 | 24 h |
| 29 October 2021 | 16:00–20:00 | 12 December 2021 | 16:00–20:00 | 6 January 2022 | 16:00–20:00 |
| 30 October 2021 | 16:00–20:00 | 13 December 2021 | 16:00–20:00 | 7 January 2022 | 16:00–20:00 |
| 31 October 2021 | 24 h | 14 December 2021 | 16:00–20:00 | 8 January 2022 | 24 h |
| 16 November 2021 | 16:00–20:00 | 15 December 2021 | 16:00–20:00 | 9 January 2022 | 16:00–20:00 |
| 18 November 2021 | 16:00–20:00 | 16 December 2021 | 16:00–20:00 | 10 January 2022 | 16:00–20:00 |
| 19 November 2021 | 24 h | 17 December 2021 | 24 h | 11 January 2022 | 16:00–20:00 |
| Behaviour | Description |
|---|---|
| Locomotion | Actively moving around enclosure at any speed |
| Resting | Inactive and stationary; eyes may be closed or open. May be standing, sitting, or lying down |
| Vigilant | Stationary paying attention to the environment or scanning/ checking environment |
| Ruminating (giraffe only) | Repeatedly chewing the cud |
| Eating | Actively eating, drinking, foraging, grazing, or browsing |
| Abnormal Repetitive | Pacing (repetitive, fixed pattern), repeated licking, head rolling, excessive locomotion (restlessness/agitated at any gait) |
| Locomotion | Actively moving around enclosure at any speed |
| Resting | Inactive and stationary; eyes may be closed or open. May be standing, sitting, or lying down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, E.; Fulwell, T.; Walsh, N.D.; Harley, J.J.; Johnson, B. The Impacts of Evening Events in Zoos: A Christmas Event at Knowsley Safari. J. Zool. Bot. Gard. 2023, 4, 21-38. https://doi.org/10.3390/jzbg4010003
Williams E, Fulwell T, Walsh ND, Harley JJ, Johnson B. The Impacts of Evening Events in Zoos: A Christmas Event at Knowsley Safari. Journal of Zoological and Botanical Gardens. 2023; 4(1):21-38. https://doi.org/10.3390/jzbg4010003
Chicago/Turabian StyleWilliams, Ellen, Tom Fulwell, Naomi Davies Walsh, Jessica J. Harley, and Bridget Johnson. 2023. "The Impacts of Evening Events in Zoos: A Christmas Event at Knowsley Safari" Journal of Zoological and Botanical Gardens 4, no. 1: 21-38. https://doi.org/10.3390/jzbg4010003
APA StyleWilliams, E., Fulwell, T., Walsh, N. D., Harley, J. J., & Johnson, B. (2023). The Impacts of Evening Events in Zoos: A Christmas Event at Knowsley Safari. Journal of Zoological and Botanical Gardens, 4(1), 21-38. https://doi.org/10.3390/jzbg4010003








