Impacts of Socialization on Bull Asian Elephant (Elephas maximus) Stereotypical Behavior
Abstract
:1. Introduction
2. Materials and Methods
3. Results
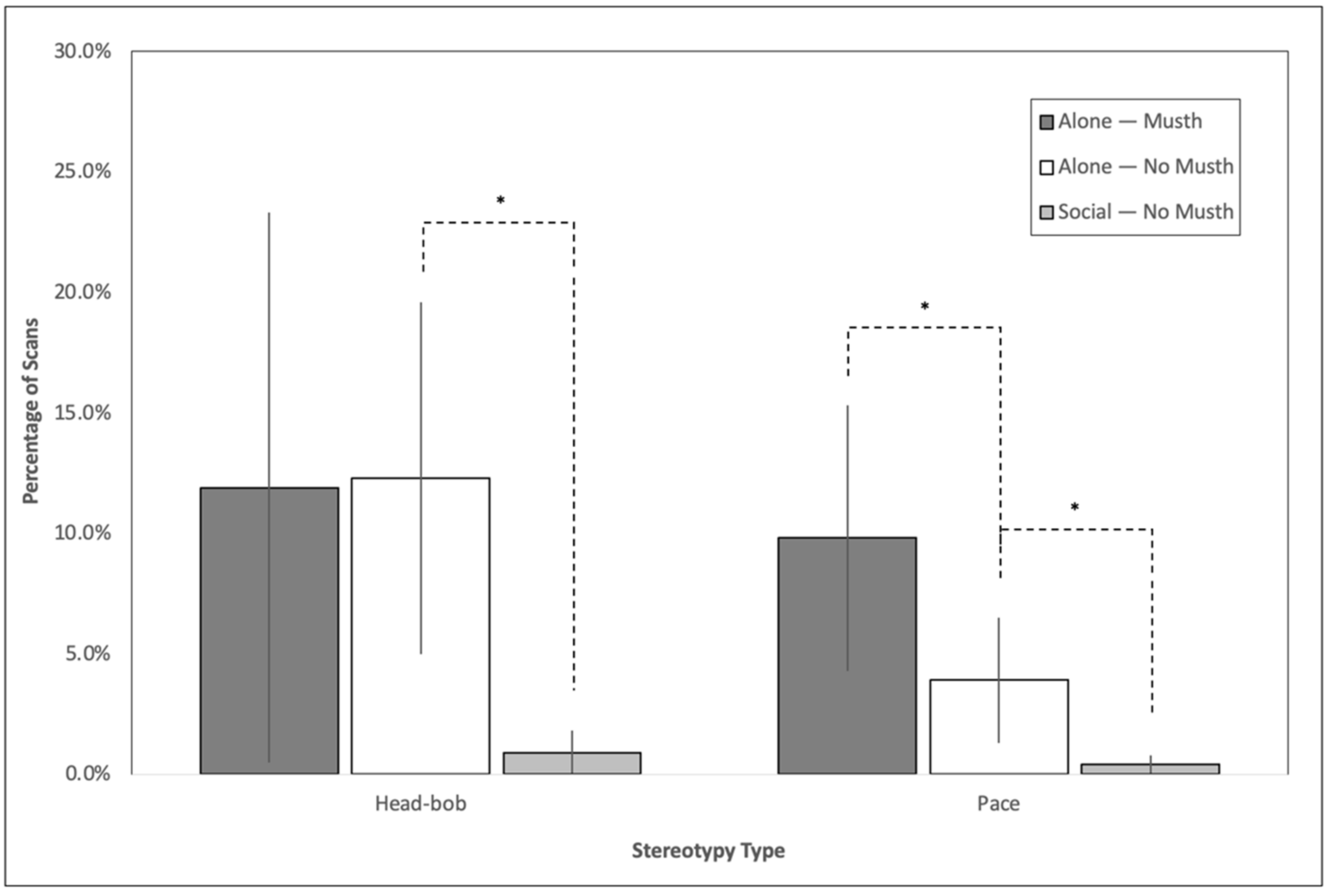
3.1. Impact of Social Housing on Stereotypy
3.2. Impact of Social Behaviors (Proximity, Affiliative Behavior, and Agonistic Behavior) on Stereotypy
3.3. Impact of Introductions on Stereotypy
3.4. Impact of Musth on Stereotypy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, K.E.; Harris, S. Adolescence in male African elephants, Loxodonta africana, and the importance of sociality. Anim. Behav. 2008, 76, 779–787. [Google Scholar] [CrossRef]
- De Silva, S.; Wittemyer, G. A comparison of social organization in Asian elephants and African savannah elephants. Int. J. Primatol. 2012, 33, 1125–1141. [Google Scholar] [CrossRef]
- Association of Zoos and Aquariums. Asian Elephant Population Analysis and Breeding and Transfer Plan; Association of Zoos and Aquariums: Silver Spring, MD, USA, 2017. [Google Scholar]
- Joseph, S.; Denver Zoo, Denver, CO, USA. Personal communication, 2018.
- Hambrecht, S.; Reichler, S. Group dynamics of young Asian elephant bulls (Elephas maximus, Linnaeus, 1758) in Heidelberg zoo—Integration of a newcomer in an established herd. Der. Zool. Gart. 2013, 82, 267–292. [Google Scholar] [CrossRef]
- Hartley, M.; Wood, A.; Yon, L. Facilitating the social behaviour of bull elephants in zoos. Int. Zoo Yearb. 2019, 53, 62–77. [Google Scholar] [CrossRef]
- Metrione, L.C.; Flora, P.; Foster, W.; Penfold, L.M.; Hilton, C.D. Relationships of dominance and tractability with serum testosterone among a bachelor group of African elephants (Loxodonta africana). In Proceedings of the American Association of Zoo Veterinarians, Salt Lake City, UT, USA, 28 September—4 October 2013; p. 116. [Google Scholar]
- Mason, G.J.; Veasey, J.S. How should the psychological well-being of zoo elephants be objectively investigated? Zoo Biol. 2010, 29, 237–255. [Google Scholar] [CrossRef] [Green Version]
- Greco, B.J.; Meehan, C.L.; Heinsius, J.L.; Mench, J.A. Why pace? The influence of social, housing, management, life history, and demographic characteristics on locomotor stereotypy in zoo elephants. Appl. Anim. Behav. Sci. 2017, 194, 104–111. [Google Scholar] [CrossRef]
- Kurt, F.; Garai, M. Stereotipies in captive Asian elephants: A symptom of social isolation. In Proceedings of the International Elephant and Rhino research Symposium, Vienna, Austria, 7–11 June 2001; pp. 57–63. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.585.8778&rep=rep1&type=pdf (accessed on 5 January 2022).
- Alonso-Spilsbury, M.; Alcántara-Barrera, A.; Escobar-Ibarra, I.; Mariscal-López, O.; Gómez, M.C.; Frieventh, J. El público afecta positivamente la conducta estereotipada de una elefante asiática en el Zoológico Zacango. In Proceedings of the XXV Congreso Panamericano de Ciencias Veterinarias, Ciudad de Panama, Panama, 3–7 October 2016. [Google Scholar]
- Mason, G.J.; Clubb, R.; Latham, N.; Vickery, S. Why and how should we use environmental enrichment to tackle stereotypic behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef] [Green Version]
- van der Harst, J.E.; Spruijt, B.M. Tools to measure and improve animal welfare: Reward-related behaviour. Anim. Welf. 2007, 16, 67–73. [Google Scholar]
- Watters, J.V. Searching for behavioral indicators of welfare in zoos: Uncovering anticipatory behavior. Zoo Biol. 2014, 33, 251–256. [Google Scholar] [CrossRef]
- Krebs, B.L.; Torres, E.; Chesney, C.; Moon, V.K.; Watters, J. Applying behavioral conditioning to identify anticipatory behaviors. J. Appl. Anim. Welf. Sci. 2017, 20, 155–175. [Google Scholar] [CrossRef]
- Joshi, R. Tusker’s social bonds in Rajaji. Hystrix 2015, 26, 41–45. [Google Scholar] [CrossRef]
- Srinivasaiah, N.; Kumar, V.; Vaidyanathan, S.; Sukumar, R.; Sinha, A. All-male groups in Asian elephants: A novel, adaptive social strategy in increasingly anthropogenic landscapes of Southern India. Sci. Rep. 2019, 9, 8678. [Google Scholar] [CrossRef] [Green Version]
- Greco, B.J.; Brown, T.K.; Andrews, J.R.; Swaisgood, R.R.; Caine, N.G. Social learning in captive African elephants (Loxodonta africana africana). Anim. Cogn. 2012, 16, 459–469. [Google Scholar] [CrossRef]
- Katugaha, H.; de Silva, M.; Santiapillai, C. A long-term study on the dynamics of the elephant (Elephas maximus) population in Ruhuna National Park, Sri Lanka. Biol. Conserv. 1999, 89, 51–59. [Google Scholar] [CrossRef]
- Santiapillai, C.; Chambers, M.; Ishwaran, N. Aspects of the ecology of the Asian elephant Elephas maximus L. in the Ruhuna National Park, Sri Lanka. Biol. Conserv. 1984, 29, 47–61. [Google Scholar] [CrossRef]
- Veasey, J.S. Assessing the psychological priorities for optimizing captive Asian elephant (Elephas maximus) welfare. Animals 2020, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Asher, L.; Williams, E.; Yon, L. Developing Behavioural Indicators, As Part of a Wider Set of Indicators, to Assess the Welfare of Elephants in UK Zoos-Defra Project WC 1081; University of Nottingham: Nottingham, UK, 2015. [Google Scholar]
- Greco, B.J.; Meehan, C.L.; Hogan, J.N.; Leighty, K.A.; Mellen, J.; Mason, G.J.; Mench, J.A. The days and nights of zoo elephants: Using epidemiology to better understand stereotypic behavior of African elephants (Loxodonta africana) and Asian elephants (Elephas maximus) in North American zoos. PLoS ONE 2016, 11, e0144276. [Google Scholar] [CrossRef]
- Mason, G. Stereotypic behaviour in captive animals: Fundamentals and implications for welfare and beyond. In Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare; Mason, G., Rushen, B.J., Eds.; CABI: Cambridge, MA, USA, 2006; pp. 325–332. [Google Scholar]
- Schiffmann, C.; Clauss, M. Impact of a new exhibit on stereotypic behavior in an elderly captive African elephant. J. Zoo Aquar. Res. 2019, 7, 37–43. [Google Scholar] [CrossRef]
- Rees, P.A. The sizes of elephant groups in zoos: Implications for elephant welfare. J. Appl. Anim. Welf. Sci. 2009, 12, 44–60. [Google Scholar] [CrossRef]
- Association of Zoos and Aquariums. Standards for Elephant Management and Care; Association of Zoos and Aquariums: Silver Spring, MD, USA, 2011. [Google Scholar]
- Meehan, C.L.; Hogan, J.N.; Bonaparte-Saller, M.K.; Mench, J.A. Housing and social environments of African (Loxodonta africana) and Asian (Elephas maximus) elephants in North American zoos. PLoS ONE 2016, 11, e0146703. [Google Scholar] [CrossRef] [Green Version]
- Meehan, C.L.; Mench, J.A.; Carlstead, K.; Hogan, J.N. Determining connections between the daily lives of zoo elephants and their welfare: An epidemiological approach. PLoS ONE 2016, 11, e0158124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado-Oviedo, N.A.; Bonaparte-Saller, M.K.; Malloy, E.J.; Meehan, C.L.; Mench, J.A.; Carlstead, K.; Brown, J.L. Evaluation of demographics and social life events of Asian (Elephas maximus) and African elephants (Loxodonta africana) in North American zoos. PLoS ONE 2016, 11, e0154750. [Google Scholar] [CrossRef] [PubMed]
- Vidya, T.N.C.; Sukumar, R. Social organization of the Asian elephant (Elephas maximus) in southern India inferred from microsatellite DNA. J. Ethol. 2005, 23, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Vidya, T.N.C.; Sukumar, R. Social and reproductive behaviour in elephants. Curr. Sci. 2005, 89, 1200–1207. Available online: https://www.jstor.org/stable/24110972 (accessed on 21 December 2021).
- Sukumar, R. The Living Elephants: Evolutionary Ecology, Behaviour, and Conservation; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Desai, A.; Johnsingh, A.J.T. Social organization and reproductive strategy of the male Asian elephants (Elephas maximus). In Abstract in A Week with Elephants; Daniel, J.C., Datye, H.S., Eds.; Bombay Natural History Society and Oxford University Press: Bombay, India, 1995. [Google Scholar]
- Schreier, A.L.; Readyhough, T.S.; Moresco, A.; Davis, M.; Joseph, S. Social dynamics of a newly integrated bachelor herd of Asian elephants (Elephas maximus): Welfare implications. J. Appl. Anim. Welf. Sci. 2021. [Google Scholar] [CrossRef]
- Thevarajah, S.J.; Readyhough, T.S.; Davis, M.; Moresco, A.; Joseph, S.; Schreier, A.L. Nighttime behavior and the length of social relationships in male Asian elephants. J. Appl. Anim. Welf. Sci. 2021. [Google Scholar] [CrossRef]
- Chelliah, K.; Sukumar, R. Interplay of male traits, male mating strategies and female mate choice in the Asian elephant. Elephas. Maximus. Behav. 2015, 152, 1113–1144. [Google Scholar] [CrossRef]
- Schulte, B.A.; Rasmussen, L.E.L. Musth, sexual selection, testosterone, and metabolites. In Advances in Chemical Signals in Vertebrates; Johnston, R.E., Ed.; Springer: New York, NY, USA, 1999; pp. 383–397. [Google Scholar]
- Ananth, D. Musth in elephants. Zoos Print J. 2000, 15, 259–262. [Google Scholar] [CrossRef]
- Keerthipriya, P.; Nandini, S.; Gautam, H.; Revathe, T.; Vidya, T. Musth and its effects on male–male and male–female associations in Asian elephants. J. Mammal. 2020, 101, 259–270. [Google Scholar] [CrossRef]
- Cooper, K.; Harder, J.; Clawson, D.; Fredrick, D.; Lodge, G.; Peachey, H.; Spellmire, T.; Winstel, D. Serum testosterone and musth in captive male African and Asian elephants. Zoo Biol. 1990, 9, 297–306. [Google Scholar] [CrossRef]
- Fernando, P.; Wikramanayake, E.D.; Janaka, H.; Jayasinghe, L.; Gunawardena, M.; Kotagama, S.W.; Weerakoon, D.; Pastorini, J. Ranging behavior of the Asian elephant in Sri Lanka. Mamm. Biol.-Z. Säugetierkunde 2008, 73, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [Green Version]
- Højsgaard, S.; Halekoh, U.; Yan, J. The R package geepack for generalized estimating equations. J. Stat. Softw. 2006, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yan, J. geepack: Yet another package for generalized estimating equations. R-News 2002, 2, 12–14. [Google Scholar]
- Yan, J.; Fine, J.P. Estimating equations for association structures. Stat. Med. 2004, 23, 859–880. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 1.4.1717; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 March 2022).
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com (accessed on 1 March 2022).
- Blackett, T.; McKenna, C.; Kavanagh, L.; Morgan, D. The welfare of wild animals in zoological institutions: Are we meeting our duty of care? Int. Zoo Yearb. 2017, 51, 187–202. [Google Scholar] [CrossRef]
- Mason, G.J.; Burn, C.C. Behavioural restriction. In Animal Welfare; Appleby, M.C., Hughes, B.O., Mench, J.A., Olsson, A., Eds.; CABI International: Oxford, UK, 2011; pp. 98–119. [Google Scholar]
- Hall-Martin, A. Role of musth in the reproductive strategy of the African elephant (Loxodonta africana). South Afr. J. Sci. 1987, 83, 616–620. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Hall, C.; Bremner-Harrison, S. Social interactions in zoo-housed elephants: Factors affecting social relationships. Animals 2019, 9, 747. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.; Denver Zoo, Denver, CO, USA. Personal communication, 2021.
- Schmidt, H.; Kappelhof, J. Review of the management of the European breeding programme for Asian elephants (Elephas maximus): Current challenges and future solutions. Int. Zoo Yearb. 2019, 53, 31–44. [Google Scholar] [CrossRef] [Green Version]
| Behavior Category | Behavior | Definition | |
|---|---|---|---|
| Agonistic | Non-Contact | Approach head high | Actor moves toward recipient to within two body lengths with head above shoulders and ears out perpendicular |
| Charge | Rapid forward lunging or rapid gait by actor towards a stationary conspecific starting from more than two body lengths away | ||
| Chase | Actor rapidly pursues recipient, who is moving away from actor, for at least 5 s | ||
| Head shake | Actor holds head above shoulders and moves vigorously from side to side, up and down, or in circular motion | ||
| Supplant | Actor approaches to within two body lengths of conspecific without making contact, causing recipient to turn away or yield ground | ||
| Contact | Grasp tail | Actor places tail of conspecific into its own trunk while recipient attempts to move away from focal animal | |
| Kick | Actor strikes at recipient with rear limb | ||
| Mount | Actor rears up on hind legs and places forelegs on recipient for 5 s or more | ||
| Push | Actor contacts conspecific with enough force to displace recipient | ||
| Spar | Two elephants mutually and simultaneously push one another backwards with force with heads and/or heads and trunks and this is sustained for at least 5 s | ||
| Trunk over back | Actor places 2/3 or more of its trunk firmly over the back or head of a conspecific | ||
| Affiliative | Approach relaxed | Actor moves to within to within two body lengths of recipient with head low and ears lying flat against its head, not associated with any other behavior | |
| Body contact | Body contact unspecified in any other behavior (e.g., side-to-side rubbing or touching) | ||
| Play | Actor voluntarily spars, wrestles with, mounts, or chases recipient without obvious intent to do harm or display dominance or for less than 5 s; does not include when following agonistic interaction | ||
| Shares food/object | Actor either feeds or uses an object in concert with another elephant that is within one body length | ||
| Trunk tangle | Actor loosely entwines its trunk with that of recipient | ||
| Trunk to mouth | Actor places its trunk in another elephant’s mouth | ||
| Trunk touch/toward | Actor extends trunk toward recipient with or without touching; not associated with any other behavior | ||
| Submissive | Allow | Actor remains still and calmly permits physical contact by conspecific, including genital investigation | |
| Back into/toward | Actor takes two steps (minimum) backward towards another elephant to within one body length, with or without touching | ||
| Lower head or ears | Actor quickly drops head and/or ears in response to approach by another elephant | ||
| Run away | Actor flees from conspecific in response to its agonistic contact, display, or approach | ||
| Turn away/yield | Actor turns body away from or yields ground as a result of actions or encroachment by another elephant | ||
| Other | Bathe/swim | Actor lies, stands, or submerges in pool (includes spraying water on self); not associated with any other ethogram behavior | |
| Drink | Actor uses trunk to bring water to its mouth and drink | ||
| Dust/mud | Actor uses trunk to throw dirt, sand, shavings, or mud onto body while standing | ||
| Enrichment interaction | Actor interacts with provided non-food enrichment items | ||
| Feed | Actor ingests presented diet items; includes manipulating food items | ||
| Follow | Actor closely trails behind recipient, who is moving away from actor (at normal walking speed) | ||
| Genital investigation | Actor sniffs or touches genitals of another elephant with its trunk | ||
| Locomotion | Actor moves directionally along a horizontal surface (not while feeding); can include slow or fast walking or running | ||
| Rest | Stationary; lying down or standing with trunk resting loosely on the ground; eyes open or closed; not performing any other behavior | ||
| Head-bob | Actor displays repetitive head rotation/movement from side to side, at least two repetitions within 10 s | ||
| Pace | Actor repeatedly walks the same line of travel, at least three times | ||
| Wallow | Actor lies or rolls in mud or dirt | ||
| Other | Actor performs any behavior not on ethogram | ||
| Out of View | Out of view | Actor cannot be seen or cannot be distinguished from other elephants | |
| Variable | Description | Reference Level |
|---|---|---|
| HeadBobProp | Numeric (binomial proportion) variable indicating proportion of scans that the focal animal was engaging in head-bobbing (x/30) | NA—response variable |
| PaceProp | Numeric (binomial proportion) variable indicating proportion of scans that the focal animal was engaging in pacing (x/30) | NA—response variable |
| TimePeriod.5mos | Categorical variable indicating which 5 month time period the observation fell within (Before, Intro.5mos, End.5mos) | Before |
| Relevel.5mos | Categorical variable indicating which 5 month time period the observation fell within (Before, Intro.5mos, End.5mos); relevelled to compare Intro.5mos to End.5mos | Intro.5mos |
| Socialized | Binary variable indicating if the focal animal was house alone (0) or with at least one conspecific (1) | Alone (0) |
| FocalMusth | Binary variable indicating if the focal animal was in musth (1) or not (0) during the time of the observation session | No musth (0) |
| AccessArea | Continuous variable indicating the size of the area that the focal animal had access to (per 1000 ft2); 2.00–47.37 | 2000 ft2 |
| InOutAccess | Categorical variable indicating if focal animal had access inside (in), outside (out), or both (both) | Both |
| AMPM | Binary variable indicating if observations took place in the afternoon (1) or morning (0) | Morning (0) |
| AffiliativeProp | Numeric (binomial proportion) variable indicating proportion of scans that the focal animal was engaging in affiliative behavior (x/30) | 0.10 increase in proportion |
| AgonisticProp | Numeric (binomial proportion) variable indicating proportion of scans that the focal animal was engaging in agonistic behavior (x/30) | 0.10 increase in proportion |
| NearProp | Numeric (binomial proportion) variable indicating the proportion of scans that the focal animal was within two body-lengths of a conspecific (x/30) | 0.10 increase in proportion |
| InOutAccess*AccessArea | Interaction term between InOutAccess (in, out, both) and AccessArea (2.00–47.37) | Both:AccessArea |
| Socialized*FocalMusth | Interaction term between Socialized (alone/0, social/1) and FocalMusth (no musth/0, musth/1) | Alone(0):No Musth(0) |
| Socialized*AccessArea | Interaction term between Socialized (alone/0, social/1) and AccessArea (2.00–47.37) | Alone(0):AccessArea |
| IndivGroup | Categorical variable indicating observations on a focal animal within a specific social unit; used for clustering observations with an independent correlation matrix | N/A—(used for clustering data) |
| Model | QIC | QICu | p-Value | Mean Effect Parameters | Corrected QIC |
|---|---|---|---|---|---|
| All Data (Alone and Social) | |||||
| TimePeriod.5mos + Socialized + FocalMusth + InOutAccess + AccessArea + AMPM + InOutAccess *AccessArea + Socialized*AccessArea + Socialized*FocalMusth | 1475.4 | 143.3 | - | 13 | 1475.7 |
| TimePeriod.5mos + Socialized + FocalMusth + InOutAccess + AccessArea + AMPM + InOutAccess *AccessArea + Socialized*FocalMusth | 1844.0 | 141.4 | 0.43 | 12 | 1844.3 |
| Social Data Only | |||||
| NearProp + AffiliativeProp + AgonisticProp + FocalMusth + InOutAccess + AccessArea + AMPM + TimePeriod.5mos + InOutAccess *AccessArea | 32.98 | 32.55 | - | 13 | 33.53 |
| NearProp + FocalMusth + InOutAccess + AccessArea + TimePeriod.5mos + InOutAccess*AccessArea | 112.13 | 31.34 | 0.19 | 12 | 112.60 |
| Alone Data Only | |||||
| TimePeriod.5mos + FocalMusth + InOutAccess + AccessArea + AMPM + InOutAccess*AccessArea | 1782 | 128 | - | 10 | 1783 |
| Model | QIC | QICu | p-Value | Mean Effect Parameters | Corrected QIC |
|---|---|---|---|---|---|
| All Data (Alone and Social) | |||||
| TimePeriod.5mos + Socialized + FocalMusth + InOutAccess + AccessArea + AMPM + InOutAccess*AccessArea + Socialized*FocalMusth + Socialized*AccessArea | 1317.7 | 190.6 | - | 13 | 1318.1 |
| TimePeriod.5mos + Socialized + FocalMusth + InOutAccess + AccessArea + AMPM + InOutAccess*AccessArea + Socialized*AccessArea | 1323.0 | 188.6 | 0.43 | 12 | 1323.3 |
| Social Data Only | |||||
| NearProp + AffiliativeProp + AgonisticProp + FocalMusth + InOutAccess + AccessArea + AMPM + TimePeriod.5mos + InOutAccess*AccessArea | 273.8 | 51.9 | - | 13 | 274.4 |
| NearProp + AffiliativeProp + AgonisticProp + FocalMusth + InOutAccess + AccessArea + AMPM + TimePeriod.5mos | 427 | 48 | 0.98 | 11 | 428 |
| NearProp + AffiliativeProp + AgonisticeProp + FocalMusth + InOutAccess + AccessArea + AMPM | 492.3 | 44.3 | 0.99 | 9 | 492.5 |
| NearProp + AffiliativeProp + AgonisticeProp + FocalMusth + InOutAccess + AccessArea | 475.3 | 42.5 | 0.96 | 8 | 475.5 |
| NearProp + AffiliativeProp + AgonisticProp + FocalMusth + InOutAccess | 309.4 | 40.5 | 0.99 | 7 | 309.6 |
| Alone Data Only | |||||
| TimePeriod.5mos + FocalMusth + InOutAccess + AccessArea + AMPM + InOutAccess *AccessArea | −1107.2 | 153.5 | - | 10 | −1106.5 |
| Elephant | Overall Percentage of Scans Engaged in Stereotypic Behavior |
|---|---|
| Individual 1 | 2.4% (95% CI: 0.5–4.3%) |
| Individual 2 | 6.2% (95% CI: 3.0–9.5%) |
| Individual 3 | Pacing: 0.5% (95% CI: 0.0–1.6%) Head-bobbing: 7.4% (95% CI: 3.7–11.1%) |
| Individual 4 | 1.3% (95% CI: 0.0–2.9%) |
| Individual 5 | 0.0% (Individual 5 did not engage in any stereotypic behaviors during our observations) |
| HeadBobProp | PaceProp | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Level | Odds Ratio | ß | SE | Wald X2 | p | Odds Ratio | ß | SE | Wald X2 | p |
| TimePeriod.5mos | Before * | - | - | - | - | - | - | - | - | - | - |
| Intro.5mos | 0.791 | −0.234 | 0.146 | 2.59 | 0.108 | 0.931 | −0.071 | 0.292 | 0.06 | 0.808 | |
| End.5mos | 0.621 | −0.476 | 0.344 | 1.92 | 0.166 | 0.144 | −1.937 | 0.454 | 18.18 | <0.001 | |
| Relevel.5mos | Before | 1.264 | 0.234 | 0.146 | 2.59 | 0.108 | 1.074 | 0.071 | 0.292 | 0.06 | 0.808 |
| Intro.5mos * | - | - | - | - | - | - | - | - | - | - | |
| End.5mos | 0.785 | −0.242 | 0.256 | 0.89 | 0.344 | 0.155 | −1.867 | 0.296 | 39.66 | <0.001 | |
| Socialized | Alone * | - | - | - | - | - | - | - | - | - | - |
| Social | 0.024 | −3.735 | 0.829 | 20.32 | <0.001 | 0.021 | −3.842 | 1.367 | 7.90 | 0.005 | |
| FocalMusth | No musth * | - | - | - | - | - | - | - | - | - | - |
| Musth | 0.945 | −0.057 | 0.166 | 0.12 | 0.730 | 3.796 | 1.334 | 0.186 | 51.25 | <0.001 | |
| InOutAccess | Both * | - | - | - | - | - | - | - | - | - | - |
| Inside | 72.39 | 4.282 | 0.471 | 82.61 | <0.001 | 1.680 | 0.519 | 1.123 | 0.21 | 0.644 | |
| Outside | 1.231 | 0.208 | 0.507 | 0.17 | 0.683 | 6.240 | 1.831 | 1.256 | 2.13 | 0.145 | |
| AccessArea | 1.087 | 0.083 | 0.065 | 1.61 | 0.205 | 0.996 | −0.004 | 0.057 | 0.01 | 0.937 | |
| AMPM | AM * | - | - | - | - | - | - | - | - | - | - |
| PM | 2.779 | 1.022 | 0.131 | 61.32 | <0.001 | 2.586 | 0.950 | 0.317 | 8.95 | 0.003 | |
| InOutAccess * AccessArea | Both:AccessArea * | - | - | - | - | - | - | - | - | - | - |
| In:AccessArea | 0.138 | −1.982 | 0.200 | 98.43 | <0.001 | 0.454 | −0.790 | 0.193 | 16.76 | <0.001 | |
| Out:AccessArea | 0.900 | −0.105 | 0.067 | 2.46 | 0.117 | 0.897 | −0.109 | 0.068 | 2.55 | 0.111 | |
| Socialized * FocalMusth | Alone:FocalMusth * | - | - | - | - | - | N/A | ||||
| Social:FocalMusth | <0.001 | −36.98 | 0.937 | 1557.4 | <0.001 | ||||||
| Socialized * AccessArea | Alone:AccessArea * | N/A | - | - | - | - | - | ||||
| Social:AccessArea | 1.120 | 0.113 | 0.057 | 3.92 | 0.048 | ||||||
| HeadBobProp | PaceProp | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Level | Odds Ratio | ß | SE | Wald X2 | p | Odds Ratio | ß | SE | Wald X2 | p |
| NearProp | 0.444 | −0.812 | 0.394 | 4.25 | 0.039 | 1.033 | 0.032 | 0.196 | 0.03 | 0.870 | |
| AffiliativeProp | 0.652 | −0.428 | 0.416 | 1.05 | 0.304 | 0.607 | −0.500 | 0.147 | 11.48 | <0.001 | |
| AgonisticProp | 2.779 | 1.022 | 0.785 | 1.69 | 0.193 | 0.025 | −3.705 | 2.346 | 2.49 | 0.114 | |
| FocalMusth | No musth * | - | - | - | - | - | - | - | - | - | - |
| Musth | <0.001 | −40.508 | 1.809 | 501.2 | <0.001 | 17.41 | 2.857 | 0.897 | 10.14 | 0.001 | |
| InOutAccess | Both * | - | - | - | - | - | - | - | - | - | - |
| Inside | <0.001 | −47.974 | 3.335 | 206.9 | <0.001 | <0.001 | −41.816 | 1.274 | 1077.7 | <0.001 | |
| Outside | 1378.8 | 7.229 | 2.368 | 9.32 | 0.002 | 0.124 | −2.089 | 0.901 | 5.37 | 0.020 | |
| AccessArea | 1.310 | 0.270 | 0.120 | 5.11 | 0.024 | N/A | |||||
| TimePeriod.5mos | Before * | - | - | - | - | - | N/A | ||||
| Intro.5mos | <0.001 | −42.551 | 1.953 | 474.9 | <0.001 | ||||||
| End.5mos | 0.225 | −1.493 | 1.465 | 1.04 | 0.308 | ||||||
| InOutAccess * AccessArea | Both:AccessArea * | - | - | - | - | - | N/A | ||||
| In:AccessArea | 23.220 | 3.145 | 0.432 | 52.90 | <0.001 | ||||||
| Out:AccessArea | 0.534 | −0.627 | 0.218 | 8.25 | 0.004 | ||||||
| HeadBobProp | PaceProp | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Level | Odds Ratio | ß | SE | Wald X2 | p | Odds Ratio | ß | SE | Wald X2 | p |
| FocalMusth | No musth * | - | - | - | - | - | - | - | - | - | - |
| Musth | 0.932 | −0.070 | 0.173 | 0.16 | 0.685 | 3.781 | 1.33 | 5.899 | 37.47 | <0.001 | |
| InOutAccess | Both * | - | - | - | - | - | - | - | - | - | - |
| Inside | 55.757 | 4.021 | 0.411 | 95.77 | <0.001 | 10.155 | 2.318 | 0.785 | 8.73 | 0.003 | |
| Outside | 0.806 | −0.216 | 0.324 | 0.44 | 0.506 | 2.512 | 0.921 | 1.229 | 0.56 | 0.453 | |
| AccessArea | 1.014 | 0.014 | 0.034 | 0.17 | 0.678 | 0.954 | −0.047 | 0.042 | 1.21 | 0.271 | |
| AMPM | AM * | - | - | - | - | - | - | - | - | - | - |
| PM | 2.467 | 0.903 | 0.040 | 501.3 | <0.001 | 2.732 | 1.005 | 0.192 | 27.36 | <0.001 | |
| TimePeriod.5mos | Before * | - | - | - | - | - | - | - | - | - | - |
| Intro.5mos | 0.892 | −0.114 | 0.086 | 1.76 | 0.185 | 0.991 | −0.009 | 0.220 | 0.00 | 0.967 | |
| End.5mos | 0.680 | −0.385 | 0.323 | 1.42 | 0.233 | 0.319 | −1.144 | 0.490 | 5.46 | 0.020 | |
| InOutAccess *AccessArea | Both:AccessArea * | - | - | - | - | - | - | - | - | - | - |
| In:AccessArea | 0.144 | −1.936 | 0.195 | 98.34 | <0.001 | 0.265 | −1.328 | 0.398 | 11.15 | <0.001 | |
| Out:AccessArea | 0.971 | −0.029 | 0.031 | 0.88 | 0.347 | 0.971 | −0.029 | 0.042 | 0.50 | 0.480 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Readyhough, T.S.; Joseph, S.; Davis, M.; Moresco, A.; Schreier, A.L. Impacts of Socialization on Bull Asian Elephant (Elephas maximus) Stereotypical Behavior. J. Zool. Bot. Gard. 2022, 3, 113-130. https://doi.org/10.3390/jzbg3010010
Readyhough TS, Joseph S, Davis M, Moresco A, Schreier AL. Impacts of Socialization on Bull Asian Elephant (Elephas maximus) Stereotypical Behavior. Journal of Zoological and Botanical Gardens. 2022; 3(1):113-130. https://doi.org/10.3390/jzbg3010010
Chicago/Turabian StyleReadyhough, Taylor S., Sharon Joseph, Maura Davis, Anneke Moresco, and Amy L. Schreier. 2022. "Impacts of Socialization on Bull Asian Elephant (Elephas maximus) Stereotypical Behavior" Journal of Zoological and Botanical Gardens 3, no. 1: 113-130. https://doi.org/10.3390/jzbg3010010
APA StyleReadyhough, T. S., Joseph, S., Davis, M., Moresco, A., & Schreier, A. L. (2022). Impacts of Socialization on Bull Asian Elephant (Elephas maximus) Stereotypical Behavior. Journal of Zoological and Botanical Gardens, 3(1), 113-130. https://doi.org/10.3390/jzbg3010010







