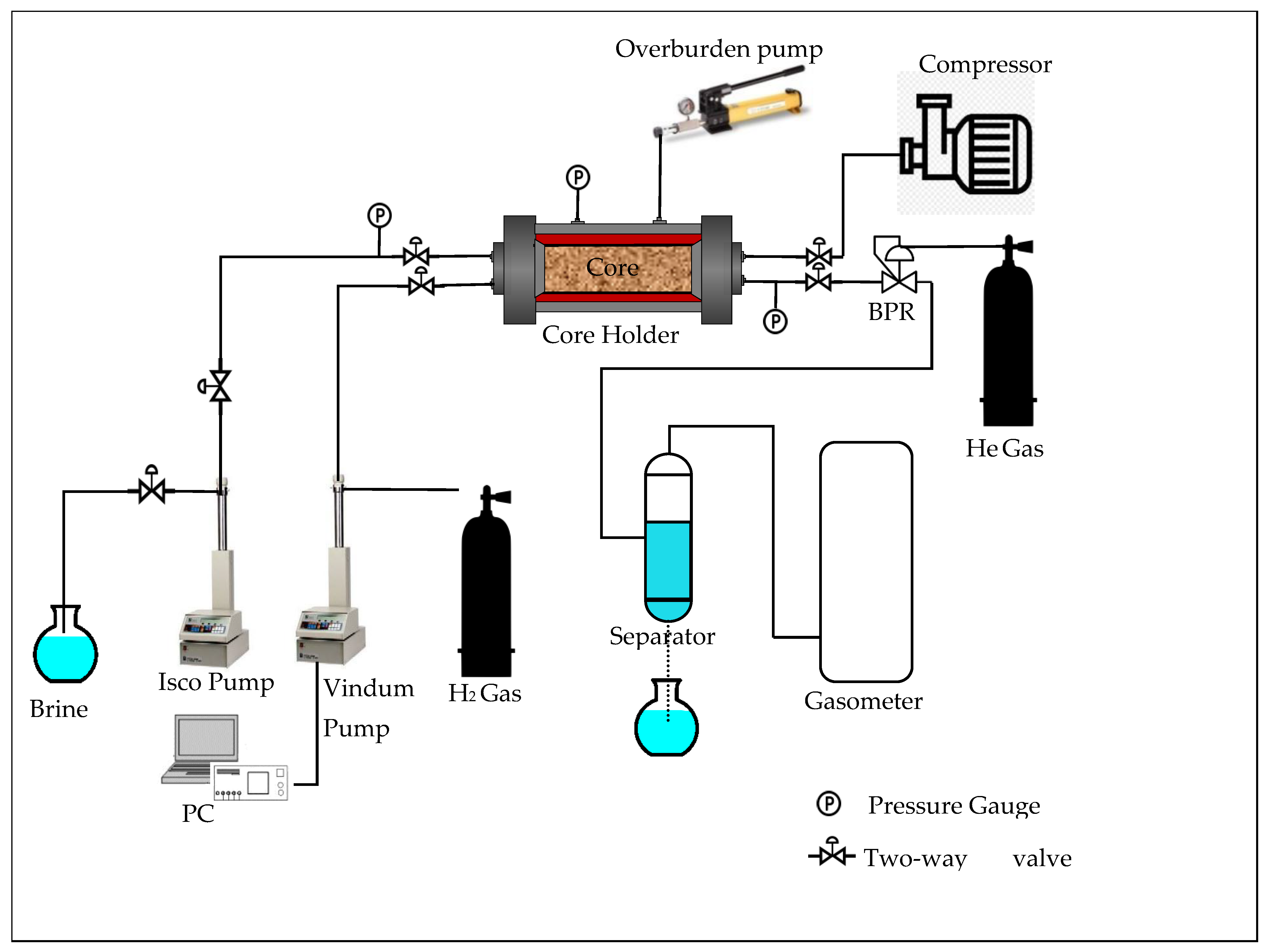
3.1. Brine and Hydrogen Absolute Permeability
The average measured absolute permeability to brine was 13.23 millidarcy (mD), based on steady-state tests conducted at three flow rates (0.25, 0.5, and 1.0 cm
3/min). Individual permeability values were highly consistent, ranging from approximately 13.1 to 13.3 mD, and exhibited no significant dependence on the flow rate, as summarized in
Table 4. This consistency indicates that Darcy’s law was obeyed throughout the experiments and that rate-dependent effects such as turbulence or fines migration were negligible in this regime.
Equation (1) calculates the brine permeability in mD derived from the Darcy equation
where
kw is the brine permeability in mD.
μl is the brine viscosity in cP.
ql is the brine flow rate at steady state conditions in cc/s.
L is the core length in cm.
P1 is the upstream pressure in atm.
P2 is the downstream pressure in atm.
∆P = P1 − P2.
A is the cross-sectional area of the porous medium in cm2.
The measured brine permeability,
kw (13.23 mD), closely matches the Klinkenberg-corrected gas permeability,
kg* (13.8 mD), with a difference of only about 4.5%.
Figure 5 illustrates a strong agreement between the brine-derived permeability and the gas-derived permeability extrapolated to infinite pressure. The minor difference in value (approximately 0.6 mD) falls within the range of experimental uncertainty. These results confirm that, once gas slippage is accounted for, the absolute permeability to gas converges with that of brine, as expected for an ideal single-phase system [
60,
61,
62]. The close correspondence between the brine and slip-corrected gas permeability values demonstrates the validity of the Klinkenberg correction and supports the conclusion that the core’s permeability is an intrinsic property, independent of the fluid phase when slip effects are properly addressed.
Measured hydrogen permeability,
kw, values ranged from 15 mD to 23 mD. We observed a strong trend of decreasing absolute permeability with increasing mean flowing pressure, with the highest permeability recorded at the lowest mean pressure (
Pm), as summarized in
Table 5. This pressure dependence is indicative of the Klinkenberg effect: the plot of gas permeability against the reciprocal of mean pressure (1/
Pm) shows an almost perfect linear correlation. This behavior reflects the influence of gas slippage along pore walls, which leads to inflated permeability measurements at lower pressures [
63,
64].
We used Equation (3), derived from Equation (2), to calculate the gas-measured permeability at the mean gas-flowing pressure
where
q1 is the brine flow rate at atmospheric pressure in cm3/s.
qg is the hydrogen flow rate at mean flowing pressure in cm3/s.
kg is the hydrogen permeability in mD.
μg is the hydrogen viscosity in cP.
P1 is the upstream pressure in atm.
P2 is the downstream pressure in atm.
Pa is the pressure at which gas flow rate is taken in atm.
Plotting the measured gas permeability,
kg, against 1/
Pm revealed a strong linear relationship with an R
2 of 0.985, as shown in
Figure 5. The standard error of the slope is 0.600 mD·atm (13.071 ± 0.600 mD·atm) and the standard error of the intercept is 0.208 mD (13.810 ± 0.208 mD). These standard errors indicate a precise fit with uncertainty that is modest relative to the parameter magnitudes and supports the accuracy and adequacy of the linear Klinkenberg model over our pressure range.
This almost perfect linear fit is consistent with Klinkenberg’s [
65] model of gas flow in porous media. The extrapolated interception of the linear fit, 13.8 mD (
Figure 5), represents the
kg*, which corresponds to the intrinsic permeability of the core in the absence of gas slippage. Practically, this value reflects the expected liquid-phase permeability at infinite pressure, where slip effects are negligible. The high degree of linearity and the strong R
2 confirm that gas slippage is the primary factor responsible for the observed pressure dependence of gas permeability in these experiments.
We used the linear form of Klingenberg’s [
66] equation, shown in Equation (4), to correct gas slippage in lab-measured gas permeability.
where
kg is the gas permeability.
b is the slip factor.
Pm = mean pressure, Equation (5).
P1 is the upstream pressure in atm.
P2 is the downstream pressure in atm.
λ is the mean flow path.
r is the pore-throat radius.
c is a proportional factor.
The relation between
kg and 1/
Pm was highly linear, having an R
2 of 0.985 with random, homoscedastic residuals. A first-order slip correction is theoretically appropriate in the slip-flow regime where the Knudsen number,
Kn (λ/r) is small but nonzero. Using a representative hydrogen mean free path, λ, of 100 nm at 1 atm [
67], which scales approximately as 1/
Pm, and pore-throat radii,
r, of 0.5 to 2 μm for chalk [
67,
68],
Kn is in the order of 0.01 to 0.2 across our conditions. This range lies primarily in the slip flow, where the linear term dominates. Consistent with this expectation, augmenting the model with a quadratic, as shown in Equation (6), term
c did not improve the goodness of fit or information criteria, and the estimated
c was not significant. By contrast, in much lower-permeability rocks or at very low pressures, deviations from linearity can occur. Ref. [
60], among others [
69,
70], developed an improved Klinkenberg model, accounting for non-linear gas slippage in tight cores, showing that the
kg vs. 1/
Pm relationship can become curved when
Kn is higher. Their analysis indicates that in ultra-tight media, a single
b-factor will not suffice, and higher-order terms or pressure-dependent slip coefficients are needed.
Non-linear slip effects can be of concern at lower pressures or in much finer throats that drive Kn ≳ 0.1 into the transition regime. Under such conditions, higher-order slip models or pore-scale simulations may be needed. Within the pressure and rock tested here, the linear Klinkenberg correction provides an adequate estimation of gas-slippage.
From the
kg vs. 1/
Pm plot, and using Equation (4), we estimated the Klinkenberg correction factor, b at 0.944 atm, shown in Equation (7) as
3.2. Hydrogen–Brine Relative Permeability
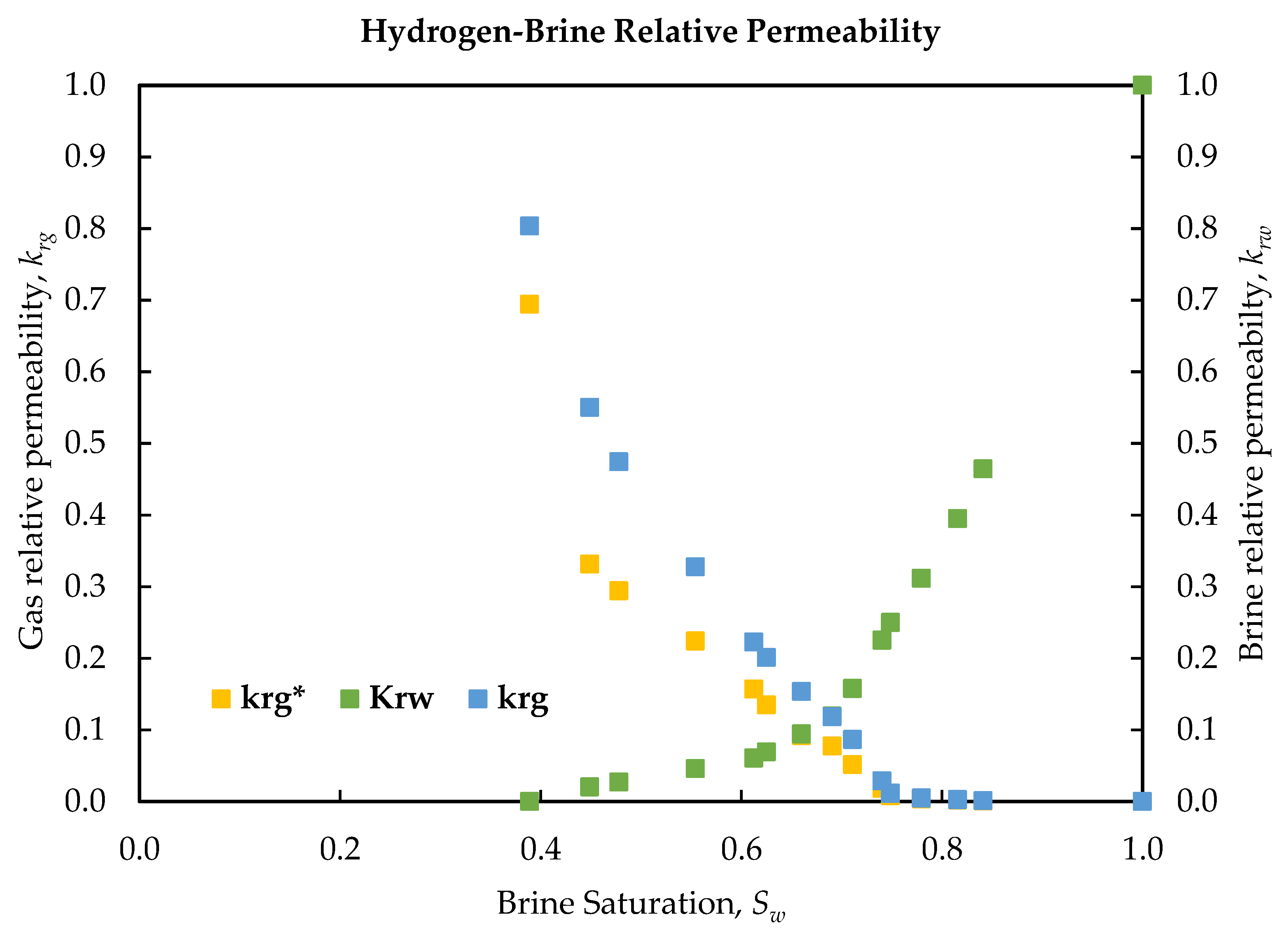
Steady-state relative permeability measurements for the hydrogen–brine system, presented in
Table 6, confirm strongly water-wet behavior. Noticeable hydrogen flow began at a
Sw of 0.84, where the
krg was 0.001 and the
krw had declined markedly to 0.464.
Sw,irr was reached at 0.39, at which point the brine became essentially immobile and the gas phase attained its maximum relative permeability of 0.8 (prior to slip correction). These results demonstrate a delayed hydrogen breakthrough, a sharp increase in
krg with increasing gas saturation, and a high
Sw,irr—all characteristic of a strongly water-wet system.
Figure 6 and
Table 7 present the experimentally determined relative permeabilities for brine and hydrogen, along with the corresponding slip-corrected values. The Klinkenberg slip corrected relative permeability,
krg*, values were obtained by applying the previously established slip correction factor to the gas-phase permeability at each saturation, thereby estimating the
krg, as it would be measured at infinite pressure (i.e., in the absence of slippage). At all saturation points,
krg* is consistently lower than the
krg, highlighting the elimination of slip-induced overestimation. Notably, the difference between
krg and
krg* becomes more pronounced at higher gas saturations (lower
Sw), where gas flow rates and Reynolds numbers are elevated and mean pressures are reduced. This systematic discrepancy emphasizes the necessity of accounting for gas slippage during two-phase flow experiments; neglecting this effect would lead to an overestimation of apparent gas mobility under reservoir (liquid-equivalent) conditions. Following correction, the hydrogen relative permeability curve is shifted downward, yielding a more accurate representation of the gas phase’s conductive capacity in the absence of slip effects. Despite this adjustment, the overall shape of the relative permeability curves remains unchanged: the gas curve initiates near zero (0) at high
Sw and increases sharply as
Sw decreases, while the water curve approaches
Sw,irr.
The high endpoint,
krg, in our H
2-brine data (0.8 at
Sw,irr of 0.39, dropping to 0.69 with slip correction) is slightly above the values reported for other gas–water systems. For instance, carbonate core tests in [
71] found CO
2 or methane–water’s relative permeabilities maxing out around 0.5–0.6 under similar saturations. Our hydrogen results reach a somewhat higher gas conductivity, which can be attributed to hydrogen’s lower interfacial tension and viscosity. Overall, the shape of the hydrogen relative permeability curve mirrors those documented for methane–brine or air–brine systems in strongly water-wet rocks [
72]. This indicates that hydrogen, like other non-wetting gases, must overcome entry pressures and then flow primarily through larger pore channels. One distinction is that hydrogen’s lower interfacial tension against water and lower viscosity can lead to slightly more efficient gas mobility. In fact, comparative analyses suggest that hydrogen may exhibit marginally higher relative permeability than methane at equivalent saturations [
41]. This is consistent with our data showing a high
krg endpoint. However, these differences are subtle and the shape of our relative permeability curve and CH
4-brine relative permeability curves are broadly similar: controlled primarily by rock wettability and pore structure, rather than gas type [
73].
Appendix A provides detailed calculations illustrating the estimation of porosity, pore volume,
Pm,
Sw,
kw,
kg,
kg*,
krw,
krg, and
krg* for a representative flow condition.
3.3. Controls on Flow: Wettability, Solubility, Diffusivity, and Pore Structure
The observed relative permeability trends can be interpreted in light of the core’s pore structure, wettability, and the hydrogen’s fluid properties. The Austin Chalk contains micron to sub-micron scale pores and throats [
67,
68], which promote extensive capillary trapping of brine. This fine-scale porosity explains the high
Sw,irr of 0.39. Water remains in the small pores and grain contacts, even after extensive hydrogen injection. As a result, hydrogen flow is largely confined to larger pore pathways at lower
Sw, which limits gas mobility until those larger channels connect. The core’s strongly water-wet nature further delays the hydrogen percolation. Hydrogen, being non-wetting in a water-wet matrix [
33], must overcome the entry capillary pressures to move through smaller pores. Once hydrogen pathways form,
krg rises sharply: a typical behavior of a strongly water-wet system where brine clings to pore walls and hydrogen flows through the central pore regions. The pronounced increase in
krg to 0.8 as at
Sw,irr reflects this behavior, as the water evacuation from the pore centers greatly boosts gas conductivity.
Hydrogen’s molecular characteristics, like low viscosity, small molecular size, and high diffusivity, also influence flow. While bulk-phase diffusion of hydrogen into the brine is minimal due to low solubility under steady-state conditions, the small molecule size manifests in the form of gas slippage. In our tests, at lower pressures, the mean free path of H2 (approximately 100 nm at 1 atm) became significant relative to the pore-throat sizes, resulting in the Klinkenberg effect. This slip flow enhanced the apparent gas permeability without altering the fundamental shape of the relative permeability curves. In essence, hydrogen’s high diffusivity and molecular free path allowed it to navigate pore walls more freely (increasing flow rates in micro-pores), but it did not change the fact that a water-wet, micro-porous structure governs the saturation at which gas can move through the rock. Hydrogen’s low solubility in brine means essentially all the injected H2 remains in the gas phase under our conditions. These factors combined (a micro-porous, strongly water-wet matrix and hydrogen’s slip-flow tendency) explain the delayed gas breakthrough and steep krg progression observed in our experiments.
Molecular diffusion of hydrogen through water can allow it to penetrate fine-scale stagnant pores or water films, producing a small “tail” of hydrogen presence, even at high water saturations, thus smoothing the
krw decline. Hydrogen’s tiny molecules also give rise to slip flow along pore walls, effectively reducing viscous drag and enhancing its mobility compared to higher molecular gases [
74]. Pore-scale modeling by [
38] indicates that hydrogen can diffuse into water-filled micro-porosities more readily than larger gas molecules, although this effect is subtle during active flow. We did not explicitly measure diffusion in our experiments, but qualitatively, if any hydrogen dissolved into the brine or diffused ahead of the displacement front, it could contribute to a smoother decline in
krw and a small hydrogen presence, even above
Sw,irr. Recent pore-scale analyses confirm these effects: when the
Kn exceeds 1 × 10
−3, hydrogen exhibits both bulk slip flow and surface (adsorption) diffusion, so that the classical no-slip Darcy law breaks down. The remaining distinctive features of hydrogen at low
Sw, which includes the rapid rise of
krg and a near-linear
krg–
Sw trend, agree qualitatively with recent models and experiments. For example, ref. [
75] shows that hydrogen’s non-wetting relative permeability curve is nearly linear at a high gas saturation. Similarly, ref. [
76] reports strong hysteresis in hydrogen–water relative permeability, with significant hydrogen flow persisting into the imbibition regime. The pore-scale phenomena of wall slip and molecular diffusion help explain why hydrogen achieves such high relative permeability in our experiments and why slight departures from methane-analog behavior occur in low-perm pores. The slight deviations observed in the low-saturation end of our hydrogen relative permeability curve can be partly attributed to pore-scale effects like interfacial slip and molecular diffusion. Our slip correction ensured that this did not distort the relative permeability magnitude, but at very low
Sw, hydrogen was flowing through the largest pores with high
Kn.
3.4. Field-Scale Implications and Reservoir Condition Considerations
The relative permeability curves derived in this work can directly provide input data for UHS models, but certain scaling considerations must be addressed. Our steady-state drainage-relative permeability functions provide input parameters for reservoir simulators, improving predictions of hydrogen injectivity and recovery. For instance, using the measured endpoint
krg will yield more realistic injectivity estimates than assuming an arbitrary value. Likewise, the high
Sw,irr we found implies that a considerable fraction of pore volume will remain occupied by brine, depending on the formation rock, which, in field models, translates to the need for cushion gas and lower working hydrogen capacity. It is important to note that we have characterized only the drainage cycle; during withdrawal (imbibition), relative permeability curves will differ due to hysteresis [
32]. Neglecting hysteresis in UHS cycles can lead to optimistic predictions. Bo et al. [
39] report that ignoring relative permeability hysteresis can overestimate the annual working gas capacity by up to 34% and the recovered hydrogen volume by 85%.
When utilizing our curves in simulations, one should implement appropriate hysteresis models or imbibition curves to account for the lower gas relative permeability and residual trapping expected in hydrogen production. While our experiments were performed on a homogeneous core in ambient laboratory conditions, reservoirs may exhibit greater heterogeneity, different wettability distributions, and elevated pressures/temperatures. In situ, higher overburden stress could reduce absolute permeability, and any fine-scale heterogeneity or partial wettability changes at the reservoir temperature might lower the effective relative permeability. The slip-corrected hydrogen–brine curves presented here are effectively intrinsic (approximating infinite pressure conditions), which makes them transferable to higher reservoir pressures without further correction for gas slippage. However, reservoir-scale modeling may still require integrating capillary pressure functions and accounting for multi-scale rock features. Nonetheless, the fundamental trends captured, i.e., a strongly water-wet system with a high Sw,irr and rapid rise in krg, are expected to remain in similar geologic formations. These experimental flow functions thus serve as a valuable calibration dataset. Based on our data, we anticipate that reservoir conditions would yield slightly lower gas slippage and slightly higher hydrogen diffusivity, but similar relative permeability shapes. History-matching our relative permeability curves in numerical simulations will constrain models of hydrogen plume migration and trapping. In particular, incorporating the measures will improve estimates of injection pressure requirements, working gas fraction, and cushion gas needs. By using laboratory-derived hydrogen–brine relative permeabilities instead of analogs from methane or CO2, UHS project simulations can achieve greater fidelity in forecasting storage performance under cyclic operations.
Our findings carry important implications for hydrogen containment in UHS. The good news is that the intrinsic permeability of the reservoir rock and the high krg at Sw,irr indicate that hydrogen can flow readily through the intended storage formation, supporting efficient injectivity and withdrawal. However, the flip side is that hydrogen’s mobility means that if any unintended pathway exists (fractures, caprock micro-perm, or wellbore leaks), hydrogen could escape more easily than a less mobile fluid. In particular, hydrogen’s high diffusivity and low viscosity raise the risk of leakage through tiny pores or defects that might retain other gases. For instance, our slip-corrected data show that hydrogen moves almost as freely as brine in the rock. Thus, any permeable conduit could transmit hydrogen nearly unhindered, underscoring the need for extremely low-permeability seals. The measured parameters from our work can be used to model worst-case leakage scenarios. The high hydrogen relative permeability in storage conditions also implies that once a crack or pathway opens, hydrogen will preferentially flow through it (being non-wetting and mobile). Therefore, safeguards like multiple sealing layers, active monitoring, and rapid pressure relief are warranted to mitigate leakage risk.
Hydrogen’s high diffusivity poses a long-term leakage concern in UHS. The small hydrogen molecules can gradually migrate through ostensibly tight caprocks and engineered seals over storage timescales [
77]. This diffusion-driven loss mechanism may undermine storage integrity, especially in sites with thinner or more porous caprock layers. Hydrogen diffuses very slowly through water-saturated caprocks, with effective diffusivities typically being around 1 × 10
−10–1 × 10
−8 m
2/s [
78], so long-term migration is controlled by molecular diffusion, rather than flow [
79]. Despite being slow, diffusion can still move gas appreciable distances over decades. A simple semi-analytical model by [
80] shows that diffusivity of approximately 1 × 10
−7 m
2/s, which is at the upper end of measured values, would drive on the order of 2.5 m of H
2 penetration per year. Additionally, reservoir simulation by [
81] found diffusion-driven plume expansion of H
2 to be a few percent under the ideal conditions and suggested that diffusion flow has a minimal impact on plume migration. Hydrogen can likewise permeate wellbore seals over time. Numerical simulations indicate that hydrogen could fully penetrate a standard 35 cm-thick cement sheath in about a week under dry conditions, due to its small molecular size and high diffusivity [
82]. Caprocks strongly suppress flux because low porosity and high tortuosity reduce the effective diffusivity to only a few percent of the bulk value [
81], and full brine saturation further slows hydrogen compared with gas-phase diffusion [
79]. Cushion gases such as methane or CO
2 can further reduce hydrogen’s effective diffusivity by diluting its partial pressure at the seal and partially blocking pore pathways, so a dense, brine-saturated, low-permeability caprock with cushion gas in the reservoir behaves closely to a diffusion barrier for H
2 over practical storage timescales [
83]. These findings show that while caprocks and well seals exhibit ultra-low permeability to convective flow, their ability to halt molecular diffusion of hydrogen is limited. Therefore, diffusion-induced losses must be considered in long-term containment risk assessments.
The plug contained minor laminations (chalk and marl), but it behaved hydraulically homogeneously. Pressure drops were smooth with consistent saturation changes, with no signatures of multi-region flow, such as dual plateaus or abrupt jumps in fractional flow. The thin laminations were parallel to the flow, so the co-injected fluids sampled an average of all layers and the measured relative permeability represents an effective composite. If strong heterogeneity were present, for example, a high-permeability streak or a fracture, we would expect an earlier gas breakthrough or a lower apparent Sw,irr. This was not observed; the breakthrough was gradual and consistent with a uniform water-wet system. The close agreement between kw and the krg* (difference about 4.5%) further indicates an evenly distributed flow. We conclude that core-scale heterogeneity had minimal impact on our results. Field-scale heterogeneity can still influence local behavior, so our curve should be viewed as representative of a homogeneous matrix.
We quantified the force balance in the displacement experiments using the dimensionless capillary number, defined in Equation (8) as
where
Nc is capillary number.
μ is gas viscosity in Pa∙s.
vg is superficial (Darcy) velocity in m/s.
σ is interfacial tension in N/m.
For hydrogen in our core, μ is approximately 9 × 10−6 Pa∙s (0.009 cP) and σ of 0.072 N/m for the H2—brine system. At the highest gas injection rate of 20 cm3/min, vg is 6.6 × 10−4 m/s, which yields Nc on the order of 10 × 10−7 to 10 × 10−8. At the lowest gas rate of 0.2 cm3/min, (vg is 6.6 × 10−4 m/s), Nc was approximately 10 × 10−9. These values are several orders of magnitude below the commonly cited threshold Nc value of 2 × 10−5 required to mobilize a trapped wetting phase. Thus, viscous forces in our tests were too small to overcome the capillary retention of brine. This is consistent with the Sw,irr and indicates a capillary-force dominated regime. The flow rates (0.2 to 20 cm3/min H2 co-injected with 0.8 to 0.05 cm3/min brine) correspond to interstitial velocities on the order of 10 × 10−6 to 10 × 10−4 m/s, which further supports that capillary forces dominated at these flow rates. As a result, hydrogen did not significantly displace brine from smaller pores, because viscous forces were insufficient to overcome capillary trapping, in line with the observed strongly water-wet behavior.
To assess experimental reliability, we quantified uncertainties and checked the repeatability of the measurements. The single-phase permeability tests showed excellent consistency. kw varied from 13.07 to 13.33 mD across three flow rates (within 2% range), indicating negligible rate-dependent effects and high repeatability. Similarly, the kr vs. 1/Pm plot was almost perfectly linear with an R2 of 0.985, demonstrating minimal scatter in the measured data. Based on equipment precision (pressure transducers ±0.25% F.S., flow measurements ±1%, and balance accuracy ±0.01 g for core mass), we estimate an uncertainty on the order of ±5% in the reported permeability values. Water saturation determinations by core weighing are accurate within approximately ±0.5% of the pore volume, given the small mass increments recorded. The stringent steady-state criteria (change in pressure stability < 1% and flow rate variation < 3% over 30 min intervals) lend confidence that each data point is representative. Overall, the precision and repeatability of these results support their validity for use in further analysis.