Machine Learning Applications in Gray, Blue, and Green Hydrogen Production: A Comprehensive Review
Abstract
1. Introduction
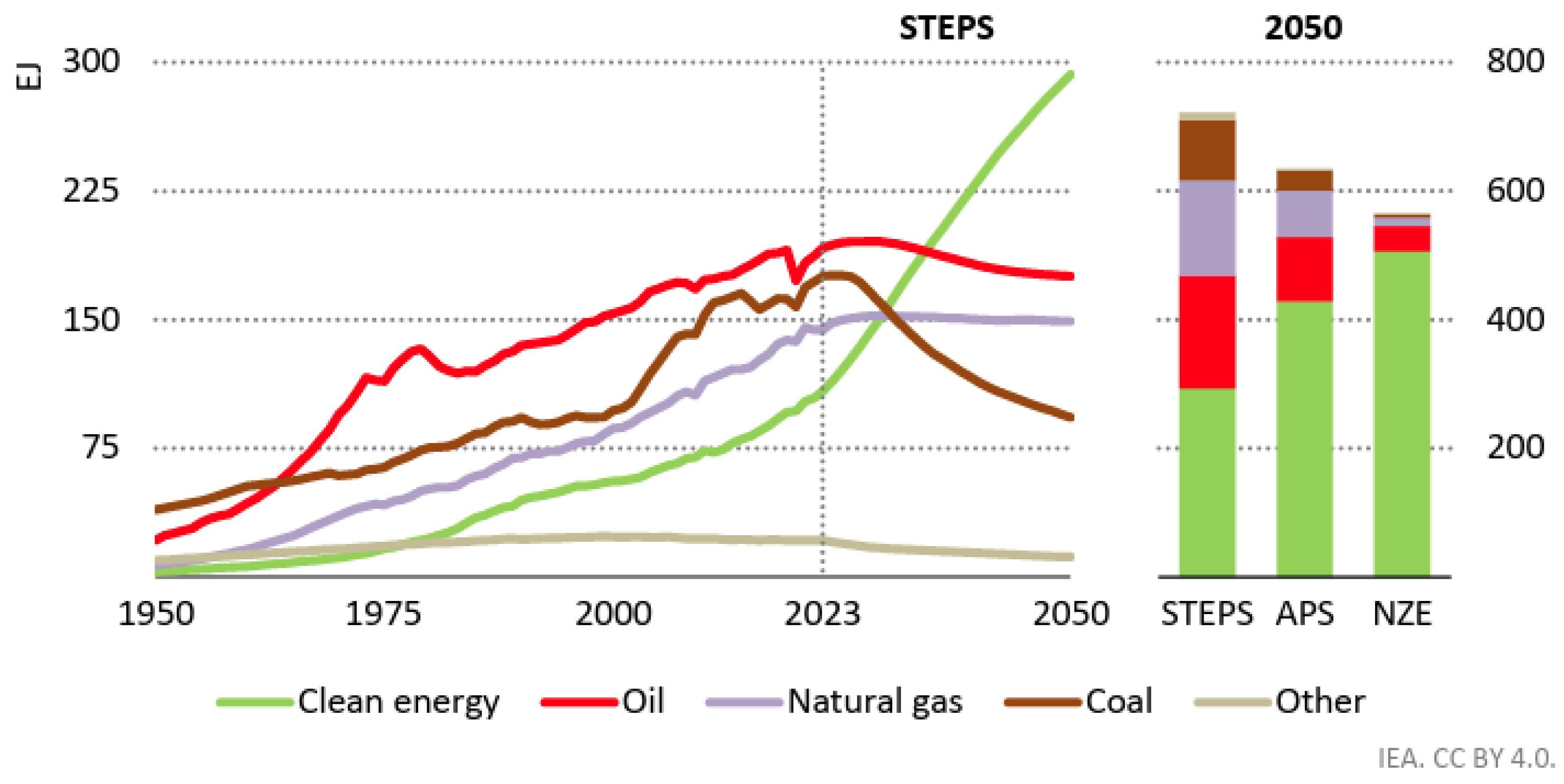
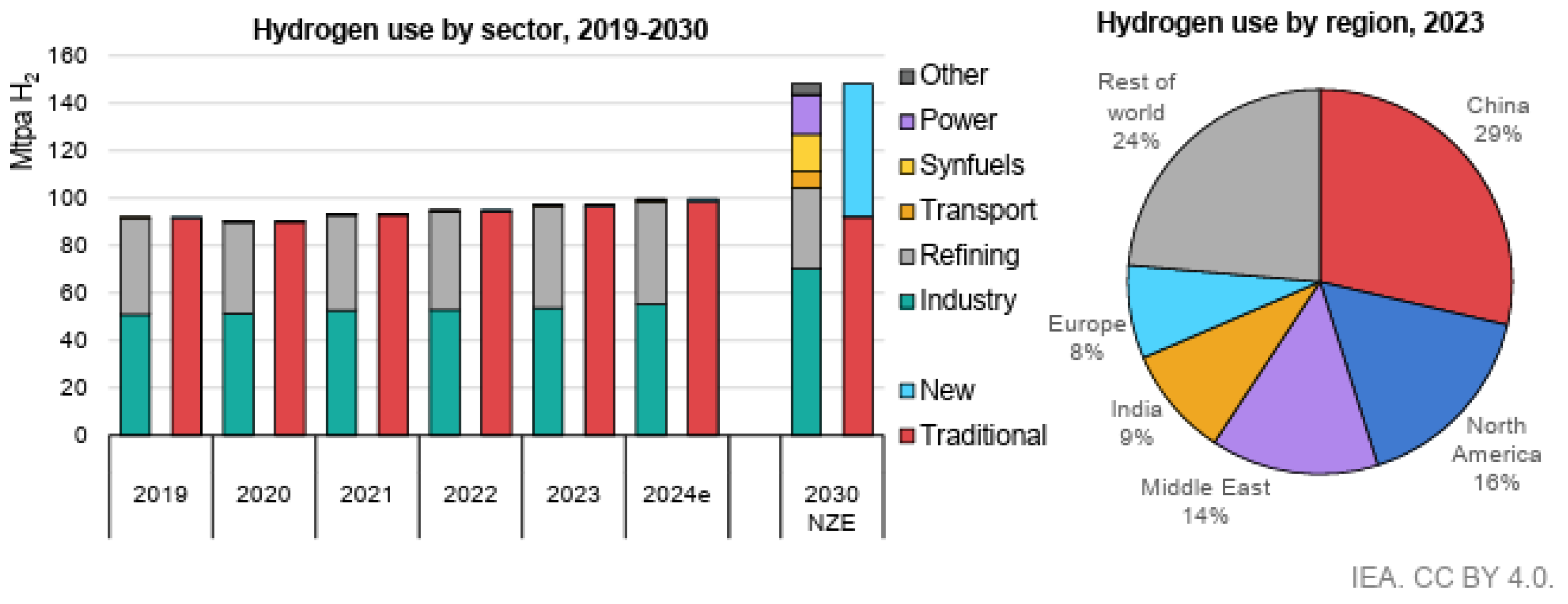
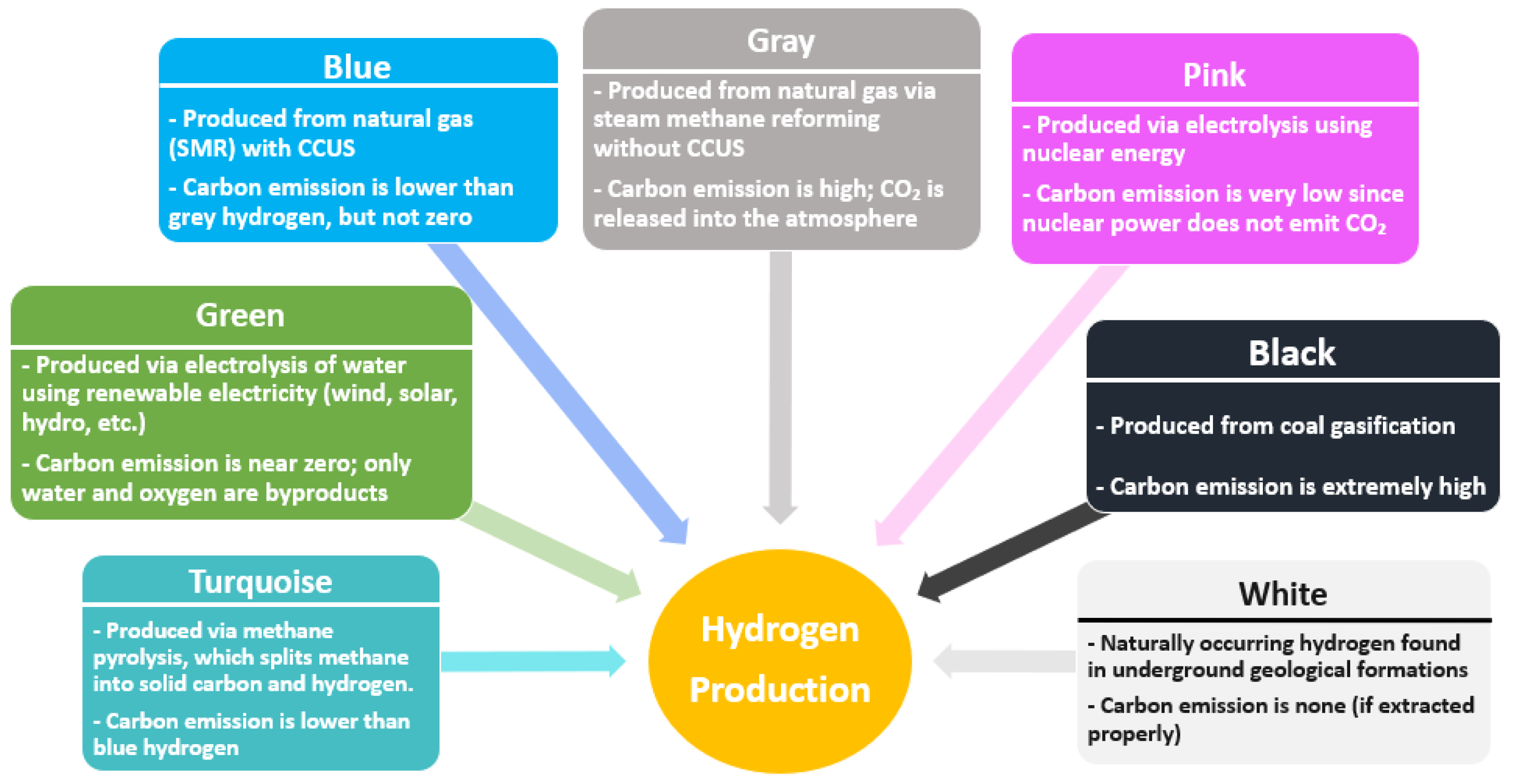
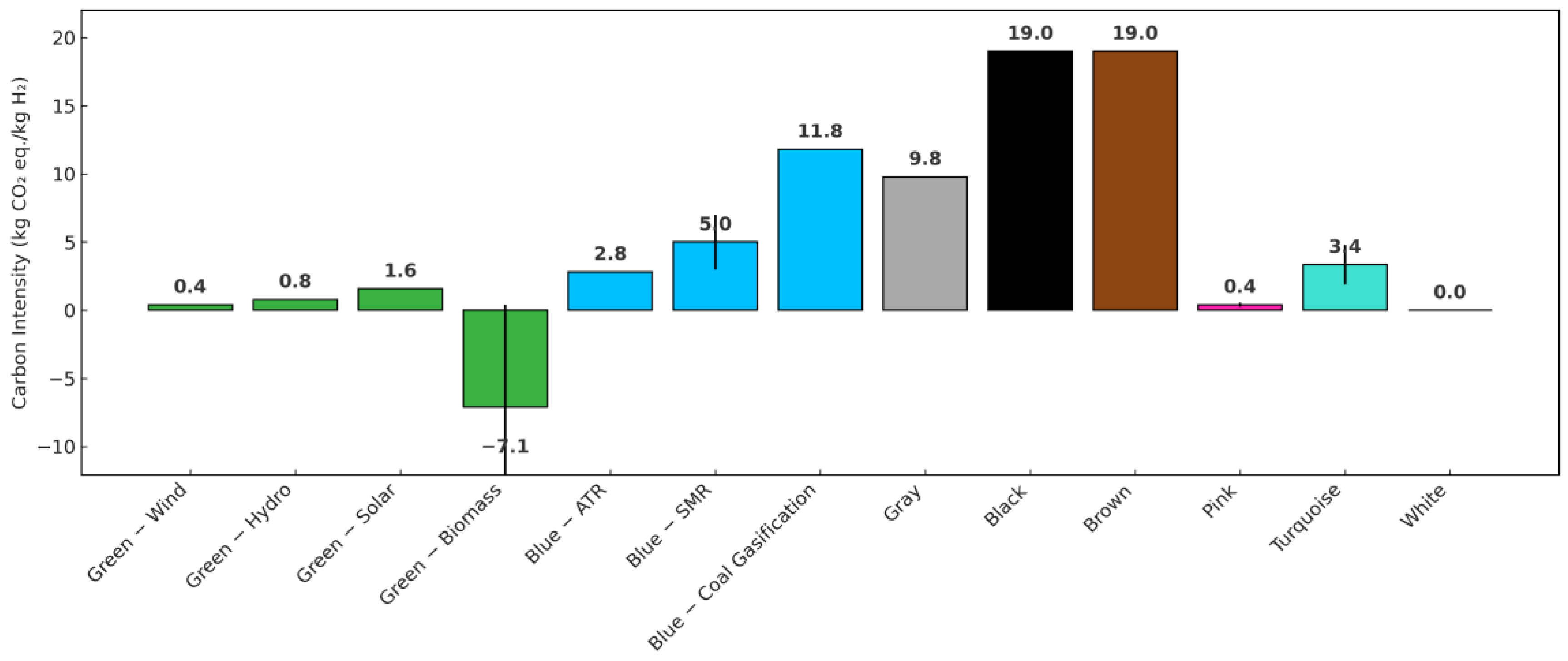
1.1. Background of Hydrogen Production
1.2. Incorporating Machine Learning with Hydrogen Production
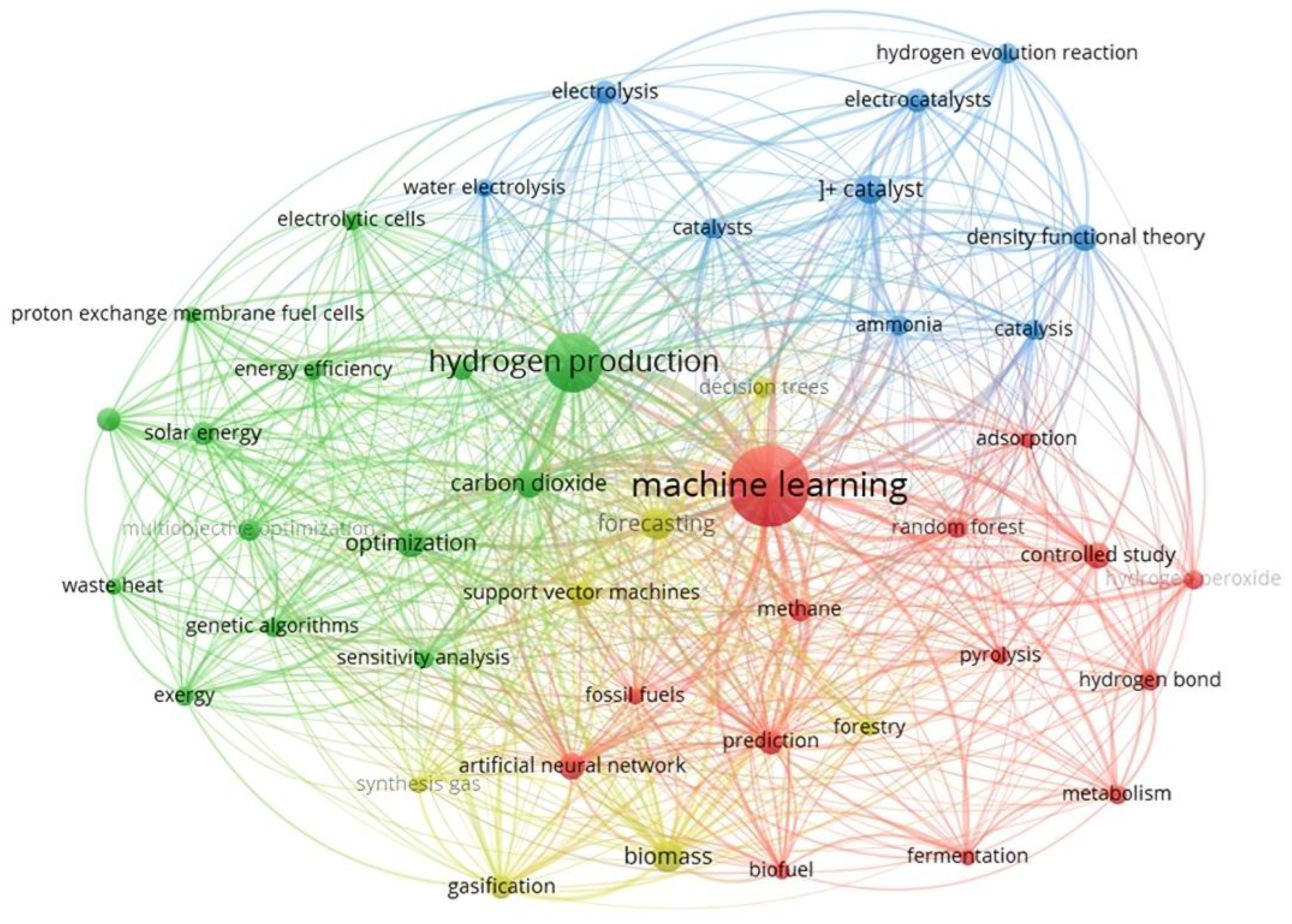
1.3. Motivation of This Review
2. Overview of Machine Learning
2.1. Brief History of ML
2.2. Categories of ML
- Supervised learning: In this approach, models are trained on labeled data, meaning each input is paired with the correct output. The algorithm learns by minimizing errors and improving accuracy through iterative training. Examples include linear regression, decision trees, and neural networks, which are commonly used in applications such as fraud detection, medical diagnosis, and stock price prediction.
- Unsupervised learning: This method deals with unlabeled data, where the algorithm identifies hidden patterns or structures without explicit output labels. Clustering and association rule learning are common techniques with applications in customer segmentation, anomaly detection, and market analysis. Examples include k-means clustering and principal component analysis (PCA).
- Reinforcement learning: Unlike the previous categories, reinforcement learning (RL) is based on reward-based learning, where an agent interacts with an environment and learns through trial and error to maximize cumulative rewards. RL is widely used in robotics, gaming (e.g., AlphaGo), and autonomous systems. Algorithms such as Q-learning and deep Q-networks (DQNs) power advanced decision-making systems.
2.3. Common ML Algorithms
3. Blue Hydrogen Production and ML Applications
3.1. Blue Hydrogen Production
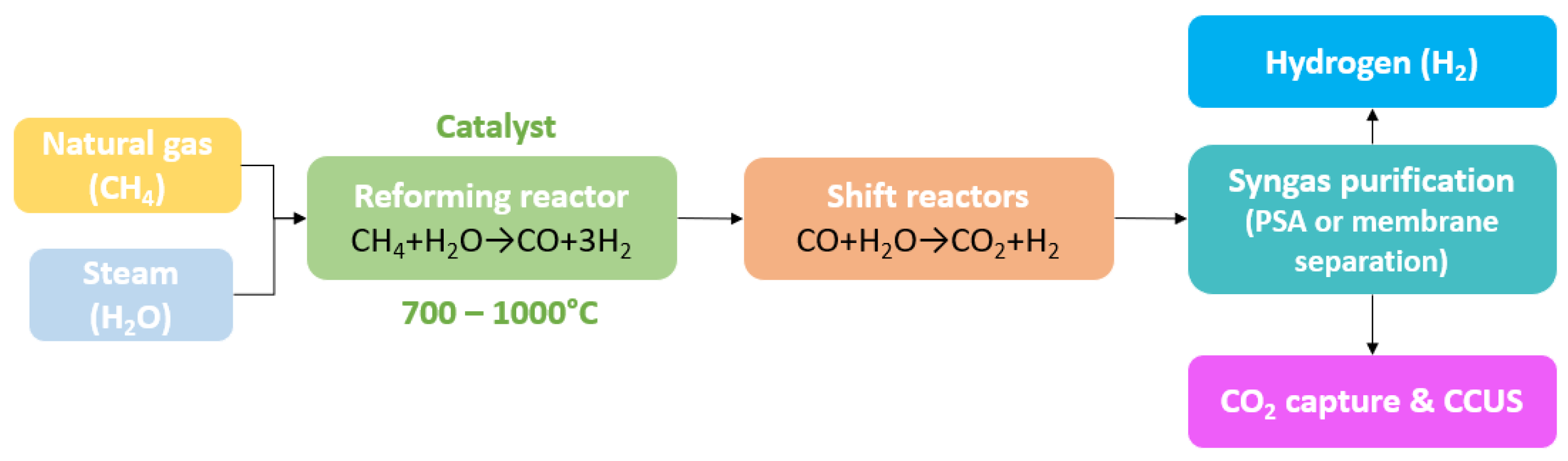
3.1.1. Steam Methane Reforming (SMR)
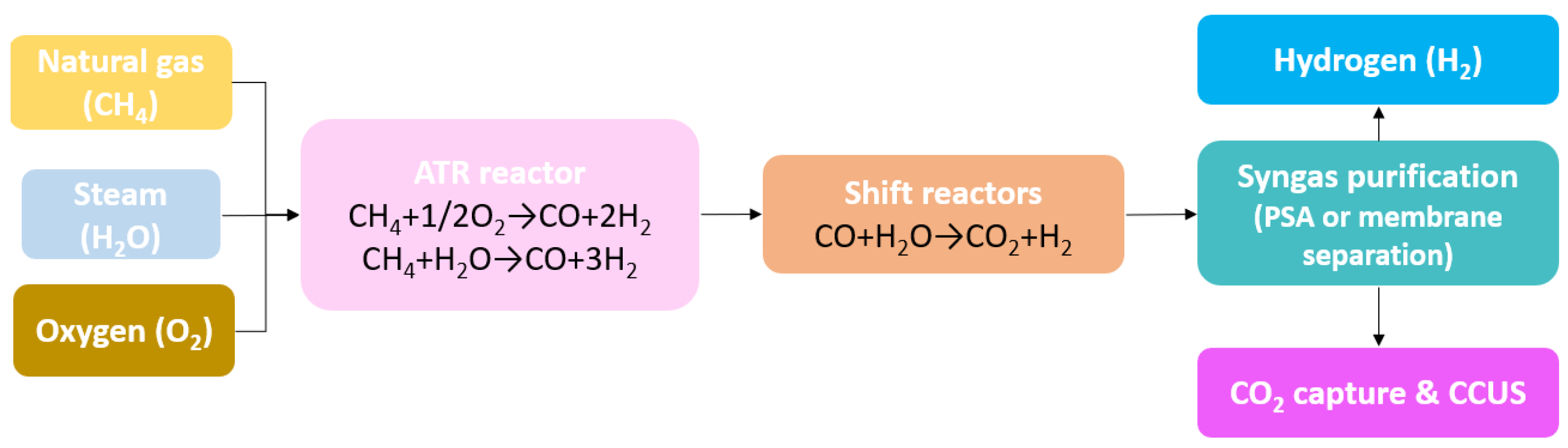
3.1.2. Autothermal Reforming (ATR)
- POX Reaction
- 2.
- Steam Reforming Reaction
- 3.
- Water–Gas Shift Reaction (WGSR)
3.2. ML Application for Blue Hydrogen
4. Gray Hydrogen Production and ML Applications
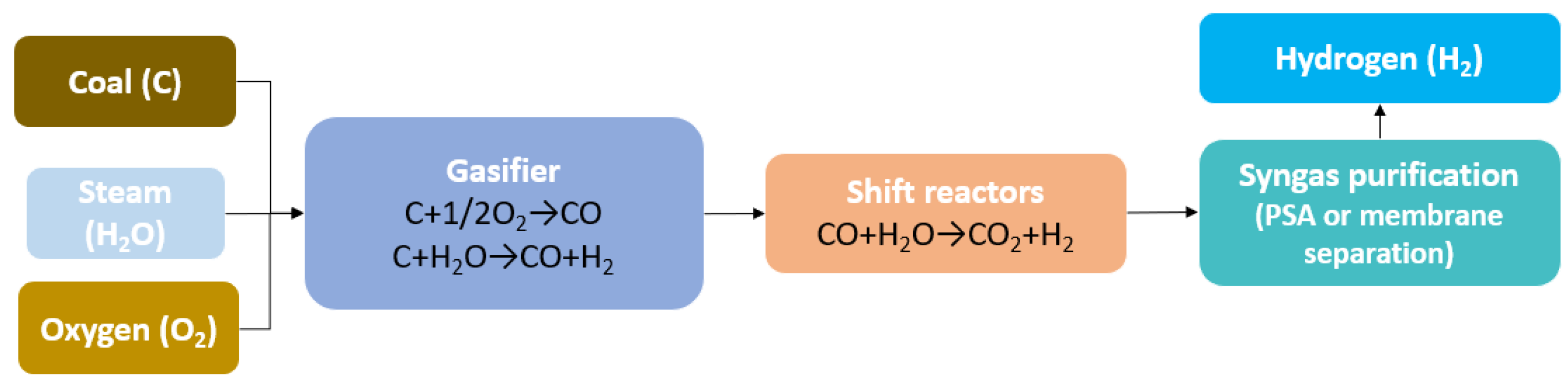
4.1. Gray Hydrogen Production Process
4.2. ML Application for Gray Hydrogen
5. Green Hydrogen Production and ML Applications
5.1. Green Hydrogen Production Process
5.1.1. Water Electrolysis
5.1.2. Biomass Gasification
5.1.3. PEC Water Splitting
5.1.4. Biohydrogen Production
5.2. ML Application for Green Hydrogen
6. Other Hydrogen Production Pathways and Their ML Applications
6.1. Pink Hydrogen Production
6.2. Turquoise Hydrogen Production
6.3. White Hydrogen Production
6.4. Black/Brown Hydrogen Production
6.5. ML Application Summary
7. Key Challenges, Opportunities, and Future Work
7.1. Key Challenges
7.2. Opportunities
7.3. Future Work
8. Conclusions
- A total of 51 peer-reviewed papers from 2012 to 2025 were analyzed, covering ML applications across multiple hydrogen production pathways.
- Green hydrogen received the most ML attention, especially in water electrolysis and biomass gasification, driven by the global shift toward carbon-neutral energy systems.
- ANNs and their variants (e.g., MLP, BPNN, RBF) were the most frequently used models, applied in over 60% of the studies.
- Ensemble learning methods like RF, GBR, and XGBoost demonstrated high predictive accuracy and are increasingly used in catalyst screening, syngas modeling, and multi-variable optimization.
- Time-series models (e.g., LSTM, Bi-LSTM) were effectively employed in forecasting applications, such as renewable-energy-driven electrolysis and biohydrogen production.
- Common input variables included process parameters (temperature, pressure, flow rates), feedstock properties (elemental composition, ash, moisture), and environmental conditions (solar irradiance, weather data).
- Key predicted outputs included hydrogen yield, CO2 capture or emission rates, syngas composition, and economic metrics such as production cost.
- Major challenges include limited real-world deployment, data availability, and a lack of model interpretability, especially in safety-critical systems.
- Future work should focus on developing robust, generalizable ML models supported by high-quality real-time datasets, emphasizing industrial integration, cost analysis, and techno-economic and life-cycle assessments and addressing current gaps in model validation and interpretability.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABR/ADA | AdaBoost regression |
| AdB | Adaptive boosting regression |
| AMT | Alternating model tree |
| ANFIS | Adaptive neuro-fuzzy inference system |
| ANN | Artificial neural network |
| ARM | Association rule mining |
| ASNN | Associative neural network |
| Bi-LSTM | Bidirectional LSTM |
| BP | Backpropagation |
| BR | Bayesian regularization |
| CNN | Convolutional neural network |
| DE | Differential evolution |
| DNN | Deep neural network |
| DRM | Dry reforming of methane |
| DT | Decision tree |
| ELM | Extreme learning machine |
| ENR | Elastic net regression |
| ETR | Ensemble tree regression |
| FFBPNN | Feed-forward backpropagation neural network |
| GA | Genetic algorithm |
| GBDT | Gradient boosting decision tree |
| GBM | Gradient boosting machine |
| GBR | Gradient boosting regression |
| GP | Genetic programming |
| GPR | Gaussian process regression |
| KNN | N-nearest neighbor |
| KR | Kernel ridge |
| LGB | LightGBM |
| LM | Levenberg–Marquardt |
| LSTM | Long short-term memory |
| LSSVM | Least squares support vector machine |
| LTS | Low temperature shift |
| MDEA | Methyl diethanolamine |
| MLP | Multilayer perceptron |
| MLR-RR | Multi-linear regression with ridge regularization |
| MOGA | Multi-objective genetic algorithm |
| MSE | Mean squared error |
| MTL | Multitask learning |
| MVR | Multivariate regression |
| NARX | Nonlinear autoregressive model with exogenous inputs neural network |
| NNs | Neural networks |
| NSGA-II | Non-dominated sorting genetic algorithm II |
| PLS | Partial least squares |
| PSA | Pressure swing adsorption |
| PSO | Particle swarm optimization |
| PZ | Piperazine |
| QSPR | Quantitative structure–property relationship |
| RBFNN | Radial basis function neural network |
| ResNet | Residual convolutional neural network |
| RF | Random forest |
| RR | Ridge regression |
| SCG | Scaled conjugate gradient |
| SE-SMR | Sorption-enhanced steam methane reforming |
| SMOreg | Sequential minimal optimization regression |
| SMR | Steam methane reforming |
| SMR | Small modular reactor |
| SVD | Singular value decomposition |
| SVM | Support vector machine |
| SVR | Support vector regression |
| TINN | Thermodynamics-informed neural network |
References
- IEA World Energy Outlook. 2024. Available online: https://www.iea.org/reports/world-energy-outlook-2024 (accessed on 11 February 2025).
- Acar, C.; Dincer, I. Review and Evaluation of Hydrogen Production Options for Better Environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An Overview of Hydrogen Production Technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- IEA Global Hydrogen Review. 2024. Available online: https://www.iea.org/reports/global-hydrogen-review-2024 (accessed on 11 February 2025).
- George Davies, W.; Babamohammadi, S.; Yang, Y.; Masoudi Soltani, S. The Rise of the Machines: A State-of-the-Art Technical Review on Process Modelling and Machine Learning within Hydrogen Production with Carbon Capture. Gas. Sci. Eng. 2023, 118, 205104. [Google Scholar] [CrossRef]
- Incer-Valverde, J.; Korayem, A.; Tsatsaronis, G.; Morosuk, T. “Colors” of Hydrogen: Definitions and Carbon Intensity. Energy Convers. Manag. 2023, 291, 117294. [Google Scholar] [CrossRef]
- United Nations Economic Commission for Europe (UNECE). Hydrogen: Technology Brief. 2022. Available online: https://unece.org/hydrogen (accessed on 20 February 2025).
- Jordan, M.I.; Mitchell, T.M. Machine Learning: Trends, Perspectives, and Prospects. Science (1979) 2015, 349, 255–260. [Google Scholar] [CrossRef]
- Sarker, I.H. Machine Learning: Algorithms, Real-World Applications and Research Directions. SN Comput. Sci. 2021, 2, 160. [Google Scholar] [CrossRef]
- Shahin, M.; Simjoo, M. Potential Applications of Innovative AI-Based Tools in Hydrogen Energy Development: Leveraging Large Language Model Technologies. Int. J. Hydrogen Energy 2025, 102, 918–936. [Google Scholar] [CrossRef]
- Kwon, H.; Park, J.; Shin, J.E.; Koo, B. Optimal Investment Strategy Analysis of On-Site Hydrogen Production Based on the Hydrogen Demand Prediction Using Machine Learning. Int. J. Energy Res. 2024, 2024. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A Brief Review of Hydrogen Production Methods and Their Challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- Alagumalai, A.; Devarajan, B.; Song, H.; Wongwises, S.; Ledesma-Amaro, R.; Mahian, O.; Sheremet, M.; Lichtfouse, E. Machine Learning in Biohydrogen Production: A Review. Biofuel Res. J. 2023, 10, 1844–1858. [Google Scholar] [CrossRef]
- Kumar Sharma, A.; Kumar Ghodke, P.; Goyal, N.; Nethaji, S.; Chen, W.-H. Machine Learning Technology in Biohydrogen Production from Agriculture Waste: Recent Advances and Future Perspectives. Bioresour. Technol. 2022, 364, 128076. [Google Scholar] [CrossRef]
- Bassey, K.E.; Ibegbulam, C. Machine learning for green hydrogen production. Comput. Sci. IT Res. J. 2023, 4, 368–385. [Google Scholar] [CrossRef]
- Allal, Z.; Noura, H.N.; Salman, O.; Vernier, F.; Chahine, K. A Review on Machine Learning Applications in Hydrogen Energy Systems. Int. J. Thermofluids 2025, 26, 101119. [Google Scholar] [CrossRef]
- Takeda, S.; Nam, H.; Chapman, A. Low-Carbon Energy Transition with the Sun and Forest: Solar-Driven Hydrogen Production from Biomass. Int. J. Hydrogen Energy 2022, 47, 24651–24668. [Google Scholar] [CrossRef]
- Devasahayam, S. Deep Learning Models in Python for Predicting Hydrogen Production: A Comparative Study. Energy 2023, 280, 128088. [Google Scholar] [CrossRef]
- Qi, H.; Cui, P.; Liu, Z.; Xu, Z.; Yao, D.; Wang, Y.; Zhu, Z.; Yang, S. Conceptual Design and Comprehensive Analysis for Novel Municipal Sludge Gasification-Based Hydrogen Production via Plasma Gasifier. Energy Convers. Manag. 2021, 245, 114635. [Google Scholar] [CrossRef]
- Haq, Z.U.; Ullah, H.; Khan, M.N.A.; Naqvi, S.R.; Ahsan, M. Hydrogen Production Optimization from Sewage Sludge Supercritical Gasification Process Using Machine Learning Methods Integrated with Genetic Algorithm. Chem. Eng. Res. Des. 2022, 184, 614–626. [Google Scholar] [CrossRef]
- Kononenko, I. Machine Learning for Medical Diagnosis: History, State of the Art and Perspective. Artif. Intell. Med. 2001, 23, 89–109. [Google Scholar] [CrossRef]
- Fradkov, A.L. Early History of Machine Learning. IFAC-Pap. 2020, 53, 1385–1390. [Google Scholar] [CrossRef]
- Zhu, X.; Goldberg, A.B. Introduction to Semi-Supervised Learning; Springer International Publishing: Cham, Switzerland, 2009; ISBN 978-3-031-00420-9. [Google Scholar]
- Zhou, Z.-H. Machine Learning; Springer Singapore: Singapore, 2021; ISBN 978-981-15-1966-6. [Google Scholar]
- Mahesh, B. Machine Learning Algorithms—A Review. Int. J. Sci. Res. (IJSR) 2020, 9, 381–386. [Google Scholar] [CrossRef]
- Rundo, F.; Trenta, F.; di Stallo, A.L.; Battiato, S. Machine Learning for Quantitative Finance Applications: A Survey. Appl. Sci. 2019, 9, 5574. [Google Scholar] [CrossRef]
- Zitnik, M.; Nguyen, F.; Wang, B.; Leskovec, J.; Goldenberg, A.; Hoffman, M.M. Machine Learning for Integrating Data in Biology and Medicine: Principles, Practice, and Opportunities. Inf. Fusion. 2019, 50, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Lary, D.J.; Alavi, A.H.; Gandomi, A.H.; Walker, A.L. Machine Learning in Geosciences and Remote Sensing. Geosci. Front. 2016, 7, 3–10. [Google Scholar] [CrossRef]
- Morgan, D.; Jacobs, R. Opportunities and Challenges for Machine Learning in Materials Science. Annu. Rev. Mater. Res. 2020, 50, 71–103. [Google Scholar] [CrossRef]
- Schweidtmann, A.M.; Esche, E.; Fischer, A.; Kloft, M.; Repke, J.; Sager, S.; Mitsos, A. Machine Learning in Chemical Engineering: A Perspective. Chem. Ing. Tech. 2021, 93, 2029–2039. [Google Scholar] [CrossRef]
- Murkin, C.; Brightling, J. Eighty Years of Steam Reforming. Johns. Matthey Technol. Rev. 2016, 60, 263–269. [Google Scholar] [CrossRef]
- Simpson, A.; Lutz, A. Exergy Analysis of Hydrogen Production via Steam Methane Reforming. Int. J. Hydrogen Energy 2007, 32, 4811–4820. [Google Scholar] [CrossRef]
- Saha, P.; Akash, F.A.; Shovon, S.M.; Monir, M.U.; Ahmed, M.T.; Khan, M.F.H.; Sarkar, S.M.; Islam, M.K.; Hasan, M.M.; Vo, D.-V.N.; et al. Grey, Blue, and Green Hydrogen: A Comprehensive Review of Production Methods and Prospects for Zero-Emission Energy. Int. J. Green. Energy 2024, 21, 1383–1397. [Google Scholar] [CrossRef]
- Oni, A.O.; Anaya, K.; Giwa, T.; Di Lullo, G.; Kumar, A. Comparative Assessment of Blue Hydrogen from Steam Methane Reforming, Autothermal Reforming, and Natural Gas Decomposition Technologies for Natural Gas-Producing Regions. Energy Convers. Manag. 2022, 254, 115245. [Google Scholar] [CrossRef]
- Bauer, C.; Treyer, K.; Antonini, C.; Bergerson, J.; Gazzani, M.; Gencer, E.; Gibbins, J.; Mazzotti, M.; McCoy, S.T.; McKenna, R.; et al. On the Climate Impacts of Blue Hydrogen Production. Sustain. Energy Fuels 2022, 6, 66–75. [Google Scholar] [CrossRef]
- Van Cappellen, L.; Croezen, H.; Rooijers, F. Feasibility Study into Blue Hydrogen Technical, Economic & Sustainability Analysis. 2018. Available online: https://www.cedelft.eu/en/publications/2149/ (accessed on 20 March 2025).
- AlHumaidan, F.S.; Absi Halabi, M.; Rana, M.S.; Vinoba, M. Blue Hydrogen: Current Status and Future Technologies. Energy Convers. Manag. 2023, 283, 116840. [Google Scholar] [CrossRef]
- Howarth, R.W.; Jacobson, M.Z. How Green Is Blue Hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Vo, N.D.; Oh, D.H.; Kang, J.-H.; Oh, M.; Lee, C.-H. Dynamic-Model-Based Artificial Neural Network for H2 Recovery and CO2 Capture from Hydrogen Tail Gas. Appl. Energy 2020, 273, 115263. [Google Scholar] [CrossRef]
- Yu, X.; Shen, Y.; Guan, Z.; Zhang, D.; Tang, Z.; Li, W. Multi-Objective Optimization of ANN-Based PSA Model for Hydrogen Purification from Steam-Methane Reforming Gas. Int. J. Hydrogen Energy 2021, 46, 11740–11755. [Google Scholar] [CrossRef]
- Tong, L.; Bénard, P.; Zong, Y.; Chahine, R.; Liu, K.; Xiao, J. Artificial Neural Network Based Optimization of a Six-Step Two-Bed Pressure Swing Adsorption System for Hydrogen Purification. Energy AI 2021, 5, 100075. [Google Scholar] [CrossRef]
- Streb, A.; Mazzotti, M. Performance Limits of Neural Networks for Optimizing an Adsorption Process for Hydrogen Purification and CO2 Capture. Comput. Chem. Eng. 2022, 166, 107974. [Google Scholar] [CrossRef]
- Nkulikiyinka, P.; Wagland, S.T.; Manovic, V.; Clough, P.T. Prediction of Combined Sorbent and Catalyst Materials for SE-SMR, Using QSPR and Multitask Learning. Ind. Eng. Chem. Res. 2022, 61, 9218–9233. [Google Scholar] [CrossRef]
- Vo, N.D.; Kang, J.-H.; Oh, D.-H.; Jung, M.Y.; Chung, K.; Lee, C.-H. Sensitivity Analysis and Artificial Neural Network-Based Optimization for Low-Carbon H2 Production via a Sorption-Enhanced Steam Methane Reforming (SESMR) Process Integrated with Separation Process. Int. J. Hydrogen Energy 2022, 47, 820–847. [Google Scholar] [CrossRef]
- Oh, H.-T.; Kum, J.; Park, J.; Dat Vo, N.; Kang, J.-H.; Lee, C.-H. Pre-Combustion CO2 Capture Using Amine-Based Absorption Process for Blue H2 Production from Steam Methane Reformer. Energy Convers. Manag. 2022, 262, 115632. [Google Scholar] [CrossRef]
- Pizoń, Z.; Kimijima, S.; Brus, G. Enhancing a Deep Learning Model for the Steam Reforming Process Using Data Augmentation Techniques. Energies 2024, 17, 2413. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Peters, D.; Çıtmacı, B.; Alnajdi, A.; Morales-Guio, C.G.; Christofides, P.D. Machine Learning-Based Predictive Control of an Electrically-Heated Steam Methane Reforming Process. Digit. Chem. Eng. 2024, 12, 100173. [Google Scholar] [CrossRef]
- Cherif, A.; Lee, J.-S.; Nebbali, R.; Lee, C.-J. Novel Design and Multi-Objective Optimization of Autothermal Steam Methane Reformer to Enhance Hydrogen Production and Thermal Matching. Appl. Therm. Eng. 2022, 217, 119140. [Google Scholar] [CrossRef]
- Gul, H.; Arshad, M.Y.; Tahir, M.W. Production of H2 via Sorption Enhanced Auto-Thermal Reforming for Small Scale Applications-A Process Modeling and Machine Learning Study. Int. J. Hydrogen Energy 2023, 48, 12622–12635. [Google Scholar] [CrossRef]
- Newborough, M.; Cooley, G. Developments in the Global Hydrogen Market: The Spectrum of Hydrogen Colours. Fuel Cells Bull. 2020, 2020, 16–22. [Google Scholar] [CrossRef]
- Chavan, P.D.; Sharma, T.; Mall, B.K.; Rajurkar, B.D.; Tambe, S.S.; Sharma, B.K.; Kulkarni, B.D. Development of Data-Driven Models for Fluidized-Bed Coal Gasification Process. Fuel 2012, 93, 44–51. [Google Scholar] [CrossRef]
- Patil-Shinde, V.; Kulkarni, T.; Kulkarni, R.; Chavan, P.D.; Sharma, T.; Sharma, B.K.; Tambe, S.S.; Kulkarni, B.D. Artificial Intelligence-Based Modeling of High Ash Coal Gasification in a Pilot Plant Scale Fluidized Bed Gasifier. Ind. Eng. Chem. Res. 2014, 53, 18678–18689. [Google Scholar] [CrossRef]
- Azzam, M.; Aramouni, N.A.K.; Ahmad, M.N.; Awad, M.; Kwapinski, W.; Zeaiter, J. Dynamic Optimization of Dry Reformer under Catalyst Sintering Using Neural Networks. Energy Convers. Manag. 2018, 157, 146–156. [Google Scholar] [CrossRef]
- Alsaffar, M.A.; Mageed, A.K.; Abdel Ghany, M.A.R.; Ayodele, B.V.; Mustapa, S.I. Elucidating the Non-Linear Effect of Process Parameters on Hydrogen Production by Catalytic Methane Reforming: An Artificial Intelligence Approach. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012078. [Google Scholar] [CrossRef]
- Le, V.T.; Dragoi, E.-N.; Almomani, F.; Vasseghian, Y. Artificial Neural Networks for Predicting Hydrogen Production in Catalytic Dry Reforming: A Systematic Review. Energies 2021, 14, 2894. [Google Scholar] [CrossRef]
- Byun, M.; Lee, H.; Choe, C.; Cheon, S.; Lim, H. Machine Learning Based Predictive Model for Methanol Steam Reforming with Technical, Environmental, and Economic Perspectives. Chem. Eng. J. 2021, 426, 131639. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Mustapa, S.I.; Kanthasamy, R.; Zwawi, M.; Cheng, C.K. Modeling the Prediction of Hydrogen Production by Co-gasification of Plastic and Rubber Wastes Using Machine Learning Algorithms. Int. J. Energy Res. 2021, 45, 9580–9594. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Alsaffar, M.A.; Mustapa, S.I.; Adesina, A.; Kanthasamy, R.; Witoon, T.; Abdullah, S. Process Intensification of Hydrogen Production by Catalytic Steam Methane Reforming: Performance Analysis of Multilayer Perceptron-Artificial Neural Networks and Nonlinear Response Surface Techniques. Process Saf. Environ. Prot. 2021, 156, 315–329. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Cho, H.; Kim, M.; Moon, I.; Kim, J. Multi-Objective Optimization of CO2 Emission and Thermal Efficiency for on-Site Steam Methane Reforming Hydrogen Production Process Using Machine Learning. J. Clean. Prod. 2022, 359, 132133. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Z.; Hsu, S.; Park, Y.; Juan, J.C. Reactor Design of Methanol Steam Reforming by Evolutionary Computation and Hydrogen Production Maximization by Machine Learning. Int. J. Energy Res. 2022, 46, 20685–20703. [Google Scholar] [CrossRef]
- Kim, C.; Won, W.; Kim, J. Early-Stage Evaluation of Catalyst Using Machine Learning Based Modeling and Simulation of Catalytic Systems: Hydrogen Production via Water–Gas Shift over Pt Catalysts. ACS Sustain. Chem. Eng. 2022, 10, 14417–14432. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Yu, L.; Zhu, F.; Cao, Y.; Liu, X.; Yao, A.; Cao, Y. Predicting Gas Production by Supercritical Water Gasification of Coal Using Machine Learning. Fuel 2022, 329, 125478. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Z.; Liu, Y. Smart Reforming for Hydrogen Production via Machine Learning. Chem. Eng. Sci. 2025, 304, 120959. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water Electrolysis Based on Renewable Energy for Hydrogen Production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen Production by Water Electrolysis Technologies: A Review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Alamiery, A. Advancements in Materials for Hydrogen Production: A Review of Cutting-Edge Technologies. ChemPhysMater 2023. [Google Scholar] [CrossRef]
- Valizadeh, S.; Hakimian, H.; Farooq, A.; Jeon, B.-H.; Chen, W.-H.; Hoon Lee, S.; Jung, S.-C.; Won Seo, M.; Park, Y.-K. Valorization of Biomass through Gasification for Green Hydrogen Generation: A Comprehensive Review. Bioresour. Technol. 2022, 365, 128143. [Google Scholar] [CrossRef]
- Pan, J.; Shahbeik, H.; Shafizadeh, A.; Rafiee, S.; Golvirdizadeh, M.; Ghafarian Nia, S.A.; Mobli, H.; Yang, Y.; Zhang, G.; Tabatabaei, M.; et al. Machine Learning Optimization for Enhanced Biomass-Coal Co-Gasification. Renew. Energy 2024, 229, 120772. [Google Scholar] [CrossRef]
- Kumar, M.; Meena, B.; Subramanyam, P.; Suryakala, D.; Subrahmanyam, C. Recent Trends in Photoelectrochemical Water Splitting: The Role of Cocatalysts. NPG Asia Mater. 2022, 14, 88. [Google Scholar] [CrossRef]
- Mohd Raub, A.A.; Bahru, R.; Mohd Nashruddin, S.N.A.; Yunas, J. Advances of Nanostructured Metal Oxide as Photoanode in Photoelectrochemical (PEC) Water Splitting Application. Heliyon 2024, 10, e39079. [Google Scholar] [CrossRef]
- Saifuddin, N.; Priatharsini, P. Developments in Bio-Hydrogen Production from Algae: A Review. Res. J. Appl. Sci. Eng. Technol. 2016, 12, 968–982. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.; Suvarna, M.; Wang, X. Machine Learning Aided Supercritical Water Gasification for H2-Rich Syngas Production with Process Optimization and Catalyst Screening. Chem. Eng. J. 2021, 426, 131285. [Google Scholar] [CrossRef]
- Sezer, S.; Özveren, U. Investigation of Syngas Exergy Value and Hydrogen Concentration in Syngas from Biomass Gasification in a Bubbling Fluidized Bed Gasifier by Using Machine Learning. Int. J. Hydrogen Energy 2021, 46, 20377–20396. [Google Scholar] [CrossRef]
- Saadetnejad, D.; Oral, B.; Can, E.; Yıldırım, R. Machine Learning Analysis of Gas Phase Photocatalytic CO2 Reduction for Hydrogen Production. Int. J. Hydrogen Energy 2022, 47, 19655–19668. [Google Scholar] [CrossRef]
- Cheng, G.; Luo, E.; Zhao, Y.; Yang, Y.; Chen, B.; Cai, Y.; Wang, X.; Dong, C. Analysis and Prediction of Green Hydrogen Production Potential by Photovoltaic-Powered Water Electrolysis Using Machine Learning in China. Energy 2023, 284, 129302. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Z.; Bai, L.; Yuan, Q.; Gou, F.; Li, Y.; Du, Z.; Chen, Y.; Liu, X.; Yu, J.; et al. Machine Learning Assisted Prediction for Hydrogen Production of Advanced Photovoltaic Technologies. DeCarbon 2024, 4, 100050. [Google Scholar] [CrossRef]
- Babay, M.-A.; Adar, M.; Chebak, A.; Mabrouki, M. Forecasting Green Hydrogen Production: An Assessment of Renewable Energy Systems Using Deep Learning and Statistical Methods. Fuel 2025, 381, 133496. [Google Scholar] [CrossRef]
- Salah, A.; Hanel, L.; Beirow, M.; Scheffknecht, G. Modelling SER Biomass Gasification Using Dynamic Neural Networks. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 19–24. [Google Scholar]
- Krzywanski, J.; Fan, H.; Feng, Y.; Shaikh, A.R.; Fang, M.; Wang, Q. Genetic Algorithms and Neural Networks in Optimization of Sorbent Enhanced H2 Production in FB and CFB Gasifiers. Energy Convers. Manag. 2018, 171, 1651–1661. [Google Scholar] [CrossRef]
- Ozbas, E.E.; Aksu, D.; Ongen, A.; Aydin, M.A.; Ozcan, H.K. Hydrogen Production via Biomass Gasification, and Modeling by Supervised Machine Learning Algorithms. Int. J. Hydrogen Energy 2019, 44, 17260–17268. [Google Scholar] [CrossRef]
- Torky, M.; Dahy, G.; Hassanein, A.E. GH2_MobileNet: Deep Learning Approach for Predicting Green Hydrogen Production from Organic Waste Mixtures. Appl. Soft Comput. 2023, 138, 110215. [Google Scholar] [CrossRef]
- Gil, M.V.; Jablonka, K.M.; Garcia, S.; Pevida, C.; Smit, B. Biomass to Energy: A Machine Learning Model for Optimum Gasification Pathways. Digit. Discov. 2023, 2, 929–940. [Google Scholar] [CrossRef]
- Meena, M.; Kumar, H.; Chaturvedi, N.D.; Kovalev, A.A.; Bolshev, V.; Kovalev, D.A.; Sarangi, P.K.; Chawade, A.; Rajput, M.S.; Vivekanand, V.; et al. Biomass Gasification and Applied Intelligent Retrieval in Modeling. Energies 2023, 16, 6524. [Google Scholar] [CrossRef]
- Oral, B.; Can, E.; Yildirim, R. Analysis of Photoelectrochemical Water Splitting Using Machine Learning. Int. J. Hydrogen Energy 2022, 47, 19633–19654. [Google Scholar] [CrossRef]
- Tajima, M.; Nagai, Y.; Chen, S.; Pan, Z.; Katayama, K. A Robust Methodology for PEC Performance Analysis of Photoanodes Using Machine Learning and Analytical Data. Analyst 2024, 149, 4193–4207. [Google Scholar] [CrossRef]
- Sahu, N.; Azad, C.; Kumar, U. Construction of Hybrid Models Based on Cascade Technique Using Basic Machine Learning Models: An Application as Photocurrent Density Predictor of the Photoelectrode in PEC Cell. Mater. Today Commun. 2024, 41, 110643. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, P.; Dey, S.; Pattanayak, P.; Singh, T. Design of Ternary Metal Oxides for Photoelectrochemical Water Splitting Using Machine Learning Techniques. J. Environ. Chem. Eng. 2025, 13, 115260. [Google Scholar] [CrossRef]
- Taheri, E.; Amin, M.M.; Fatehizadeh, A.; Rezakazemi, M.; Aminabhavi, T.M. Artificial Intelligence Modeling to Predict Transmembrane Pressure in Anaerobic Membrane Bioreactor-Sequencing Batch Reactor during Biohydrogen Production. J. Environ. Manag. 2021, 292, 112759. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Zhou, J.L.; Altaee, A.; Li, D. Machine Learning Modeling and Analysis of Biohydrogen Production from Wastewater by Dark Fermentation Process. Bioresour. Technol. 2022, 343, 126111. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, P.; Chowdhury, M.R.; Rajasekhar, N.; Radhakrishnan, T.K.; Samsudeen, N. Deep Learning Based Modelling and Control of a Microbial Electrolysis Cell for Enhanced Bio Hydrogen Production. Int. J. Hydrogen Energy 2024. [Google Scholar] [CrossRef]
- Office of Nuclear Energy Nine Mile Point Begins Clean Hydrogen Production. Available online: https://www.energy.gov/ne/articles/nine-mile-point-begins-clean-hydrogen-production (accessed on 23 March 2025).
- Fernández-Arias, P.; Antón-Sancho, Á.; Lampropoulos, G.; Vergara, D. Emerging Trends and Challenges in Pink Hydrogen Research. Energies 2024, 17, 2291. [Google Scholar] [CrossRef]
- Diab, J.; Fulcheri, L.; Hessel, V.; Rohani, V.; Frenklach, M. Why Turquoise Hydrogen Will Be a Game Changer for the Energy Transition. Int. J. Hydrogen Energy 2022, 47, 25831–25848. [Google Scholar] [CrossRef]
- Muradov, N.; Vezirolu, T. From Hydrocarbon to Hydrogen?Carbon to Hydrogen Economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Steinberg, M. Fossil Fuel Decarbonization Technology for Mitigating Global Warming. Int. J. Hydrogen Energy 1999, 24, 771–777. [Google Scholar] [CrossRef]
- Dagle, R.A.; Dagle, V.; Bearden, M.D.; Holladay, J.D.; Krause, T.R.; Ahmed, S. An Overview of Natural Gas Conversion Technologies for Co-Production of Hydrogen and Value-Added Solid Carbon Products; Richland, WA, USA, 2017. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Bazmi, A.A.; Zahedi, G. Underground Coal Gasification: From Fundamentals to Applications. Prog. Energy Combust. Sci. 2013, 39, 189–214. [Google Scholar] [CrossRef]
- Jiang, L.; Xue, D.; Wei, Z.; Chen, Z.; Mirzayev, M.; Chen, Y.; Chen, S. Coal Decarbonization: A State-of-the-Art Review of Enhanced Hydrogen Production in Underground Coal Gasification. Energy Rev. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Schneider, S.; Bajohr, S.; Graf, F.; Kolb, T. State of the Art of Hydrogen Production via Pyrolysis of Natural Gas. ChemBioEng Rev. 2020, 7, 150–158. [Google Scholar] [CrossRef]
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally Friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Kim, J.; Rweyemamu, M.; Purevsuren, B. Machine Learning-Based Approach for Hydrogen Economic Evaluation of Small Modular Reactors. Sci. Technol. Nucl. Install. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Salimian, A.; Grisan, E. Deep Learning Analysis of Plasma Emissions: A Potential System for Monitoring Methane and Hydrogen in the Pyrolysis Processes. Int. J. Hydrogen Energy 2024, 58, 1030–1043. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, S.; Wu, L.; Hondo, E.; Tang, C.; Jiang, J.; Ho, G.W.; Kawi, S.; Wang, C.-H. Exploring the Role of Process Control and Catalyst Design in Methane Catalytic Decomposition: A Machine Learning Perspective. Int. J. Hydrogen Energy 2024, 72, 601–613. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Katterbauer, K.; Al Shehri, A.; Sun, S.; Hoteit, I. Deep Learning–Assisted Phase Equilibrium Analysis for Producing Natural Hydrogen. Int. J. Hydrogen Energy 2024, 50, 473–486. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Yi, Q. Bridging Uncertainty Gaps with Artificial Intelligence-Assisted Syngas Precise Prediction in Coal Gasification. Chem. Eng. Sci. 2025, 301, 120734. [Google Scholar] [CrossRef]
- Ceylan, Z.; Ceylan, S. Application of Machine Learning Algorithms to Predict the Performance of Coal Gasification Process. In Applications of Artificial Intelligence in Process Systems Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 165–186. [Google Scholar] [CrossRef]

| Algorithm | Concept | Sample Applications | Advantages | Limitations |
|---|---|---|---|---|
| LR | One of the simplest ML algorithms used for predicting continuous numerical values. It assumes a linear relationship between input features (independent variables) and the target variable (dependent variable). The algorithm fits a straight line that best represents the relationship between the input and output. | Predicting house prices based on size, location, and other features; forecasting sales trends in retail and e-commerce; stock price prediction in financial markets. | Simple and easy to interpret; works well when the relationship between variables is approximately linear. | Fails for nonlinear relationships; sensitive to outliers, which can distort predictions. |
| DT | A tree-like structure used for both classification and regression. It splits data into branches based on conditions, forming a flowchart-like decision model. Each node represents a decision based on a feature, and branches lead to possible outcomes. | Credit risk assessment (loan approvals); medical diagnosis (classifying diseases based on symptoms); customer segmentation (targeted marketing. | Easy to interpret and visualize; handles both numerical and categorical data; works well for small to medium-sized datasets. | Prone to overfitting on complex datasets; highly sensitive to noisy data (small changes in data can lead to different tree structures). |
| RF | An ensemble learning algorithm that builds multiple decision trees and combines their results to make more accurate predictions. It reduces overfitting by averaging multiple trees trained on different subsets of data. | Fraud detection in banking; predicting customer churn in telecom and subscription-based businesses; medical imaging analysis (cancer detection from MRI scans). | Higher accuracy than a single decision tree; handles missing data well and works on large datasets; reduces overfitting by combining multiple trees. | Computationally expensive for large datasets; harder to interpret compared to a single decision tree. |
| SVM | A powerful classification algorithm that works by finding the best decision boundary (hyperplane) for separating different classes. It aims to maximize the margin between data points of different classes. | Text classification (spam email detection); image recognition (face detection); medical diagnostics (classifying tumors as benign or malignant). | Effective for high-dimensional data; works well for small datasets with clear class separation. | Computationally expensive for large datasets; sensitive to noisy data and requires careful feature scaling. |
| K-means | An unsupervised learning algorithm that groups similar data points into k clusters. It minimizes the distance between data points within a cluster and assigns new data to the closest cluster. | Customer segmentation in marketing; anomaly detection (fraudulent transactions); image segmentation in computer vision. | Fast and scalable for large datasets; works well when clusters are clearly defined. | Sensitive to outliers; requires the number of clusters (k) to be predefined. |
| ANN | Inspired by the human brain, consisting of layers of interconnected neurons. These models use backpropagation to adjust weights and improve accuracy. | Speech recognition (Google Assistant, Siri); autonomous driving (object detection in self-driving cars); medical diagnostics (AI-driven X-ray analysis). | Handles complex problems like speech and image recognition; self-learning capabilities from vast amounts of data. | Requires large datasets for training; computationally expensive (needs GPUs). |
| Gradient Boosting (XGBoost, LightGBM, CatBoost) | Combines multiple weak models (decision trees) to create a strong predictive model. It corrects previous mistakes iteratively using gradient descent. | Financial modeling (credit scoring); weather forecasting; medical outcome prediction. | Handles missing data and outliers well. | Computationally expensive for big data; prone to overfitting if not carefully tuned. |
| Feature | SMR | ATR |
|---|---|---|
| Feedstock | Natural gas (CH4) | Natural gas (CH4) |
| Process complexity | Lower (relies on external heating) | Higher (requires O2 supply) |
| CO2 capture efficiency | Moderate (~75–85% with CCUS) | Higher (~95% with CCUS) |
| Energy requirements | Higher (requires external heat input) | Lower (self-sustaining heat generation) |
| CO2 emissions | Moderate (requires CCUS) | Lower (easier CO₂ separation) |
| Industrial maturity | Widely used globally | Emerging but growing |
| Capital costs | Lower (simpler design) | Higher (complex setup) |
| Category | No. | Reference | Algorithms | Dataset | Inputs | Output(s) | Key Findings |
|---|---|---|---|---|---|---|---|
| SMR | 1 | Vo et al. (2020) [41] | Dynamic-model-based ANN, SVD, FFBPNN | 108 (cryogenic unit); 291 (membrane); 35 (PSA) | Membrane area, adsorption time, purge-to-feed ratio | H2 purity, CO2 capture rate, H2 productivity, H2 production cost, energy consumption for CO2 capture | ANN models provided high accuracy (<2% error) and significant computational cost reduction. |
| 2 | Yu et al. (2021) [42] | ANN-GA | 100 | Adsorption pressure, part of adsorption time, feed flow rate, length of activated carbon layer, ratio of purge to feed | Purity of hydrogen, recovery rate, productivity of the PSA process | The optimized PSA process achieved hydrogen purity above 99% while balancing recovery and productivity. | |
| 3 | Tong et al. (2021) [43] | ANN | 112 | Adsorption pressure, adsorption step time | H2 purity and recovery | ANN can effectively predict and optimize PSA 1-based hydrogen purification. | |
| 4 | Streb and Mazzotti (2022) [44] | ANN | 20,000 | Feed composition (mol fractions of CO2, CO, CH4, N2, Ar, and H2), adsorption time, light purge duration, evacuation pressure, recycle ratio | H2 purity, H2 recovery, CO2 purity, CO2 recovery, CO2 specific energy consumption, productivity | ANN successfully used for multi-objective constrained optimization: H2 purity ≥ 99–99.97%; CO2 purity ≥ 96%; H2 and CO2 recovery ≥ 90%. | |
| 5 | Nkulikiyinka et al. (2022) [45] | QSPR, MTL, ASNN, DNN, LSSVM | 446 | Molecular descriptors of materials, CaO or Ni concentration, calcination/carbonation temperature and time, synthesis method, BET surface area, steam-to-carbon ratio | Methane conversion, CO2 capture capacity | ASNN with GSFrag descriptors + multitask learning gave the most accurate predictions. | |
| 6 | Vo et al. (2022) [46] | ANN | 402 | Inlet temperature, velocity, steam-to-carbon ratio, purge-to-feed ratio, adsorption pressure | H2 purity and recovery, CO2 capture efficiency, H2 production cost, energy consumption | The ANN-based SE-SMR model reduces simulation time from 2 h to 20 s, achieving 99.99% H2 purity and 90.3% CO2 capture efficiency. | |
| 7 | Oh et al. (2022) [47] | ANN-DE | 480 | MDEA concentration, PZ concentration, flash drum pressure, PZ ion flow rate in lean-amine solvent | Reboiler duty, electricity consumption, total equivalent work | ANNs and DE successfully optimize pre-combustion CO2 capture in SMR-based blue hydrogen production. | |
| 8 | Pizoń et al. (2024) [48] | ANN | 10,475 | Temperature, steam-to-CH4 ratio, N2-to-CH4 ratio, CH4 flow rate, nickel catalyst mass | Concentration of H2, CO, CO2, and CH4 | The ANN model performs better than traditional kinetic models, showing MSE = 0.00022 compared to alternative models. | |
| 9 | Wang et al. (2024) [49] | RNN, LSTM | 100,000 | Electric current, reactor temperature, flow rate of CH4, H2, CO2, and CO | Reactor temperature, flow rate of CH4, H2, CO2, and CO | The LSTM-RNN model can accurately predict reactor dynamics and drive model predictive control for H2 production. | |
| ATR | 10 | Cherif et al. (2022) [50] | MOGA | N/A | Catalyst configuration (Ni/Al2O3 or Pt/Al2O3) | H2 yield, maximum wall temperature | Optimized catalyst configuration has 46% increase in H2 yield and 27% increase in CH4 conversion. |
| 11 | Gul et al. (2023) [51] | LM, BR, SCG | N/A | Concentration of CH4, CO, CO2, H2, H2O, and N2, CaCO3 and CaO (solid phase), reactor temperature | H2 yield, CO2 capture efficiency, H2 purity, CH4 conversion | Sorption-enhanced autothermal reforming (SEATR) process achieved 97% H2 purity (compared to 66% in conventional ATR) and 94% CH4 conversion (compared to 77% in conventional ATR). |
| No. | Reference | Algorithms | Dataset | Inputs | Output(s) | Key Findings |
|---|---|---|---|---|---|---|
| 1 | Chavan et al. (2012) [53] | MVR, ANN | 106 | Fixed carbon, volatile matter, mineral matter/ash content, air feed per kg of coal, steam feed per kg of coal, bed temperature | Gas production rate, heating value of the product gas | ANN models outperformed MVR models. The air feed rate was the most influential factor for both gas production and heating value. |
| 2 | Patil-Shinde et al. (2014) [54] | GP, ANN, PCA | 36 | Fuel ratio, ash content, specific surface area of coal, activation energy of gasification, coal feed rate, gasifier bed temperature, ash discharge rate, air/coal ratio | CO + H2 generation rate, syngas production rate, carbon conversion, heating value of syngas | Both GP and ANN models performed well, with R2 between 0.920 and 0.996. Air/coal ratio, temperature, ash discharge rate, and coal feed rate were the most influential inputs. |
| 3 | Azzam et al. (2018) [55] | ANN-GA | 2000 | Reaction temperature, pressure, catalyst diameter | CH4 conversion, CO2 conversion, H2/CO ratio, molar percentage of solid carbon | ANN and GA provide accurate and efficient optimization; high temperatures favor DRM performance but increase catalyst degradation. |
| 4 | Alsaffar et al. (2020) [56] | ANN-MLP | 30 | Gas hourly space velocity, O2 concentration in the feed, reaction temperature, CH4/CO2 ratio | H2 yield, CH4 conversion | The best-performing ANN architecture was 4-9-2, achieving a sum of squares error of 0.076 and R2 > 0.9. |
| 5 | Le et al. (2021) [57] | ANN-DE | 100 | Hydrocarbon type, catalyst composition, reaction temperature, support material properties, process conditions | Hydrocarbon conversion, H2 yield, catalyst stability | Hydrocarbon type affects H2 yield. The best ANN model had MSE < 0.05 and relative error < 3.36%. |
| 6 | Byun et al. (2021) [58] | SVR, DT, GPR | 10,000 | Number of reactors, temperature, H2 permeance, membrane area, sweep gas flow rate, steam-to-carbon ratio, compressor capital cost, labor cost, natural gas cost, electricity cost | H2 production rate, CO2 emission rate, unit H2 production cost | Reactor count and operating temperature have the strongest influence on hydrogen production. The GPR model outperformed SVR and DT. |
| 7 | Ayodele et al. (2021) [59] | ANN (RBFNN and MLP) | 30 | Gasification temperature, rubber seed shell particle size, high-density polyethylene particle size, amount of plastic in the mixture | H2 production | One-layer MLP showed the best performance with an R2 of 0.990 and the lowest sum of squares error. |
| 8 | Ayodele et al. (2021) [60] | ANN-MLP | 17 | Methane partial pressure, steam partial pressure, reaction temperature | H2 yield and CH4 conversion | ANN with 3–17–15–2 structure provides the best prediction for H2 yield (R2 = 0.997) and CH4 conversion (R2 = 0.996). |
| 9 | Hong et al. (2022) [61] | DNN-PSO | 10,514 for operation, 10,000 for simulation | Natural gas feed flow rate, demineralized water flow rate, air flow rate, natural gas fuel flow rate, PSA recovery rate, system pressure, off-gas pressure, SMR reactor inlet/outlet temperature, LTS reactor inlet temperature, air-to-fuel ratio | H2 production, H2 purity, thermal efficiency, CO2 emission, SMR conversion efficiency | The hybrid DNN model achieves an R2 score of 0.94; higher thermal efficiency comes at the cost of higher CO2 emissions. |
| 10 | Chen et al. (2022) [62] | NNs | 60 | Inlet temperature, steam-to-carbon ratio, Reynolds number | H2 yield, methanol conversion | Steam-to-carbon ratio has the most significant impact on H2 yield; NNs achieve high prediction accuracy. |
| 11 | Kim et al. (2022) [63] | ANN | 419 | A total of 34 input features (catalyst composition, operating conditions, catalyst preparation conditions) | CO conversion | The ANN model predicts one-pass CO conversion with high accuracy (R2 = 0.997). The best-performing catalysts include Pt/Co(10 wt%)/Al2O3, Pt/Co(20 wt%)/Al2O3, and Pt/Ce(5 wt%)/TiO2. |
| 12 | Liu et al. (2022) [64] | GBR, RF, SVR, DT, ANN, ABR | 3536 | Elements (C, H, O, N, S), moisture, ash, volatile, fixed carbon, temperature, concentration ratio, equivalence ratio, residence time | H2, CO, CH4, CO2 gas yields | GBR was the most accurate model. Operating conditions (especially temperature and residence time) contributed 88.55% to gas yield predictions. |
| 13 | Huang et al. (2025) [65] | LR, RR, Lasso, ENR, DT, RF, GBR, ETR, XGBoost, KNN, MLP | 1386 | Temperature, steam-to-carbon ratio, oxygen-to-carbon ratio, pressure | H2 yield, CO2 yield, heat duty | XGBoost outperformed all other models. Temperature was the most influential factor for H2 yield. |
| Electrolyzer Type | Electrolyte | Temperature (°C) | Efficiency (%) | Advantages | Challenges |
|---|---|---|---|---|---|
| AEL | KOH or NaOH solution | 60–80 | 65–75 | Low-cost, mature technology | Low current density |
| PEM | Solid polymer membrane | 50–80 | 75–80 | Fast response, compact | Uses expensive catalysts (Pt, Ir) |
| SOEC | Ceramic oxide | 700–1000 | 80–85 | High efficiency, uses heat | High degradation, expensive materials |
| Category | No. | Reference | Algorithms | Dataset | Inputs | Output(s) | Key Findings |
|---|---|---|---|---|---|---|---|
| Water electrolysis | 1 | Li et al. (2021) [74] | NNs, RF, SVR | 718 | Feedstock composition, operational conditions (temperature, pressure, reaction time, solid content), catalyst properties | H2 yield, CO2 yield, CH4 yield, CO yield | NNs outperformed RF and SVR in optimizing H2 production from supercritical water gasification. |
| 2 | Sezer and Özveren (2021) [75] | ANN-LM | 370,656 | Carbon content, H2 content, O2 content, gasifier temperature, steam flow rate, fuel (biomass) flow rate | H2 mole fraction in syngas, total exergy value of syngas | The ANN model achieved high accuracy (R2 = 0.9999 for training and test data sets). | |
| 3 | Haq et al. (2022) [22] | GPR, ETR, ANN, SVM, GA | 125 | Proximate analysis of sewage sludge, ultimate analysis of sewage sludge, supercritical water gas operation conditions | H2 yield, CO2 yield, CH4 yield, CO yield | The GPR model is the best for predicting H2 yield; temperature is the most influential factor for H2 production. | |
| 4 | Saadetnejad et al. (2022) [76] | RF, DT | 549 | Photocatalyst properties (semiconductor material, band gap energy, co-catalyst type and loading) and reaction conditions (temperature, pressure, CO2/H2O molar ratio) | Band gap energy of the photocatalyst, total gas production rate | Best semiconductors for gas-phase CO2 reduction are CeO2, SrTiO3, ZnS, ZrO2. | |
| 5 | Cheng et al. (2023) [77] | SVM, Prophet | 9840 | Temperature, atmospheric pressure, relative humidity, cloud cover, precipitation, fixed month index, full timestamp and time-series structure (for Prophet only) | Hydrogen production | ML is effective for regional hydrogen production forecasting, especially when integrated with local climate data. SVM outperformed Prophet. | |
| 6 | Yang et al. (2024) [78] | ELM, RF, SVM, GA, LSTM, RBF, BPNN | 1095 | Solar irradiance, temperature, sunshine hours | Photovoltaic (PV) power generation, H2 production | LSTM performed best with R2 = 0.8402. HJT PV technology produced most H2 with the lowest cost. | |
| 7 | Babay et al. (2025) [79] | SVR, RF, MLP, LSTM-CNN | N/A | Solar irradiance, ambient temperature, photovoltaic (PV) panel temperature, panel type, seasonal data | H2 production | Polycrystalline panels showed higher H2 output than monocrystalline and amorphous silicon. RF gave the best accuracy. | |
| Biomass or organic waste | 8 | Salah et al. (2016) [80] | NARX | N/A | Mass flow of fuel and steam into gasifier, fuel mass and air and O2 flow into regenerator, continuous and discontinuous mass flow from generator | Product gas flow rate, temperature and pressure of gasifier, temperature and pressure of regenerator | The model achieved low prediction errors, demonstrated real-time adaptability, and helped find the key operating parameters. |
| 9 | Krzywanski et al. (2018) [81] | ANN-GA | 25 | Reactor type, CaO/C mole ratio, H2O/C mole ratio, reaction temperature | Volumetric H2 concentration in syngas | Developed [4–3–3–1] ANN-GA model predicted H2 concentrations with high accuracy: <±8% relative error. | |
| 10 | Ozbas et al. (2019) [82] | LR, KNN, SVR, DT | 2036 | Time, temperature, concentration of CO, CO2, CH4, and O2, higher heating value of syngas | Hydrogen concentration in syngas | LR has the highest accuracy with R2 = 0.99. The highest H2 concentration in syngas reached 35% vol. | |
| 11 | Torky et al. (2023) [83] | MobileNet-CNN, Xception-CNN, DNN, Mask-RCNN | 23,628 | Image data, waste characteristics (material type, waste category, physical properties, environmental conditions), estimated weight parameters (volume, density) | Waste classification (recyclable, organic, or harmful), estimated waste weight (dry or wet), H2 production | MobileNet-CNN achieved 93% accuracy for waste classification and 98% accuracy in distinguishing dry vs. wet organic waste. | |
| 12 | Gil et al. (2023) [84] | GPR | 30 | Process parameters (temperature, steam-to-air ratio, stoichiometric ratio, steam-to-biomass ratio), biomass properties (C%, H%, O%, ash content) | H2 vol%, CO vol%, CH4 vol%, gas yield, combustible gas concentration | The GPR model achieved high predictive accuracy, with R2 values ranging from 0.82 to 0.98 for different gasification parameters. | |
| 13 | Meena et al. (2023) [85] | ANN, SVM, DT, RF, GB | N/A | Process parameters (temperature, equivalence ratio, steam-to-biomass ratio, pressure), biomass properties (C%, H%, O%, ash content, volatile matter), type of gasifying agent, catalyst type, time | H2 vol%, CO vol%, CH4 vol%, CO2 vol%, syngas heating value, syngas yield, tar content, gasification efficiency | RF and GB models showed the highest accuracy, with R2 values exceeding 0.95 for predicting H2 yield and syngas composition. | |
| 14 | Pan et al. (2024) [70] | GBR, RF, DT, KR, GA | 458 | Feedstock composition (C, H2, N2, O2, S, volatile matter, fixed carbon, ash, moisture %), temperature, biomass/coal blending ratio, equivalence ratio, gasifying agent type | Syngas yield, H2, CO2, CH4, and CO2 content, syngas lower heating value | GBR showed the highest accuracy (R2 up to 0.99) for predicting syngas composition and heating value. | |
| PEC | 15 | Oral et al. (2022) [86] | ARM, RF, DT | 10,560 | Electrode materials, synthesis methods, doping elements, co-catalyst, second-layer materials, calcination conditions, electrolyte type and pH, irradiation conditions, applied bias voltage | Band gap energy, photocurrent density | ML successfully identified patterns and optimized conditions. RF performed well in predicting band gap energy. ARM and DT helped identify key parameters for enhancing PEC efficiency |
| 16 | Tajima et al. (2024) [87] | SVR, GPR, DT | 75 (Fe2O3), 32 (BiVO4), 58 (WO3/BiVO4) | X-ray diffraction, Raman spectroscopy, UV/Vis absorbance, photoelectrochemical impedance spectroscopy | Photocurrent density | GPR achieved highest prediction accuracy across all tested photoanode materials (hematite, BiVO4, and WO3/BiVO4) with R2 among 0.85–0.99, even for small datasets (30–70 samples). | |
| 17 | Sahu et al. (2024) [88] | MLP, ABR, RR, ENR | 2593 | Band gap of photoelectrode material, working electrode area, light intensity, power of light source, pH, filter condition, molarity | Photocurrent density | The hybrid model ABR + MLP performs best with R2 = 0.9686. | |
| 18 | Mishra et al. (2025) [89] | KNN, RF, AdB, GBR, XGBoost | 85 | Material properties (Shannon ionic radius, density, electronegativity, etc.), experimental conditions (light density, applied bias voltage, preparation method) | Band gap energy, photocurrent density | The XGB model performed best for both band gap prediction and photocurrent density prediction. | |
| Biohydrogen | 19 | Taheri et al. (2021) [90] | ANN, ANFIS | 119 | Organic loading rate, effluent pH, mixed liquor suspended solids, mixed liquor volatile suspended solids | Transmembrane pressure | ANFIS slightly outperformed ANN (R2 = 0.93 vs. R2 = 0.88). |
| 20 | Hosseinzadeh et al. (2022) [91] | GBM, SVR, RF, AdaBoost | 210 | Acetate (A), butyrate (B), A/B ratio, ethanol, iron, nickel, pH, biomass proportion, hydraulic retention, chemical oxygen demand | H2 production (yield or rate) during dark fermentation | All four ML models showed high accuracy (R2 > 0.88), with RF having the highest (R2 = 0.902). | |
| 21 | Venkatesh et al. (2024) [92] | LSTM, Bi-LSTM | 5600 | Applied voltage, sequential input data | Current density (directly correlates to H2 production rate) | Bi-LSTM outperforms LSTM in modeling and controlling biohydrogen production in a microbial electrolysis cell. |
| Category | No. | Reference | Algorithms | Dataset | Inputs | Output(s) | Key Findings |
|---|---|---|---|---|---|---|---|
| Pink hydrogen | 1 | Kim et al. (2022) [103] | CART implemented in Minitab software | NA | 61 inputs (heat consumption at H2 generation plant, electricity rating of SMRs, heat supplied to plants, operating years, tax rate, inflation, etc.) | H2 production cost (USD/kg) | ML can identify key economic drivers in nuclear H2 production. Heat consumption is the most important factor. |
| Turquoise hydrogen | 2 | Salimian and Grisan (2024) [104] | ResNet-50 | 4975 | Plasma emission spectra (200–1100 nm) reshaped as 32 × 32 tensors | H2 and CH4 concentration | The model performed well in predicting CH4 concentration but was less accurate for low H2 concentrations. |
| 3 | Wen et al. (2024) [105] | RF, XGBoost, DT, ADA, GBDT, LGB, KNN, SVR, Lasso, RR, ENR, MLP, MLR | 2733 | wt% of Fe, Ni, Cu, Co, Al2O3, SiO2, TiO2, MgO; calcination temperature, CH4 concentration, gas hourly space velocity, reaction temperature and time | CH4 conversion and H2 yield | RF and XGBoost achieved the highest accuracy with R2 = 0.9999 for CH4 conversion and R2 = 0.9996 for the H2 yield model. | |
| White hydrogen | 4 | Zhang et al. (2024) [106] | TINN | 5041 | Thermodynamic properties (critical temperature and pressure, acentric factor, mole fraction), process conditions (temperature, total molar density, pore radius) | Number of equilibrium phases, compositional mole fractions of gas and liquid phase | The TINN model achieves ~20× speedup in phase equilibrium computation compared to traditional iterative flash calculation methods. |
| Black hydrogen | 5 | Zhao et al. (2025) [107] | BP-MLP, SVR, MLR-RR, DT, RF, XGBoost, GPR | 750 | Coal composition (C, H, N, O, S, Cl, volatile matter, fixed carbon, ash content, moisture content), temperature, pressure, steam-to-coal ratio, oxygen-to-coal ratio | Syngas component proportions: H2, CO, CO2, CH4, N2, and others, hydrogen-to-carbon ratio | The BP-MLP showed the best performance. The steam-to-coal ratio, moisture content, and Cl content were the most influential features for H2 prediction. |
| 6 | Ceylan and Ceylan (2021) [108] | SMOreg, GPR, Lazy K-Star, Lazy IBk, AMT, RF | 106 | Mineral matter, fixed carbon, volatile matter, air feed, steam feed, bed temperature | Gas yield, heating value | RF performed best with R2 = 0.9998 for gas yield and R2 = 0.9730 for heating value. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Gao, S.; Yang, G. Machine Learning Applications in Gray, Blue, and Green Hydrogen Production: A Comprehensive Review. Gases 2025, 5, 9. https://doi.org/10.3390/gases5020009
Du X, Gao S, Yang G. Machine Learning Applications in Gray, Blue, and Green Hydrogen Production: A Comprehensive Review. Gases. 2025; 5(2):9. https://doi.org/10.3390/gases5020009
Chicago/Turabian StyleDu, Xuejia, Shihui Gao, and Gang Yang. 2025. "Machine Learning Applications in Gray, Blue, and Green Hydrogen Production: A Comprehensive Review" Gases 5, no. 2: 9. https://doi.org/10.3390/gases5020009
APA StyleDu, X., Gao, S., & Yang, G. (2025). Machine Learning Applications in Gray, Blue, and Green Hydrogen Production: A Comprehensive Review. Gases, 5(2), 9. https://doi.org/10.3390/gases5020009






