Abstract
Plant responses to air pollution have been extensively studied in urban environments. Nevertheless, detailed and holistic studies assessing their retaliation to air contaminants are still limited. The present study evaluates the effect of criteria pollutants (SO2, NO2, PM10 and O3) on the overall biochemistry and resource allocation strategy of plants in order to categorize the dominant roadside species (Mangifera indica, Psidium guajava, Ficus religiosa, Azadirachta indica, Dalbergia sissoo, Cascabela thevetia and Bougainvillea spectabilis) of the Indo-Gangetic Plains (IGP), with different morphologies and habits, into species that are tolerant and sensitive to the prevailing air pollutants. This study was performed at three different land-use sites (industrial, commercial and reference) in Varanasi for two seasons (summer and winter). It was inferred that NO2 and PM10 consistently violated the air quality standards at all the sites. The fifteen assessed parameters reflected significant variations depending upon the site, season and plant species whereupon the enzymatic antioxidants (superoxide dismutase and catalase) and resource utilization parameters (leaf area and leaf dry matter content) were remarkably affected. Based on the studied parameters, it was entrenched that deciduous tree species with compound leaves (D. sissoo > A. indica) were identified as the less sensitive, followed by a shrub (C. thevetia > B. spectabilis), while evergreen species with simple leaves were the most sensitive. It was also substantiated that the morphology of the foliage contributed more toward the differential response of the plants to air pollutants than its habit.
Keywords:
air pollutants; trees; shrubs; sites; biochemical; resource utilization; morphology; habit; tolerant 1. Introduction
Rapid industrialization and urbanization are the leading issues behind the alteration and deterioration of air quality and other environmental facets. Developing countries are quickly urbanizing, with a population increase from 751 million (30% of the world’s population) in 1950 to 4.2 billion (55%) in 2018, an expansion of 4.6 times. The population is expected to reach 6.4 billion (68%) in 2050 [1]. Urbanization is essential for economic upturns, poverty eradication and overall human development. Furthermore, growing urbanization has posed a substantial obstacle to the United Nations Sustainable Development Goals [2]. One of the impediments is urban air pollution, which is a major public health concern worldwide. Every year, ambient air pollution leads to 4.2 million fatalities worldwide [3].
Attributed to their perennial nature and use in roadside plantations, trees and shrubs experience consistent exposure to the air pollutants [4]. Differential plant responses to air pollutants prove efficient proxies for monitoring air pollutants and providing abatement measures [5]. Tree plantation is a cost-effective measure to lower the pollutant burden, both gaseous and particulate, as trees provide a surface for absorption, deposition and dispersion, thereby reducing their human exposure [6]. The most widely assessed parameter for tracing the responses of perennial vegetation to air pollutants is the air pollution tolerance index (APTI), which compiles the changes occurring in the pH of the leaf extract, chlorophyll, ascorbic acid (AsA) and relative water content (RWC) of a leaf [7,8]. However, it is well-established that various abiotic adversities, including air pollution, generate oxidative stress in the exposed plants, leading to the increased generation of ROS, which ultimately harms plant functioning and causes cell death [9]. Plants involve different defense mechanisms to combat oxidative stress which are responsible for their tolerance/susceptibility toward pollutants. The impacts of air pollution on plants manifest as a decrease in photosynthetic pigments, changes in metabolism, enzyme activities and impairments in physiological and metabolic processes [10]. As a consequence of air pollution, several biochemical anomalies occur in plants which encompass the generation of oxidative stress due to the increased production of ROS and a decline in photosynthetic pigments, which further adversely affects the photosynthetic processes [11]. Numerous enzymatic and non-enzymatic antioxidant defense mechanisms can lessen the negative effects of oxidative stress [10].
The present study was conducted with the aim of analyzing the impact of criteria pollutants, namely, sulfur dioxide (SO2), nitrogen dioxide (NO2), particulate matter (PM10) and ozone (O3), on seven perennial species (five trees and two shrubs), based on their dominance in roadside plantation at different geographical locations of Varanasi city (Uttar Pradesh) with variable land-use patterns.
2. Materials and Methods
2.1. Experimental Site Details
The experiment was conducted from December 2021 to May 2022, and the sites were selected on the basis of land-use patterns, namely, the Ramnagar Industrial area (R.I.A) (25°15′06″ N, 83°03′57″ E), Banaras Railway station (B.R.S) (25°18′18″ N, 82°58′28″ E) and Banaras Hindu University (B.H.U) (25°16′46″ N, 82°59′46″ E) of Varanasi city, respectively (Figure 1). R.I.A is an industrial site dominated by industrial and transport activities. B.R.S is commercial site largely affected by the emissions from vehicles and public transport, while B.H.U is a reference site with the lowest pollutant load. The city observes a continuous violation of air quality standards which is most severe for particulate matter (PM).
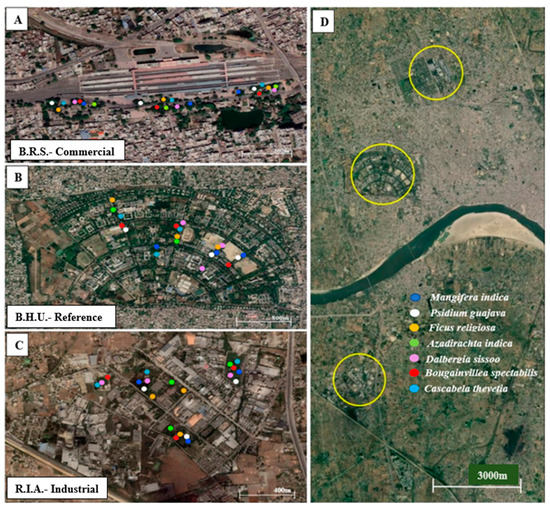
Figure 1.
Map locations of different sites under study along with the sampling points. (A) Banaras Railway Station—the commercial site, (B) Banaras Hindu University—the reference site, (C) Ramnagar Industrial Area—the industrial site. (D) relative location of the three sites under study.
Varanasi experiences a prolonged hot and humid climate with a short low-temperature regime. There are three different seasons (summer, monsoon and winter). In this study, two prominent seasons were selected based on their temperature variations and pollutant loads, namely, summer (March–May) and winter (December–February). During the study period, the maximum temperature averaged between 24.24 °C and 40.70 °C, while the minimum temperature was between 9.39 °C and 26.52 °C. The total rainfall during the experimental period was recorded at 48.3 mm. The meteorological data for the experimental site were collected from the Indian Meteorology Division (IMD) observatory at Banaras Hindu University, Varanasi, India.
The monsoon season experiences a dip in pollutant load due to frequent rainfall (a total rainfall of 662.6 mm from June 2022 to August 2022); hence, it is not considered in the study.
2.2. Plant Details
Seven perennial species were selected from the experimental sites based on their dominance in roadside plantation. Among these species, five were trees and two were shrubs. The trees included the simple-leaved evergreen species Mangifera indica L. and Psidium guajava L., simple-leaved deciduous species Ficus religiosa L., compound-leaved semi-evergreen species Azadirachta indica A. Juss. and compound-leaved deciduous species Dalbergia sissoo Roxb. The shrub species included needle-leaved Cascabela thevetia L. and broad-leaved Bougainvillea spectabilis Willd. (Table 1). Prior to sample collection, each plant species was geotagged, and samples were collected from the same plant during each season at all the sites.

Table 1.
Details of the test plants under study.
2.3. Pollutant Monitoring
Throughout the entire experimental period, PM10 monitoring was conducted for alternate weeks for an 8 h period at two sub-sites of each experimental location, using a high-volume sampler (Envirotech APM 460BL, Envirotech Instrument Pvt. Ltd., New Delhi, India). The sampler flow rate was fixed at 1.1 m3 min−1 with a glass fiber filter paper (TISCH Scientific, USA) that had a pore size of 2.7 µm and dimensions of 20.3 × 25.4 cm, following the prescribed guidelines for National Ambient Air Quality Standards (NAAQS) by the Central Pollution Control Board (CPCB) [12]. The PM concentration was calculated using the methodology of Mukherjee and Agrawal [13].
The concentration of gaseous pollutants was estimated passively using wet chemical methods, following the methodology mentioned in the CPCB guidelines for the NAAQS [12]. SO2 was estimated using the improved West and Gaeke method [14], NO2 with the modified Jocob and Hochheiser method [15] and O3 was quantified using the method of Hangartner et al. [16].
2.4. Plant Sampling
In each area, samples were collected randomly from three biological replicates of each species to minimize the local microclimatic effects. Fully expanded leaves with no sign of pest infection and damage (n = 10) were collected between 8.00 and 10.00 h every month during each season. The leaves were collected from the outer canopy and from all sides at a height of 1.5–2 m above the ground surface. The samples were carefully sealed in plastic bags and brought to the laboratory under cold conditions. The samples were washed properly and stored at −20 °C till further processing.
2.5. Photosynthetic Pigments
The protocols of Maclachlan and Zalik [17] and Duxbury and Yentsch [18] were used to estimate the photosynthetic pigments. Leaf extracts were extracted in 80% acetone, and their absorbance at 480, 510, 645 and 663 nm displayed carotenoids (carotene and xanthophyll), chlorophyll b (Chl b) and chlorophyll a (Chl a), respectively. The calculations used are as follows:
Total chlorophyll (mg g−1 FW) = Chlorophyll a + Chlorophyll b
where V is the volume of the extract (mL), W is the fresh weight of the sample (g) and d is the path length of light (cm).
2.6. Enzymatic and Non-Enzymatic Antioxidants
Enzyme extraction from the leaf samples was carried out as per the protocol of Takshak and Agrawal [19], with slight modifications. A leaf sample (0.1 g) was homogenized in 10 mL (0.1 M) potassium phosphate buffer (pH 7.8) consisting of 1% polyvinylpyrrolidone (PVP), 0.5% Triton-X 100 and 0.5 mM ethylenediaminetetraacetic acid (EDTA) and centrifuged twice at 13,000× g at 4 °C for 30 min. The supernatant was used further for the analysis of enzymatic antioxidants and proteins.
Catalase (CAT) activity was determined by following the method of Aebi [20], using potassium phosphate buffer (0.1 M) at pH 7.0 with 200 mM H2O2 and 100 µL extracted enzyme. The decreasing absorbance was recorded at 240 nm. To assess the CAT activity, 0.036 mM−1cm−1 was used as extinction coefficient in the relevant calculations. Superoxide dismutase (SOD) activity was estimated by a 50% reduction of nitro blue tetrazolium (NBT), following the methodology of Pandey et al. [21] with appropriate amendments. The reducing mixture contained 0.2 mM methionine, 2.25 mM NBT, 60 µM riboflavin, 3 mM EDTA, 0.1 M potassium phosphate buffer (pH 7.8), 1.5 M sodium carbonate (Na2CO3) and enzyme extract. The absorbance was recorded at 560 nm.
To estimate the ascorbic acid (AsA) content in the leaf extract, a modified Keller and Schwager [22] method was utilized. A 10 mL solution, prepared by adding 0.75% oxalic acid and 0.05% EDTA, was used to extract AsA. The AsA concentration was estimated using its standard graph, following the spectrophotometric estimation of reduction of a 2,6-Dichlorophenolindophenol (DCPIP) reagent by the leaf extract at 520 nm. Phenol was estimated using the modified method of Bray and Thorpe (1954) [23], in which leaf samples were extracted in 80% acetone to estimate the concentration of total polyphenolics in the leaf extract. The content of total polyphenolics was determined using a Gallic acid standard graph, following the spectrophotometric determination of a blue-color complex generated by interaction of leaf extract with Folin–Ciocalteu reagent (1 N) in the presence of sodium carbonate (5%) at 650 nm. The leaf sample was homogenized in methanol (10 mL) with a drop of conc. HCl and 1% CaCO3 to quantify anthocyanins [24]. The absorbance of the supernatant was measured at 535 and 650 nm after centrifugation. Flavonoid content was estimated following the protocol of Flint et al. [25].
2.7. Physio-Chemical Parameters
For the determination of the leaf pH, 1 g leaf sample was homogenized in 10 mL of double-distilled water and centrifuged. The supernatant was used to measure the pH using a pH meter (CyberScan-510, Thermo Scientific, Eutech Instruments, Waltham, MA, USA), following the protocol of Mukherjee and Agrawal [13]. The membrane stability index (MSI) was interpreted using the method of Gupta et al. [26], measuring the difference in the electrical conductivity (EC) of the leaf discs. The leaves were punched uniformly, avoiding the mid rib and in equal number. The leaf discs were incubated at room temperature for 2 h in 10 mL double-distilled water. The conductivity (EC1) of the incubated solution was recorded by a portable conductivity meter (Model 306, Systronics Limited, Ahmedabad, India). The solution was autoclaved at 120 °C and a pressure of 15 psi for 15 min, and the electrical conductivity (EC2) was then recorded again after cooling to room temperature. The following formula was used to calculate the MSI:
Leaf discs were punched from fresh leaves and weighed to estimate fresh weight. After soaking the discs in distilled water for 16 h, the saturated weight of the discs was quantified. The discs were oven-dried and weighed again. The relative water content (RWC) was calculated using the formula of Smart and Bingham [27], as follows:
The net water content (NWC) was calculated from the difference between the leaf fresh weight and the leaf dry weight.
2.8. Resource Utilization
The leaf dry matter content (LDMC) was estimated by dividing the dry weight of the leaves by their fresh weight. The leaf area of fresh leaf samples was calculated using Image J software (National Institutes of Health, Bethesda, MD, USA), and leaf mass per unit area (LMA) was calculated as the ratio of the dry weight of the leaves by the leaf area.
2.9. Statistical Analysis
The data from the leaf response parameters to air pollutants were analyzed using a one-way univariate analysis of variance (ANOVA) to investigate the effect of site on different parameters. Tukey’s post hoc test was used after the one-way ANOVA for distinct measurements. A three-way ANOVA was used to assess the effect of the interaction between size and season on all of the evaluated plant attributes. To gain a better understanding of the relationship between air contaminants and plant performance, the Varimax rotation criterion with Kaiser normalization was used to perform a principal component analysis (PCA). In order to clusterize the plants based on the extent of the correlation and the similarity between them, a hierarchal correlation cluster analysis (HCA) was performed. A Pearson correlation measured the similarity between the plants. All the statistical analyses were performed using IBM SPSS Statistics21 and Origin 2023.
3. Results
3.1. Pollutant Concentrations
The concentrations of the criteria pollutants for December 2021 to May 2022 are provided in Table 2. The concentration of SO2 was found to be within the NAAQS of India for all the sites and seasons, being the highest in R.I.A for the summer season (43.7 µg m−3), followed by the winter season (34.2 µg m−3). It was observed that NO2 constantly violated the World Health Organization (W.H.O) standard (10 µg m−3), while B.R.S and R.I.A were both found to violate the WHO and NAAQS standards in both seasons [28,29]. The highest concentration of NO2 was reported for R.I.A during both summer and winter, 89.7 µg m−3 and 86.2 µg m−3, respectively. Similarly, PM10 was minimally six times above the world standard, following the trend-R.I.A > B.R.S > B.H.U. For O3, the highest exceedance was observed for B.R.S in the winter season (49.3 µg m−3), followed by the summer (40.3 µg m−3). It was at a minimum in the summer season for B.H.U (5.4 µg m−3), followed by R.I.A (16.7 µg m−3). Major contributors of air quality degradation include vehicular exhaust, construction and demolition activities, the resuspension of road dust, biomass burning, and commercial and household activities.

Table 2.
Average concentrations (Mean ± S.D. µg m−3) of various air pollutants at different sampling sites for the study period Dec 2021–May 2022.
3.2. Plant Responses to Pollutant Load
3.2.1. Photosynthetic Pigments
The total chlorophyll content is reflective of a plant’s physiological health as it performs a key role in the process of photosynthesis [30]. Significant effects of site and season were observed on the chlorophyll a/b and carotenoid contents of the studied plants except A. indica and D. sissoo in the winter season (Table 3).

Table 3.
F-ratio and level of significance of three-way ANOVA test for various parameters under study.
The total chlorophyll content was found to be negatively correlated with an increasing pollutant concentration (R.I.A < B.R.S < B.H.U) in all the plants (Figure 2). The decline was most severe in P. guajava for R.I.A (68.59%) and B.R.S (53.79%), while it was the least severe in A. indica for B.R.S (1.87%) and R.I.A (6.93%) in the winter season. In the summer season, the reduction was pronounced in M. indica for R.I.A (65.94%), and it was at a minimum in B. spectabilis (19.27%). All the plants at R.I.A showed a higher variation for chlorophyll a/b, and the effect was more prominent during the winter season (Figure 2). The order of reduction during the winter season was F. religiosa > P. guajava > C. thevetia > A. indica > D. sissoo > M. indica > B. spectabilis. For the summer season, P. guajava was found to be the most affected plant, followed by F. religiosa and C. thevetia. Conversely, the carotenoid content was found to be positively correlated with the pollutant load in all the species except A. indica and D. sissoo, which demonstrated reduced carotenoid contents (15.76–25.48%) for both seasons (Figure 2). In the summer season, B. spectabilis showed a maximum increase in the carotenoid content at B.R.S (59.05%) and R.I.A (44.29%), while in the winter season, M. indica showed a maximum increase at R.I.A (37%).
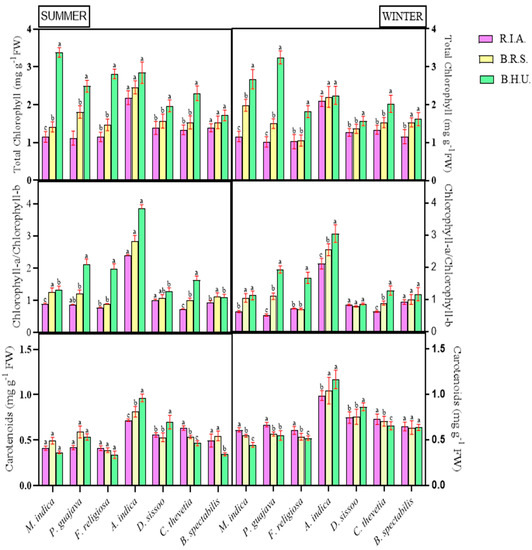
Figure 2.
Seasonal and spatial variations in the photosynthetic pigment—total chlorophyll, chlorophyll a/b and carotenoid contents in different plant species during the study period (Mean ± SE). Bars with different letters show significant differences (p < 0.05) between the sites at specific season.
3.2.2. Enzymatic and Non-Enzymatic Antioxidants
To scavenge ROS, a plant’s antioxidant defense system involves both enzymatic and non-enzymatic components. The enzymatic antioxidants revealed significant impacts of location and season. (Table 3). Catalase showed positive correlations with an increase in pollutant concentrations and hence followed the trend R.I.A > B.R.S > B.H.U in both seasons except in D. sissoo. The increase was maximum in P. guajava (22.21–166.05%), while it was minimum in D. sissoo (6.83–19.15%) at both sites in the winter season. During the summer season, M. indica (174.28%) and P. guajava (40.69%) showed a maximum increase in the catalase activity, whereas it was minimum for D. sissoo (10.28%) and A. indica (4.25%) at R.I.A. and B.R.S., respectively.
The SOD activity reflected a similar trend to catalase, i.e., it showed a significant increase with an increasing concentration of pollutants (Figure 3).
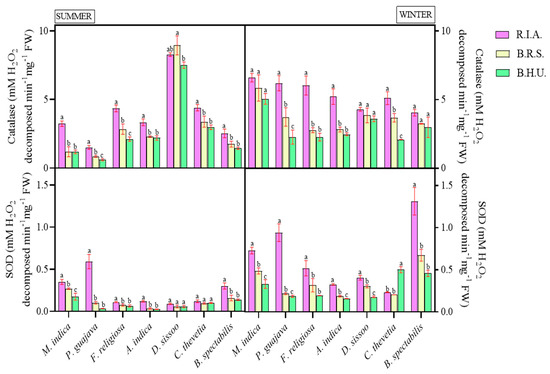
Figure 3.
Seasonal and spatial variations in enzymatic activity—catalase and superoxide dismutase in different plant species during the study period (Mean ± SE). Bars with different letters indicate significant differences (p < 0.05) between the sites at specific season.
During the study period, all the studied plants exhibited significant effects of site and season (Table 3). The assessed non-enzymatic antioxidants showed an increase with increasing pollutant load with an exception in anthocyanin and phenol contents (Figure 4). Significant increments were reported in the AsA contents in all the plants in both seasons, which ranged between 17.97 and 295.93% in the summer and between 23.42 and 270.26% in the winter. The increase was maximum for R.I.A in both the seasons in M. indica, followed by F. religiosa, whereas the increase was minimum in P. guajava for both the sites and seasons. The flavonoid content increased with the pollutant concentration, which was higher for R.I.A in both the seasons, ranging between 26.73 and 90.11% in the summer and between 29.39 and 74.74% in the winter (Figure 4). For B.R.S, the flavonoids ranged between 15.90 and 64.46% in the summer and between 18.73 and 41.89% in the winter.
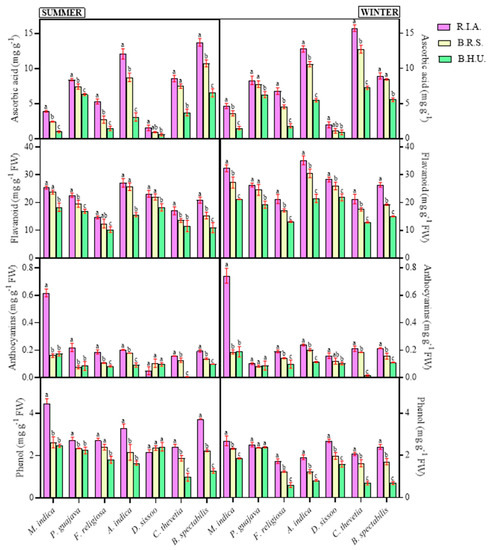
Figure 4.
Seasonal and spatial variations in the non-enzymatic antioxidants—ascorbic acid, flavonoids, anthocyanins and phenol in different plant species during the study period (Mean ± SE). Bars with different letters show significant differences (p < 0.05) between the sites at specific season.
The concentration of anthocyanin increased significantly with the pollutant concentration, and the increase was conspicuous in the summer for most of the species except in P. guajava (Figure 4). The highest anthocyanin concentration was traced in the leaves from site R.I.A, followed by B.R.S and B.H.U. C. thevetia showed a maximum increase in both the seasons, whereas a minimum was reported in D. sissoo. The phenolic content increased with an increasing pollutant load and followed a similar pattern to other non-enzymatic antioxidants (R.I.A > B.R.S > B.H.U) except in D. sissoo, which demonstrated a negative correlation between phenolic content and pollutant concentration in the summer season (Figure 4). The phenolic content increased between 3.81 and 252.30% in the winter, being minimum in P. guajava and maximum in B. spectabilis. In the summer season, an increment of 5.46–190.08% was observed, with similar plants exhibiting the minimum and maximum changes as in the winter season. D. sissoo showed a decrease in phenol concentration in the summer season by 2.79% at B.R.S and 10.89% at R.I.A.
3.2.3. Physio-Chemical Parameters
The physio-chemical properties are often altered to a significant level upon exposure to air pollutants. All analyzed parameters established significant effects of site and season on the plants under consideration except for pH (Table 3). Here, the pH of the leaf extract showed a reduction during the winter season from R.I.A (11.70–24.80%), followed by B.R.S (5.70–15.78%). It was at a minimum in the simple leaf evergreen species (M. indica and P. guajava), whereas it was maximum in the shrub species (C. thevetia and B. spectabilis) for both R.I.A and B.R.S compared to B.H.U (Figure 5). A similar trend was observed for the summer season, wherein the decline was higher in R.I.A (7.30–24.74%), followed by B.R.S (5.94–12.99%).
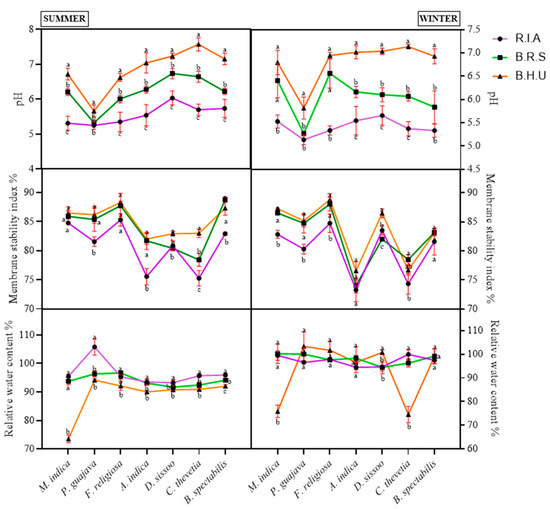
Figure 5.
Seasonal and spatial variations in the physio-chemical parameters—pH, membrane stability index and relative water content in different plant species during the study period (Mean ± SE). Bars with different letters show significant differences (p < 0.05) between the sites at specific season.
The MSI was reduced during the winter season in all the plants (Figure 5). The decline was the highest in the plants from R.I.A and maximum in the evergreen simple leaf species, followed by the semi-evergreen simple leaf, while it was minimum in the compound leaf and shrub species (P. guajava > M. indica > F. religiosa > A. indica > D. sissoo > C. thevetia > B. spectabilis). In B.R.S, both the shrubs showed an increase in MSI, while others showed decline, with highest decline found in D. sissoo (5.13%).
The RWC also declined in all the species at both the sites during winter season (Figure 5). The reduction ranged between 1.66 and 6.54% except for C. thevetia and M. indica, which demonstrated an increase in RWC. In B.R.S, the decline ranged between 13.03 and 47.21% except in M. indica, P. guajava and F. religiosa. During the summer season, a uniform increment was observed at both the sites (R.I.A: 2.72–29.83%; B.R.S: 1.01–27.50%) and in all the plants, being maximum in M. indica and minimum in D. sissoo at both sites. The NWC showed low significance with respect to sites and seasons (Table 3).
3.2.4. Resource Utilization Parameters
Plants’ resource utilization strategies are reflected by their leaf functional traits, among which LMA and LDMC are of prime importance [31]. In the present study, significant effects of site and season on all the plants were observed for resource utilization (Table 3).
The leaf area was reduced at both the sites, the reduction being more at R.I.A (16.78–36.37%) compared to B.RS (5.12–26.64%) and B.H.U during the summer season except for P. guajava and B. spectabilis (Figure 6). In the winter season, similar reductions were observed as in the summer season for both the sites with the same exceptions. The LMA significantly increased at both the sites during the winter (9.04–30.76%) and summer seasons (4.29–80.92%) except in M. indica (−17.83 and −31.97% in R.I.A and −5.53% and −11.92% in B.R.S in summer and winter, respectively) and B. spectabilis (−73.91 and 75.72% in R.I.A and −54.99 and 59.52% in B.R.S in winter and summer, respectively) (Figure 6). The LDMC was enhanced (9.39–36.47%) with an increasing pollutant load at both the sites during the winter season except in M. indica and B. spectabilis at R.I.A, with the addition of C. thevetia in B.R.S (Figure 6). Conversely, during the summer season, LDMC decreased at both sites with an exception of P. guajava and A. indica.
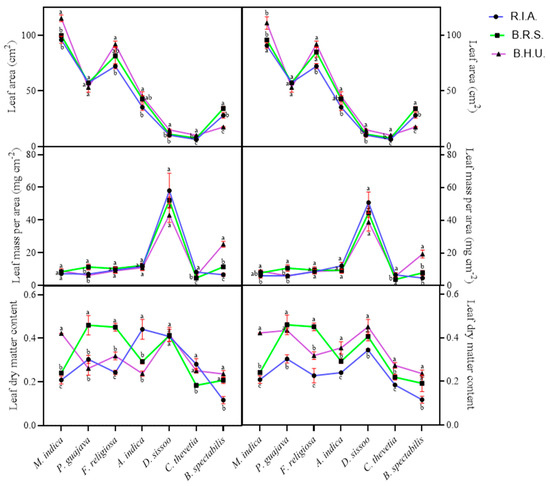
Figure 6.
Seasonal and spatial variations in the resource utilization—leaf area, leaf mass per area and leaf dry matter content in different plant species during the study period (Mean ± SE). Bars with different letters show significant differences (p < 0.05) between the sites at specific season.
4. Discussion
Trees have been extensively used in urban air pollution abatement and are consistently exposed to the increasing air pollution with an aid to provide a surface for the absorption and adsorption of the contaminants present in the surroundings. Henceforth, it is essential to choose suitable and efficient plant species for designing green belt or urban plantation based on their overall response to the prevailing pollutants. The present study provides a detailed assessment of seven commonly used perennial plant species at three different land-use areas in two dominant seasons of the Indo-Gangetic plains and will conclude their susceptibility to air pollutants based on the HCA data (Figure 7).
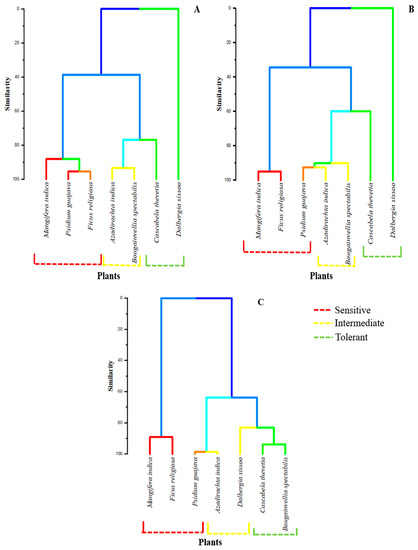
Figure 7.
Hierarchical correlation cluster analysis of the plants under study at different sites: (A) Ramnagar Industrial Area, (B) Banaras Railway Station and (C) Banaras Hindu University. Similarity between the colors reflects a higher correlation.
This study concerns the relative performance of the dominant urban plant species in different land uses from the most polluted (R.I.A) location to a comparatively less polluted (B.H.U) location. Local weather conditions play a major role in determining the pollutant concentration over a region. The dust load was found to be at a maximum in the winter season at all the sites, attributing to the foggy conditions, wetness of the foliar surface and slow-moving wind. A consistently high concentration of PM in the commercial and industrial sites might be due to the heavy tailpipe emissions of the transportation sector and re-suspended soil dust in both seasons, with an additional increase in the burning of biomass during the winter season [32]. Conversely, the regional wind movement is high in summer season, which may be responsible for the re-suspension of dust from the foliar surface and hence less accumulation of the dust load [33]. The concentration of gaseous pollutants was also invariably higher during the winter season at all sites, which was found to be the highest at R.I.A and the lowest at B.H.U. This rise in the concentration of pollutants can be correlated with their restricted dispersion at the higher atmospheric levels which, in turn, enhances their ground level concentration [34]. Due to a sudden drop in temperature, a stronger and prolonged inversion layer develops, which worsens the air quality in the lower troposphere.
4.1. Photosynthetic Pigments
The total chlorophyll content was observed to decrease significantly with an increasing pollutant load, found to be minimum at R.I.A and maximum at B.H.U. This lends credence to the assertion by Wei et al. [35], which states that the chloroplast is the primary site of strike by air pollutants, mainly PM, SO2 and NO2. Their entry into leaf tissues is regulated by stomatal apertures where they denature chlorophyll upon entering and reduce its concentration. It is also established that the phytotoxic behavior of SO2 leads to a reduction in chlorophyll concentration via the interruption of the chloroplast layer, which will ultimately cause leaching of the pigment [36]. As they are acidic in nature, SO2 and NO2 will cause the acidification of the chlorophyll which, in turn, form pheophytin through the dispersion of magnesium by protons obtained from the acidic pollutants [37] and will further contribute to the reduction of the pigment concentration [38]. The increased PM10 concentration at the industrial and commercial site might also have enhanced the degradation of chlorophyll content as a result of adhered heavy metals and the polycyclic aromatic hydrocarbons present on them, which have solubilized in the cell sap [39]. In the summer season, the concentration of chlorophyll was higher than in the winter due to the shorter photoperiod and higher PM load imparting a shading effect. It has been proposed that a plant’s sensitivity is linked to the synthesis and breakdown of chlorophyll. Thus, plants with a high chlorophyll content are considered to be resistant to air pollution. In our study, the decline in the chlorophyll content was observed to be the most in P. guajava and M. indica, while it was the least in A. indica and B. spectabilis, thereby establishing that simple leaf and evergreen species are more susceptible to prevalent air pollutants in the area.
By enhancing the concentration of carotenoids, plants appear to be attempting to shield chlorophyll from damage. When cells are exposed to air pollution, the cell membrane is harmed, and the amount of thylakoid and chlorophyll in the chloroplast is reduced. In this study, carotenoid contents were significantly increased with the pollutant load in both the shrubs, while trees with compound leaves (A. indica and D. sissoo) showed a decline in carotenoids with an increasing pollutant load in both seasons. The decrease in carotenoids in the polluted areas might reflect the sensitivity of the carotenoids to SO2, as reported by Chauhan [4]. The trees with simple leaves (M. indica and F. religiosa) showed an insignificant increase in carotenoid content in summer, while the concentration increased with the pollutant load in the winter season. The increased concentration of carotenoids, the accessory photosynthetic pigments in both seasons at polluted sites, may be attributed to a reduction in the photooxidative stress on the primary photosynthetic pigments, which is consistent with the findings of Pellegrini et al. [40]. Further, the concentration of carotenoids can be correlated with the morphology of the leaves such that the concentration increased in the simple leaves and decreased in the compound leaves. The species with simple leaves showed more sensitivity to the pollutants due to their higher surface area for pollutant impingement compared to the compound leaves.
4.2. Enzymatic and Non-Enzymatic Antioxidants
To counteract oxidative damage, plants have devised enzymatic and non-enzymatic antioxidant defense machinery to scavenge hazardous ROS [41]. Antioxidative enzymes such as SOD and CAT may scavenge destructive ROS molecules or resist plants by triggering a non-enzymatic antioxidant mechanism [42]. SOD combats oxidative stress by converting superoxide radicals in mitochondria and chloroplasts into O2 and H2O2 [43]. Further, the CAT is involved in the protection of plant peroxidation by converting damaging intracellular H2O2 to H2O and O2 without using cell energy [44]. Plants increase their antioxidants in order to enhance their defensive property against the applied stress and also in response to the oxidative stress generated in them [45]. In this study, it was observed that the concentration of both the enzymes catalase and superoxide dismutase increased with an increase in the pollutant concentration. The enhancement was maximum for the simple leaf species (P. guajava and M. indica) during both seasons, reflecting a higher oxidative stress in them and a corresponding increase in the level of antioxidative enzymes, which is in line with the study of Tripathi and Gautam [46]. AsA acts as a protectant against the SO2-induced reactive oxygen species (H2O2, O2− and OH−), thereby safeguarding carbon fixation enzymes in addition to the inactivation of chlorophyll [47]. It in addition to leaf pH, is important in determining the sensitivity of a plant to SO2 [48]. Its reducing power increases with an increasing pH and decreases with a reduction in the pH value. Thus, AsA may protect chloroplasts and chlorophyll activities from pollutants due to its pH-dependent reducing activity. In our study, the increase in AsA was maximum for M. indica and F. religiosa for both seasons, indicating higher oxidative stress in them.
Biological and non-biological stresses tend to accumulate the phenolic metabolites in plant tissues [49]. They are vital antioxidants that defend against the development and progression of the oxidation chain and reactive oxygen species by lowering or suppressing lipid auto-oxidation or degrading peroxides [50]. Despite the fact that phenols and flavonoids are present in plants as byproducts of related pathways, their concentrations may be elevated in response to oxidative stress. In our study, both the flavonoid and phenolic contents increased maximally in the shrub species (C. thevetia and B. spectabilis), thereby establishing a higher resistance in response to exposed stress. This is in accordance with the observations of Massad et al. [51] that the phenol production is boosted under stress at the expense of other primary metabolites, and this protects plants from stress caused by air pollution.
4.3. Physio-Chemical Parameters
Our study revealed a significant decline in the leaf pH with an increase in the pollutant concentration. This might be due to the impregnation of different air pollutants into the leaf cells, whereupon the plants with maximum decrease may be highly sensitive to air pollutants. The decline in pH can also be ascribed to the increased solubilization of SO2 in the cell sap, which consequently lowers the pH [8]. Pollutant absorption results in acidification of intracellular pH, which is frequently seen in sensitive species. The reduction was minimum in both evergreen simple leaf species (M. indica and P. guajava) and maximum in shrubs, making them sensitive. Our findings are consistent with findings by Joshi et al. [52], which revealed a direct correlation between a low leaf pH and the susceptibility of different plants to air pollution. Plants in a polluted environment (season and location) decrease their leaf pH in an attempt to convert carbohydrates (hexose) to ascorbic acid, hence inducing a mechanism to combat the generated oxidative stress. As a consequence of the pollutant load, membrane lipids frequently undergo phase transitions, affecting the permeability of the membrane and causing solute leakage, which is measured by MSI [26]. The MSI declined with an increasing pollutant load, and it was minimum in simple leaf evergreen species (P. guajava). A significant decline was observed in the RWC of the plants subjected to air pollution stress, reflecting their sensitivity to the subjected stress. This occurs in order to maintain the osmotic potential of the cells. The simple leaf species (M. indica, P. guajava and F. religiosa) maintained a relatively higher RWC among all species during the study period. The plants attempt to raise their RWC to maintain their physiological homeostasis in order to withstand abiotic stress, such as air pollution. The RWC was reported to analog with the protoplasmic permeability, which was frequently observed in the sensitive species [7]. The early senescence of leaves is a result of water and dissolved nutrient loss caused by pollutant-induced enhanced permeability in cells [53]. As a result, it is anticipated that plants with a higher RWC in contaminated sites will be resistant to pollutants [54].
4.4. Resource Utilization Strategy
The resource utilization strategy of the plants under study reflected that the tolerant/less sensitive species invest more resources in build up biomass at the expense of reducing their leaf area by increasing the LMA [13]. This is in line with our observation that the leaf area decreased with an increasing pollutant load while the LMA increased except in the simple leaf species (P. guajava, M. indica and B. spectabilis), making them sensitive to the exposed pollution concentration. LDMC is a significant determinant of a plant species’ resource utilization strategy, i.e., its position in a fundamental trade-off between rapid assimilation and growth at one extreme and the efficient conservation of resources inside well-protected tissues at the other [55]. The present study showed a seasonal difference in the LDMC of the plants under study. However, in both seasons, the simple leaf species (M. indica and B. spectabilis) were noted to be more sensitive when compared to the compound leaf species (A. indica).
On the basis of the Pearson correlation coefficient, the hierarchical correlation cluster analysis (HCA) established the differential responses of plants under study (Figure 7). The species with simple leaves (M. indica, P. guajava and F. religiosa) were classified as more sensitive in both the polluted sites (R.I.A and B.R.S) irrespective of their habit (evergreen or semi-evergreen), followed by the compound leaf species (A. indica and D. sissoo). Among both the sites, the similarity was the highest between P. guajava and F. religiosa, while it was the lowest between M. indica and B. spectabilis at R.I.A. It was maximum between M. indica and P. guajava, whereas minimum for M. indica and B. spectabilis for B.R.S. For B.H.U, the similarity was the highest between C. thevetia and B. spectabilis and the lowest between F. religiosa and D. sissoo.
4.5. Principal Component Analysis
The principal component analysis shown in Figure 8 was performed individually for each of the three sites: the components were extracted with a 56.64%, 57.32% and 57.57% explained variance for R.I.A, B.R.S and B.H.U, respectively. The PCA analysis was performed for the winter season only as it was the period with the highest pollutant concentration. For R.I.A, the PC1 (eigenvalue 5.35) with a 33.43% variance was loaded with TC, Caro, Cat, SOD, RWC, LA and LDMC (Figure 8A). The PC2 with an eigenvalue of 3.71 and a 23.21% variance showed higher loading for Chla/b, AsA, Flav, Antho, Phe, pH, MSI, NWC and LMA. Most of the antioxidants (enzymatic and non-enzymatic) and leaf area showed a higher correlation with the simple leaf species, i.e., M. indica, P. guajava and F. religiosa, while the compound leaf species (D. sissoo and A. indica) were more correlated with the photosynthetic pigments, LMA and LDMC. Both the shrub species were negatively correlated with all the parameters except RWC and AsA. For B.R.S, PC1 and PC2 showed eigenvalues of 4.78 and 4.39, respectively, with a 29.89% variance for PC1 and a 27.43% variance for PC2 (Figure 8B). PC1 was loaded with Caro, Cat, SOD, AsA, Phe and MSI, whereas PC2 reflected loading of TC, Chla/b, Flav, Antho, pH, RWC, NWC, LA, LMA and LDMC. F. religiosa, P. guajava, and B. spectabilis showed a higher positive correlation with the resource utilization parameters and a negative correlation with the other analyzed parameters. In B.H.U, PC1, with an eigenvalue of 5.52 and a 34.50% variance, was loaded with Cat, AsA, Flav, Antho, Phe, MSI, NWC, LA and LDMC, while PC2, with an eigenvalue of 3.69 and a 23.07% variance, demonstrated a higher loading of TC, Caro, Chla/b, SOD, pH, RWC and LMA (Figure 8C). D. sissoo, B. spectabilis and C. thevetia showed a higher correlation with the enzymatic antioxidants, physio-chemical and resource utilization parameters, while P. guajava and M. indica showed less correlation with all the studied parameters.
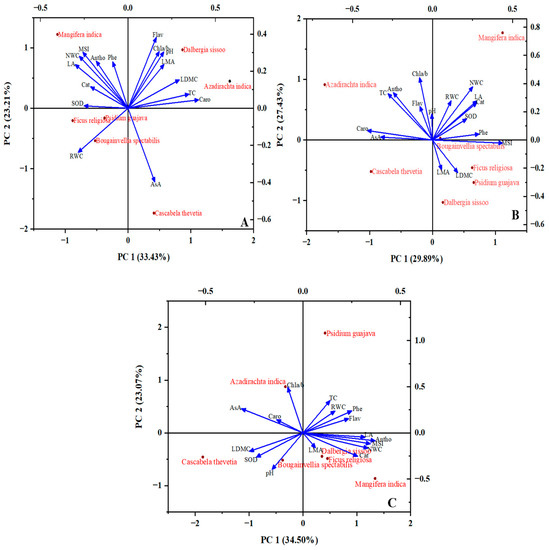
Figure 8.
Principal component analysis bi-plot demonstrating the relationship between the plant species and parameters analyzed at different sites under study—(A) Ramnagar Industrial Area, (B) Banaras Railway Station and (C) Banaras Hindu University. TC—total chlorophyll; Chla/b—chlorophyll a/b; Caro—carotenoid; AsA—ascorbic acid; LDMC—leaf dry matter content; SOD—superoxide dismutase; LMA—leaf mass per area; Cat—catalase; NWC—net water content; MSI—membrane stability index; Antho—anthocyanin; LA—leaf area; Flav—flavonoids; Phe—phenol; RWC—relative water content.
The differential response of plants appears to depend more on the leaf morphology than their habit. Leaf morphology is responsible for the per area load to exposed pollutants. The habit played a secondary role in response to the air pollutants in which the deciduous and semi-deciduous trees appeared to be less sensitive; this can be attributed to their leafless periods, during which their exposed surface to pollutants is drastically reduced and hence their adsorption and absorption are also reduced.
5. Conclusions
Seven dominant perennial species of Indo-Gangetic plains were assessed for their differential responses to air pollutants based on their morphological and biochemical parameters. The concentrations of NO2 and PM10 were consistently above the air quality standards in Varanasi. Pollution load and seasonal fluctuations had a major impact on differences in leaf functional characteristics. The analyzed photosynthetic pigments, enzymatic and non-enzymatic antioxidants, pH, MSI, RWC and resource utilization strategy of the plant revealed that the prevailing pollutant concentration had a more negative influence on evergreen trees with simple leaves than deciduous trees with compound leaves. The habit of the trees may have contributed to the observed responses indirectly, as the trees with simple leaves and evergreen habit will have a longer leaf lifespan which, in turn, will be expose them to the prevailing pollutant load for a longer period; therefore, more pollutant will be adsorbed/absorbed on their foliage. The trees with compound leaves and deciduous habit will have dual ease for the pollutant load, as they have less exposed surface and a shorter leaf lifespan, reducing the exposure time period and hence minimizing the impact of pollutants. Based on the assessed responses, it is inferred that in spite of different habits, plants with simple leaves tend to appear more sensitive to air pollutants than compound leaves. As the habit evolved from evergreen to deciduous with semi-evergreen in between, a resistance was induced against prevailing air pollution. Among both the perennial species, shrubs were more tolerant than trees when exposed to pollutant stress. Depending on the assessed parameters, C. thevetia among the shrubs and D. sissoo among the trees, followed by A. indica, were found to be less sensitive to the prevailing air pollutant concentration. It is highly recommended to promote mixed plantation with more compound leaf species in order to efficiently ameliorate the air pollutants from different land-use areas.
Author Contributions
Conceptualization, H.S. and M.A.; methodology, H.S. and P.S.; validation, H.S.; investigation, H.S.; resources, M.A. and S.B.A.; data curation, H.S.; writing—original draft preparation, H.S. and P.S.; writing—review and editing, M.A. and S.B.A.; visualization, M.A. and S.B.A.; supervision, M.A.; funding, H.S. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the CSIR project [Sanction no. 38(1500)/21/EMR-II]. The financial assistance was in the form of a Junior and Senior Research Fellowship (IF190187) by DST-INSPIRE, Government of India, to H.S. and J.C. Bose National Fellowship (Sanction no. JCB/2021/000040) by SERB, New Delhi to M.A. is gratefully acknowledged. S.B.A. acknowledges the support of CSIR, New Delhi for financial assistance in the form of Emeritus Scientist project (Award no. 21(1136)/22/EMR-II).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy issues.
Acknowledgments
Authors are thankful to the Head, Department of Botany and the coordinator, Institute of Eminence & ISLS, Banaras Hindu University, Varanasi, for providing the necessary facilities.
Conflicts of Interest
The authors declare no personal circumstances or interest that may be perceived as influencing the representation or interpretation of research results.
References
- United Nations. World Urbanization Prospects 2018—Highlights; Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- Fuso Nerini, F.; Sovacool, B.; Hughes, N.; Cozzi, L.; Cosgrave, E.; Howells, M.; Tavoni, M.; Tomei, J.; Zerriffi, H.; Milligan, B. Connecting climate action with other Sustainable Development Goals. Nat. Sustain. 2019, 2, 674–680. [Google Scholar] [CrossRef]
- WHO. Ambient Air Pollution—A Major Threat to Health and Climate; WHO: Geneva, Switzerland, 2021. Available online: https://www.who.int/airpollution/ambient/en/ (accessed on 20 January 2021).
- Chauhan, A. Photosynthetic pigment changes in some selected trees induced by automobile exhaust in Dehradun, Uttarakhand. N. Y. Sci. J. 2010, 3, 45–51. [Google Scholar]
- Rai, P.K.; Panda, L.L.; Chutia, B.M.; Singh, M.M. Comparative assessment of air pollution tolerance index (APTI) in the industrial (Rourkela) and non industrial area (Aizawl) of India: An ecomanagement approach. Afr. J. Environ. Sci. Technol. 2013, 7, 944–948. [Google Scholar] [CrossRef]
- Alahabadi, A.; Ehrampoush, M.H.; Miri, M.; Aval, H.E.; Yousefzadeh, S.; Ghaffari, H.R.; Ahmadi, E.; Talebi, P.; Fathabadi, Z.A.; Babai, F.; et al. A comparative study on capability of different tree species in accumulating heavy metals from soil and ambient air. Chemosphere 2017, 172, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Rao, D.N.; Agrawal, M.; Pandey, J.; Naryan, D. Air pollution tolerance index of plants. J. Environ. Manag. 1991, 32, 45–55. [Google Scholar] [CrossRef]
- Dadkhah-Aghdash, H.; Rasouli, M.; Rasouli, K.; Salimi, A. Detection of urban trees sensitivity to air pollution using physiological and biochemical leaf traits in Tehran, Iran. Sci. Rep. 2022, 12, 15398. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar] [CrossRef]
- Ram, S.S.; Majumder, S.; Chaudhuri, P.; Chanda, S.; Santra, S.C.; Chakraborty, A.; Sudarshan, M. A review on air pollution monitoring and management using plants with special reference to foliar dust adsorption and physiological stress responses. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2489–2522. [Google Scholar] [CrossRef]
- Sharma, M.; Panwar, N.; Arora, P.; Luhach, J.; Chaudhry, S. Analysis of biological factors for determination of air pollution tolerance index of selected plants in Yamuna Nagar, India. J. Environ. Biol. 2013, 34, 509–514. [Google Scholar]
- NAAQS. Guidelines for the Measurement of Ambient Air Pollutants Volume-I; Central Pollution Control Board, Ministry of Environment & Forests, Govt. of India: New Delhi, India, 2011.
- Mukherjee, A.; Agrawal, M. Use of GLM approach to assess the responses of tropical trees to urban air pollution in relation to leaf functional traits and tree characteristics. Ecotoxicol. Environ. Saf. 2018, 152, 42–54. [Google Scholar] [CrossRef]
- West, P.W.; Gaeke, G.C. Fixation of sulfur dioxide as disulfitomercurate (II) and subsequent colorimetric estimation. Anal. Chem. 1956, 28, 1816–1819. [Google Scholar] [CrossRef]
- Jacobs, M.B.; Hochheiser, S. Continuous sampling and ultramicrodetermination of nitrogen dioxide in air. Anal. Chem. 1958, 30, 426–428. [Google Scholar] [CrossRef]
- Hangartner, M.; Kirchner, M.; Werner, H. Evaluation of passive methods for measuring ozone in the European Alps. Analyst 1996, 121, 1269–1272. [Google Scholar] [CrossRef]
- Maclachlan, S.; Zalik, S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can. J. Bot. 1963, 41, 1053–1062. [Google Scholar] [CrossRef]
- Duxbury, A.C.; Yentsch, C.S. Plankton Pigment Nomographs; Canadian Science Publishing: Ottawa, ON, Canada, 1956. [Google Scholar]
- Takshak, S.; Agrawal, S.B. Defence strategies adopted by the medicinal plant Coleus forskohlii against supplemental ultraviolet-B radiation: Augmentation of secondary metabolites and antioxidants. Plant Physiol. Biochem. 2015, 97, 124–138. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Pandey, A.; Jaiswal, D.; Agrawal, S.B. Ultraviolet-B mediated biochemical and metabolic responses of a medicinal plant Adhatoda vasica Nees. at different growth stages. J. Photochem. Photobiol. B Biol. 2021, 216, 112142. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; Schwager, H. Air pollution and ascorbic acid. Eur. J. For. Pathol. 1977, 7, 338–350. [Google Scholar] [CrossRef]
- Bray, H.G.; Thorpe, W. Analysis of phenolic compounds of interest in metabolism. Methods Biochem. Anal. 1954, 1, 27–52. [Google Scholar] [CrossRef]
- Gupta, A.; Agrawal, S.B.; Agrawal, M. Evaluation of Toxicity of Tropospheric Ozone on Tomato (Solanum lycopersicum L.) Cultivars: ROS Production, Defense Strategies and Intraspecific Sensitivity. J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Flint, S.D.; Jordan, P.W.; Caldwell, M.M. Plant protective response to enhanced UV-B radiation under field conditions: Leaf optical properties and photosynthesis. Photochem. Photobiol. 1985, 41, 95–99. [Google Scholar] [CrossRef]
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant. 2013, 35, 1837–1842. [Google Scholar] [CrossRef]
- Smart, R.E.; Bingham, G.E. Rapid estimates of relative water content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef]
- WHO. WHO Air Quality Guidelines Global Update Published by World Health Organization on the Internet; WHO: Geneva, Switzerland, 2005.
- CPCB. National Ambient Air Quality Standards; Central Pollution Control Board: New Delhi, India, 2009; Available online: http://cpcb.nic.in/National_Ambient_Air_Quality_Standards.php (accessed on 11 April 2017).
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Ji, Y.; Gao, J. Climate factors determine the utilization strategy of forest plant resources at large scales. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, M. World air particulate matter: Sources, distribution and health effects. Environ. Chem. Lett. 2017, 15, 283–309. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, B.; Roy, L.B.; Shekhar, S.; Singh, R.K. Tree responses to foliar dust deposition and gradient of air pollution around opencast coal mines of Jharia coalfield, India: Gas exchange, antioxidative potential and tolerance level. Environ. Sci. Pollut. Res. 2021, 28, 8637–8651. [Google Scholar] [CrossRef]
- Orru, H.; Ebi, K.L.; Forsberg, B. The interplay of climate change and air pollution on health. Curr. Environ. Health Rep. 2017, 4, 504–513. [Google Scholar] [CrossRef]
- Wei, S.Z.; Yuan, H.J.; Zan, Y.L. Research on the SO2 resistance in three kinds of foliage plants. Chin. Agric. Sci. Bull. 2014, 30, 152–156, (In Chinese with English abstract). [Google Scholar]
- Uka, U.N.; Belford, E.J.; Hogarh, J.N. Roadside air pollution in a tropical city: Physiological and biochemical response from trees. Bull. Natl. Res. Cent. 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Saxena, P.; Kulshrestha, U. Biochemical effects of air pollutants on plants. In Plant Responses to Air Pollution; Springer: Berlin/Heidelberg, Germany, 2016; Volume 59. [Google Scholar] [CrossRef]
- Jyothi, S.J.; Jaya, D.S. Evaluation of air pollution tolerance index of selected plant species along roadsides in Thiruvananthapuram, Kerala. J. Environ. Biol. 2010, 31, 379–386. [Google Scholar]
- Prusty, B.A.K.; Mishra, P.C.; Azeez, P.A. Dust accumulation and leaf pigment content in vegetation near the national highway at Sambalpur, Orissa, India. Ecotoxicol. Environ. Saf. 2005, 60, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, E.; Hoshika, Y.; Dusart, N.; Cotrozzi, L.; Gérard, J.; Nali, C.; Vaultier, M.N.; Jolivet, Y.; Lorenzini, G.; Paoletti, E. Antioxidative responses of three oak species under ozone and water stress conditions. Sci. Total Environ. 2019, 647, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Anjum, S.A.; Farooq, M.; Xie, X.Y.; Liu, X.J.; Ijaz, M.F. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci. Hortic. 2012, 140, 66–73. [Google Scholar] [CrossRef]
- Davies, K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB life 2000, 50, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Ahmad, P. Catalase: A versatile antioxidant in plants. In Oxidative Damage to Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 131–148. [Google Scholar] [CrossRef]
- Gapińska, M.; Skłodowska, M.; Gabara, B. Effect of short-and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol. Plant. 2008, 30, 11–18. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Gautam, M. Biochemical parameters of plants as indicators of air pollution. J. Environ. Biol. 2007, 28, 127. [Google Scholar]
- Tanaka, K.; Otsubo, T.; Kondo, N. Participation of hydrogen peroxide in the inactivation of Calvin-cycle SH enzymes in SO2-fumigated spinach leaves. Plant Cell Physiol. 1982, 23, 1009–1018. [Google Scholar] [CrossRef]
- Chaudhary, C.S.; Rao, D.N. A study of some factors in plants controlling their susceptibility to SO2 pollution. Proc. Indian Natl. Sci. Acad. USA 1977, 43, 236–241. [Google Scholar]
- Nayak, R.; Biswal, D.; Sett, R. Biochemical changes in some deciduous tree species around Talcher thermal power station, Odisha, India. J. Environ. Biol. 2013, 34, 521. [Google Scholar]
- Kováčik, J.; Bačkor, M. Changes of phenolic metabolism and oxidative status in nitrogen-deficient Matricaria chamomilla plants. Plant Soil 2007, 297, 255–265. [Google Scholar] [CrossRef]
- Massad, T.J.; Trumbore, S.E.; Ganbat, G.; Reichelt, M.; Unsicker, S.; Boeckler, A.; Gleixner, G.; Gershenzon, J.; Ruehlow, S. An optimal defense strategy for phenolic glycoside production in Populus trichocarpa–isotope labeling demonstrates secondary metabolite production in growing leaves. New Phytol. 2014, 203, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Chauhan, A.; Joshi, P.C. Impact of industrial air pollutants on some biochemical parameters and yield in wheat and mustard plants. Environmentalist 2009, 29, 398–404. [Google Scholar] [CrossRef]
- Masuch, G.; Kicinski, H.G.; Kettrup, A.; Boos, K.S. Single and Combined Effects of Continuous and Discontinuous O3 and SO2 Immission on Norway Spruce Needles: I. Histological and Cytological Changes. Int. J. Environ. Anal. Chem. 1988, 32, 187–212. [Google Scholar] [CrossRef]
- Govindaraju, M.; Ganeshkumar, R.S.; Muthukumaran, V.R.; Visvanathan, P. Identification and evaluation of air-pollution-tolerant plants around lignite-based thermal power station for greenbelt development. Environ. Sci. Pollut. Res. 2012, 19, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Vaieretti, M.V.; Diaz, S.; Vile, D.; Garnier, E. Two measurement methods of leaf dry matter content produce similar results in a broad range of species. Ann. Bot. 2007, 99, 955–958. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).