High Stromal Senescence During the Window of Implantation Is Linked to Plasma Cell Presence and Cluster Formation in the Endometrium
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Tissue Collection and Preparation
2.3. Immunohistochemistry
2.4. Image Analysis
2.5. Sample Size and Power Analysis
2.6. Statistical Analysis
3. Results
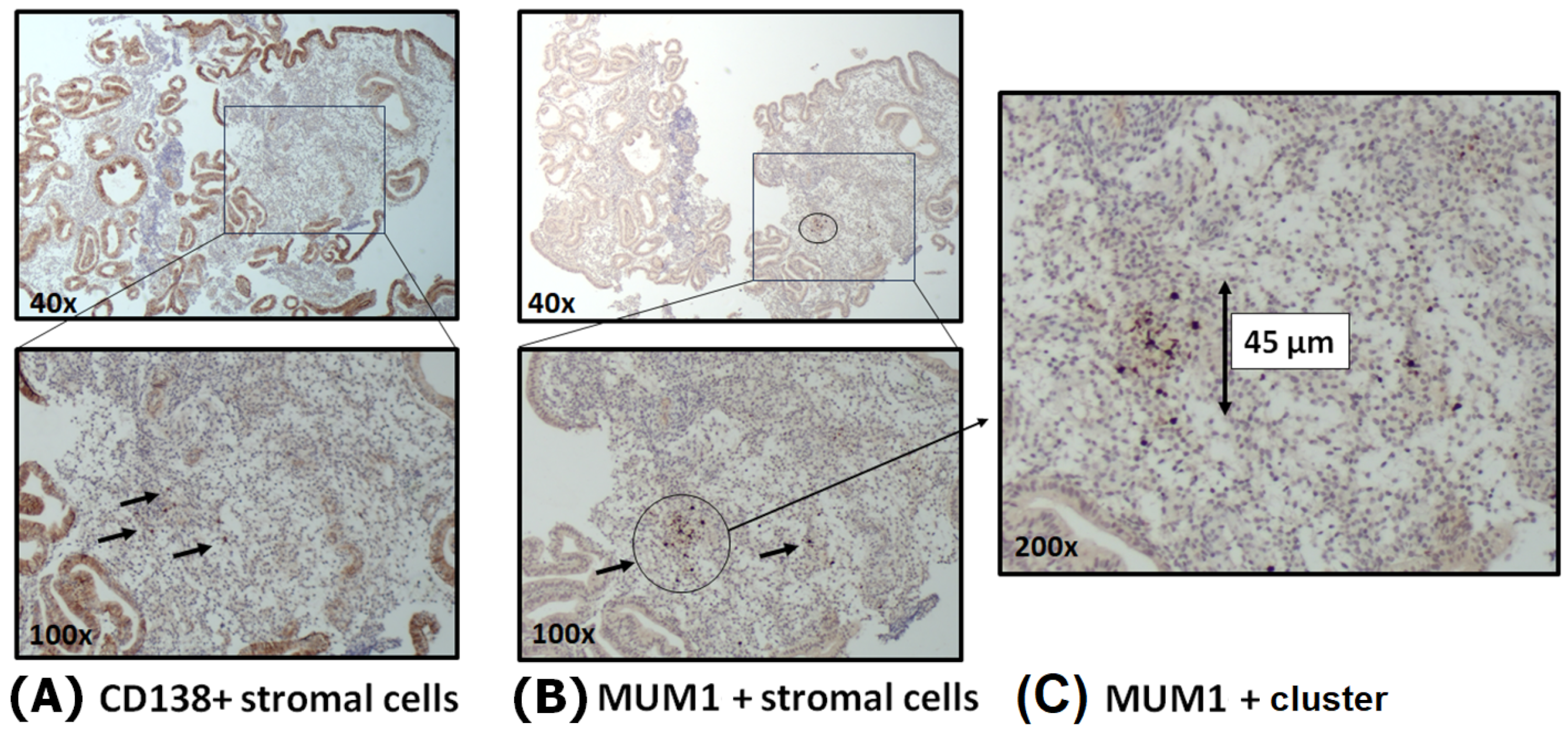
3.1. Presence of Plasma Cells in the Endometrium
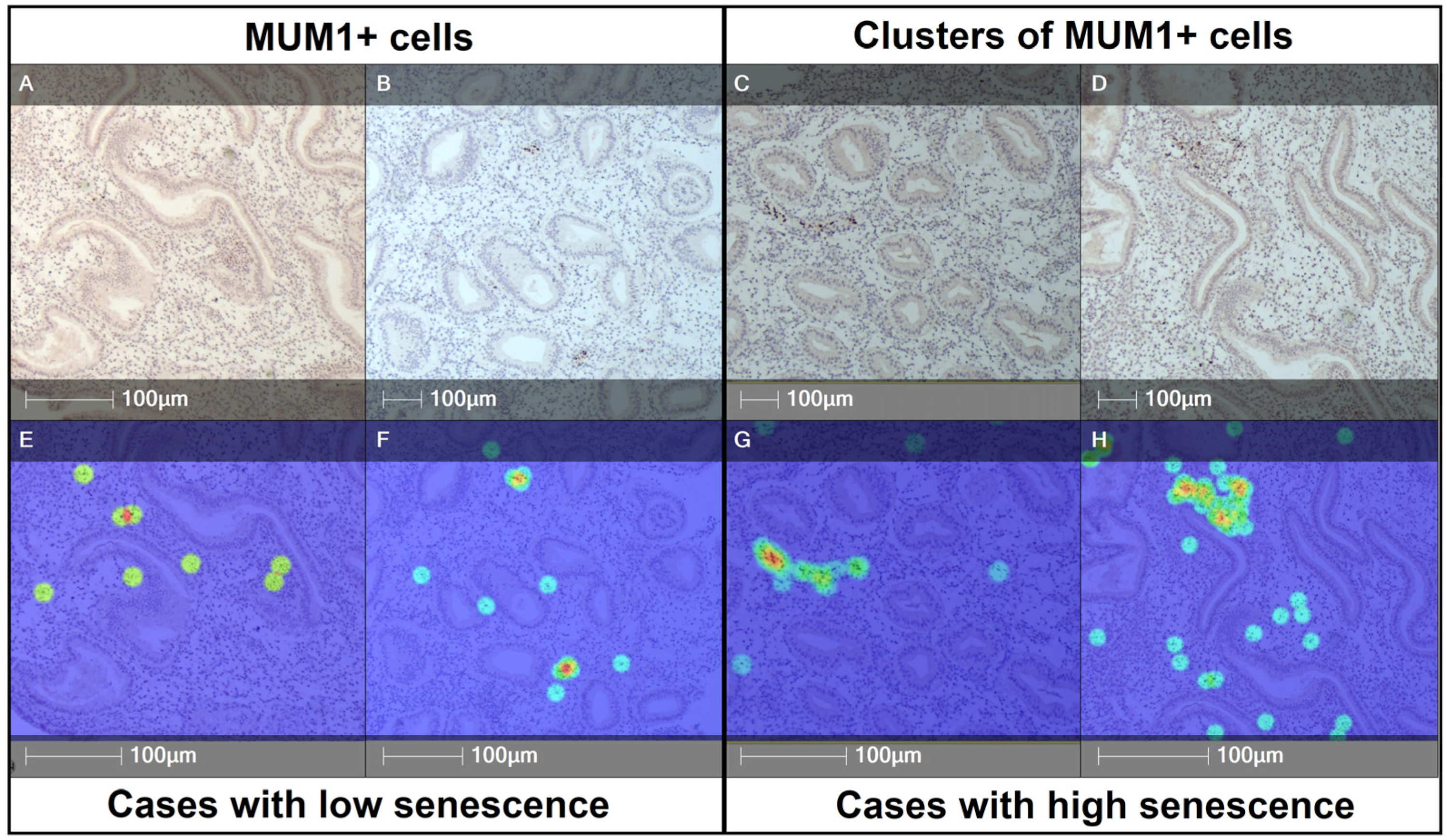
3.2. Plasma Cell Density in Low- and High-Senescence Groups
3.3. Plasma Cell Clustering and Spatial Distribution
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CE | Chronic endometritis |
| LH | Luteinizing hormone |
| p16^INK4a^ (p16) | Cyclin-dependent kinase inhibitor 4A |
| SASP | Senescence-associated secretory phenotype |
| SD | Standard deviation |
| SPSS | Statistical Package for the Social Sciences |
| HALO | Digital image analysis software (Indica Labs) |
References
- Koot, Y.E.; Teklenburg, G.; Salker, M.S.; Brosens, J.J.; Macklon, N.S. Molecular aspects of implantation failure. Biochim. Biophys. Acta 2012, 1822, 1943–1950. [Google Scholar] [CrossRef]
- Lessey, B.A.; Young, S.L. Homeostasis imbalance in the endometrium of women with implantation defects: The role of estrogen and progesterone. Semin. Reprod. Med. 2014, 32, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Volovsky, M.; Seifer, D.B. Current status of ovarian and endometrial biomarkers in predicting ART outcomes. J. Clin. Med. 2024, 13, 3739. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, M.; Lee, S.K. Role of endometrial immune cells in implantation. Clin. Exp. Reprod. Med. 2011, 38, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Moldenhauer, L.M.; Green, E.S.; Care, A.S.; Hull, M.L. Immune determinants of endometrial receptivity: A biological perspective. Fertil. Steril. 2022, 117, 1107–1120. [Google Scholar] [CrossRef]
- Bayer-Garner, I.B.; Korourian, S. Plasma cells in chronic endometritis are easily identified with syndecan-1. Mod. Pathol. 2001, 14, 877–879. [Google Scholar] [CrossRef]
- Ughade, P.A.; Shrivastava, D. Unveiling the role of endometrial CD138: A comprehensive review on its significance in infertility and early pregnancy. Cureus 2024, 16, e54782. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Liu, W.; Chen, Y.; Yang, X.; Wu, J.; Xu, M.; You, G.; Lian, R.; Huang, C.; et al. The effect of the number of endometrial CD138+ cells on the pregnancy outcomes of infertile patients in the proliferative phase. Front. Endocrinol. 2025, 15, 1437781. [Google Scholar] [CrossRef]
- Cicinelli, E.; Haimovich, S.; De Ziegler, D.; Raz, N.; Ben-Tzur, D.; Andrisani, A.; Ambrosini, G.; Picardi, N.; Cataldo, V.; Balzani, M.; et al. MUM-1 immunohistochemistry has high accuracy and reliability in the diagnosis of chronic endometritis: A multi-centre comparative study with CD-138 immunostaining. J. Assist. Reprod. Genet. 2022, 39, 219–226. [Google Scholar] [CrossRef]
- Santoro, A.; Travaglino, A.; Inzani, F.; Angelico, G.; Raffone, A.; Maruotti, G.M.; Straccia, P.; Arciuolo, D.; Castri, F.; D’Alessandris, N.; et al. The role of plasma cells as a marker of chronic endometritis: A systematic review and meta-analysis. Biomedicines 2023, 11, 1714. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Xiao, X.; Li, M. Correlation of hysteroscopic findings of chronic endometritis with CD138 immunohistochemistry and their correlation with pregnancy outcomes. J. Assist. Reprod. Genet. 2024, 41, 2477–2483. [Google Scholar] [CrossRef]
- Van Golde, R.; Van Haren, A.; Stevens, L.; Brentjens, S.; Sofia, X.; Bert, D.; Servaas, M. Higher incidence of CD138+ cells indicating chronic endometritis in patients with recurrent implantation failure. Hum. Reprod. 2025, 40, deaf097.755. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Huang, C.; Lian, R.; Chen, C.; Liu, S.; Li, L.; Diao, L.; Markert, U.R.; Zeng, Y. Evaluation of peripheral and uterine immune status of chronic endometritis in patients with recurrent reproductive failure. Fertil. Steril. 2020, 113, 187–196.e1. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhang, L.; Yang, J.; Yang, M.; Li, Q.; Chen, Q. Uterine NK cell polarization associates with chronic endometritis and predisposition to recurrent implantation failure. Int. J. Womens Health 2025, 17, 4255–4266. [Google Scholar] [CrossRef]
- Burton, D.G.; Krizhanovsky, V. Physiological and pathological consequences of cellular senescence. Cell. Mol. Life Sci. 2014, 71, 4373–4386. [Google Scholar] [CrossRef]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.D. P16INK4A—More than a senescence marker. Life 2022, 12, 1332. [Google Scholar] [CrossRef]
- Tomari, H.; Kawamura, T.; Asanoma, K.; Egashira, K.; Kawamura, K.; Honjo, K.; Nagata, Y.; Kato, K. Contribution of senescence in human endometrial stromal cells during proliferative phase to embryo receptivity. Biol. Reprod. 2020, 103, 104–113. [Google Scholar] [CrossRef]
- Deryabin, P.; Griukova, A.; Nikolsky, N.; Borodkina, A. The link between endometrial stromal cell senescence and decidualization in female fertility: The art of balance. Cell. Mol. Life Sci. 2020, 77, 1357–1370. [Google Scholar] [CrossRef]
- Lucas, E.S.; Vrljicak, P.; Muter, J.; Diniz-da-Costa, M.M.; Brighton, P.J.; Kong, C.S.; Lipecki, J.; Fishwick, K.J.; Odendaal, J.; Ewington, L.J.; et al. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun. Biol. 2020, 3, 37. [Google Scholar] [CrossRef]
- Rawlings, T.M.; Makwana, K.; Taylor, D.M.; Molè, M.A.; Fishwick, K.J.; Tryfonos, M.; Odendaal, J.; Hawkes, A.; Zernicka-Goetz, M.; Hartshorne, G.M.; et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. eLife 2021, 10, e69603. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Parvanov, D.; Ganeva, R.; Vidolova, N.; Stamenov, G. Decreased number of p16-positive senescent cells in human endometrium as a marker of miscarriage. J. Assist. Reprod. Genet. 2021, 38, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Parvanov, D.; Ganeva, R.; Arsov, K.; Decheva, I.; Handzhiyska, M.; Ruseva, M.; Vidolova, N.; Scarpellini, F.; Metodiev, D.; Stamenov, G. Association between endometrial senescent cells and immune cells in women with repeated implantation failure. J. Assist. Reprod. Genet. 2023, 40, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Takeuchi, T.; Mizuta, S.; Matsubayashi, H.; Ishikawa, T. Chronic endometritis: Potential cause of infertility and obstetric and neonatal complications. Am. J. Reprod. Immunol. 2016, 75, 13–22. [Google Scholar] [CrossRef]
- Hosseini, S.; Abbasi, H.; Salehpour, S.; Saharkhiz, N.; Nemati, M. Prevalence of chronic endometritis in infertile women undergoing hysteroscopy and its association with intrauterine abnormalities: A cross-sectional study. JBRA Assist. Reprod. 2024, 28, 430–434. [Google Scholar] [CrossRef]
- Zhang, C.; Meng, S.; Tu, X.; Wang, Y.; Meng, D.; Zhang, W.; Li, Y.; He, W. Analysis of the risk factors of chronic endometritis in infertile women. BMC Womens Health 2025, 25, 378. [Google Scholar] [CrossRef]
- Yan, X.; Jiao, J.; Wang, X. The pathogenesis, diagnosis, and treatment of chronic endometritis: A comprehensive review. Front. Endocrinol. 2025, 16, 1603570. [Google Scholar] [CrossRef]
- Cicinelli, E.; Resta, L.; Nicoletti, R.; Tartagni, M.; Marinaccio, M.; Bulletti, C.; Colafiglio, G. Detection of chronic endometritis at fluid hysteroscopy. J. Minim. Invasive Gynecol. 2005, 12, 514–518. [Google Scholar] [CrossRef]
- Bouet, P.E.; El Hachem, H.; Monceau, E.; Gariépy, G.; Kadoch, I.; Sylvestre, C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: Prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil. Steril. 2016, 105, 106–110. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, R.; Luo, Y.; Luo, C. Analysis of the diagnostic value of CD138 for chronic endometritis, the risk factors for the pathogenesis of chronic endometritis and the effect of chronic endometritis on pregnancy: A cohort study. BMC Womens Health 2016, 16, 60. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, W.; Liu, S.; Liu, K.; Wang, J.; Qin, P.; Liu, Y.; Jiang, Q. The combination of CD138/MUM1 dual-staining and artificial intelligence for plasma cell counting in the diagnosis of chronic endometritis. Am. J. Reprod. Immunol. 2023, 89, e13671. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes. Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. Nat. Commun. 2017, 8, 2129. [Google Scholar] [CrossRef]
- Chemerinski, A.; Garcia de Paredes, J.; Blackledge, K.; Douglas, N.C.; Morelli, S.S. Mechanisms of endometrial aging: Lessons from natural conceptions and assisted reproductive technology cycles. Front. Physiol. 2024, 15, 1332946. [Google Scholar] [CrossRef]
- Taylor, R.N.; Berga, S.L.; Zou, E.; Washington, J.; Song, S.; Marzullo, B.J.; Bagchi, I.C.; Bagchi, M.K.; Yu, J. Interleukin-1β induces and accelerates human endometrial stromal cell senescence and impairs decidualization via the c-Jun N-terminal kinase pathway. Cell Death Discov. 2024, 10, 288. [Google Scholar] [CrossRef]
- Kawamura, K.; Matsumura, Y.; Kawamura, T.; Araki, H.; Hamada, N.; Kuramoto, K.; Yagi, H.; Onoyama, I.; Asanoma, K.; Kato, K. Endometrial senescence is mediated by interleukin-17 receptor B signaling. Cell Commun. Signal. 2024, 22, 363. [Google Scholar] [CrossRef]
- Alqahtani, S.; Alqahtani, T.; Venkatesan, K.; Sivadasan, D.; Ahmed, R.; Sirag, N.; Elfadil, H.; Mohamed, H.A.; T.A., H.; Elsayed Ahmed, R.; et al. SASP modulation for cellular rejuvenation and tissue homeostasis: Therapeutic strategies and molecular insights. Cells 2025, 14, 608. [Google Scholar] [CrossRef]
- Ohtani, N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): Can it be controlled by senolysis? Inflamm. Regen. 2022, 42, 11. [Google Scholar] [CrossRef]
- Chen, L.; Yi, Y.; Nie, J. Multi-omic insight into the involvement of cell aging related genes in the pathogenesis of endometriosis. Sci. Rep. 2025, 15, 14103. [Google Scholar] [CrossRef]
- Delenko, J.; Hyman, N.; Chatterjee, P.K.; Safaric Tepes, P.; Shih, A.J.; Xue, X.; Gurney, J.; Baker, A.G.; Wei, C.; Munoz Espin, D.; et al. Targeting cellular senescence to enhance human endometrial stromal cell decidualization and inhibit their migration. Biomolecules 2025, 15, 873. [Google Scholar] [CrossRef]
- Feng, T.; Xie, F.; Lee, L.M.Y.; Lin, Z.; Tu, Y.; Lyu, Y.; Yu, P.; Wu, J.; Chen, B.; Zhang, G.; et al. Cellular senescence in cancer: From mechanism paradoxes to precision therapeutics. Mol. Cancer 2025, 24, 213. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Parvanov, D.; Metodiev, D.; Ganeva, R.; Ruseva, M.; Handzhiyska, M.; Safir, J.; Jelezarsky, L.; Vidolova, N.; Stamenov, G.; Hadjidekova, S. High Stromal Senescence During the Window of Implantation Is Linked to Plasma Cell Presence and Cluster Formation in the Endometrium. Immuno 2026, 6, 3. https://doi.org/10.3390/immuno6010003
Parvanov D, Metodiev D, Ganeva R, Ruseva M, Handzhiyska M, Safir J, Jelezarsky L, Vidolova N, Stamenov G, Hadjidekova S. High Stromal Senescence During the Window of Implantation Is Linked to Plasma Cell Presence and Cluster Formation in the Endometrium. Immuno. 2026; 6(1):3. https://doi.org/10.3390/immuno6010003
Chicago/Turabian StyleParvanov, Dimitar, Dimitar Metodiev, Rumiana Ganeva, Margarita Ruseva, Maria Handzhiyska, Jinahn Safir, Lachezar Jelezarsky, Nina Vidolova, Georgi Stamenov, and Savina Hadjidekova. 2026. "High Stromal Senescence During the Window of Implantation Is Linked to Plasma Cell Presence and Cluster Formation in the Endometrium" Immuno 6, no. 1: 3. https://doi.org/10.3390/immuno6010003
APA StyleParvanov, D., Metodiev, D., Ganeva, R., Ruseva, M., Handzhiyska, M., Safir, J., Jelezarsky, L., Vidolova, N., Stamenov, G., & Hadjidekova, S. (2026). High Stromal Senescence During the Window of Implantation Is Linked to Plasma Cell Presence and Cluster Formation in the Endometrium. Immuno, 6(1), 3. https://doi.org/10.3390/immuno6010003





