Investigating the Dichotomous Nature of Nitric Oxide During the Enteral Phase of Trichinella spiralis Infection in Mice: An Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. The Parasite
2.2. Animals and T. spiralis Infection
2.3. Drug Regimen: Dosage Schedule
2.4. Experimental Design
2.5. Assessment Measures
2.5.1. Parasitological Analysis
- Isolation and Counting of Adult Worms
2.5.2. Histopathological Analysis
2.5.3. Immunohistochemical Analysis
2.5.4. Biochemical Analysis
- Assessment of serum nitric oxide (NO) levels
- Assessment of the serum cytokines, IFN-γ, and TNF-α
2.6. Statistical Analysis
2.7. Ethical Statement
3. Results
3.1. Parasitological Results
Adult Worm Count
3.2. Histopathological Results
3.2.1. Histopathological Features
3.2.2. Inflammatory Response Score
3.3. Immunohistochemical (IHC) Results
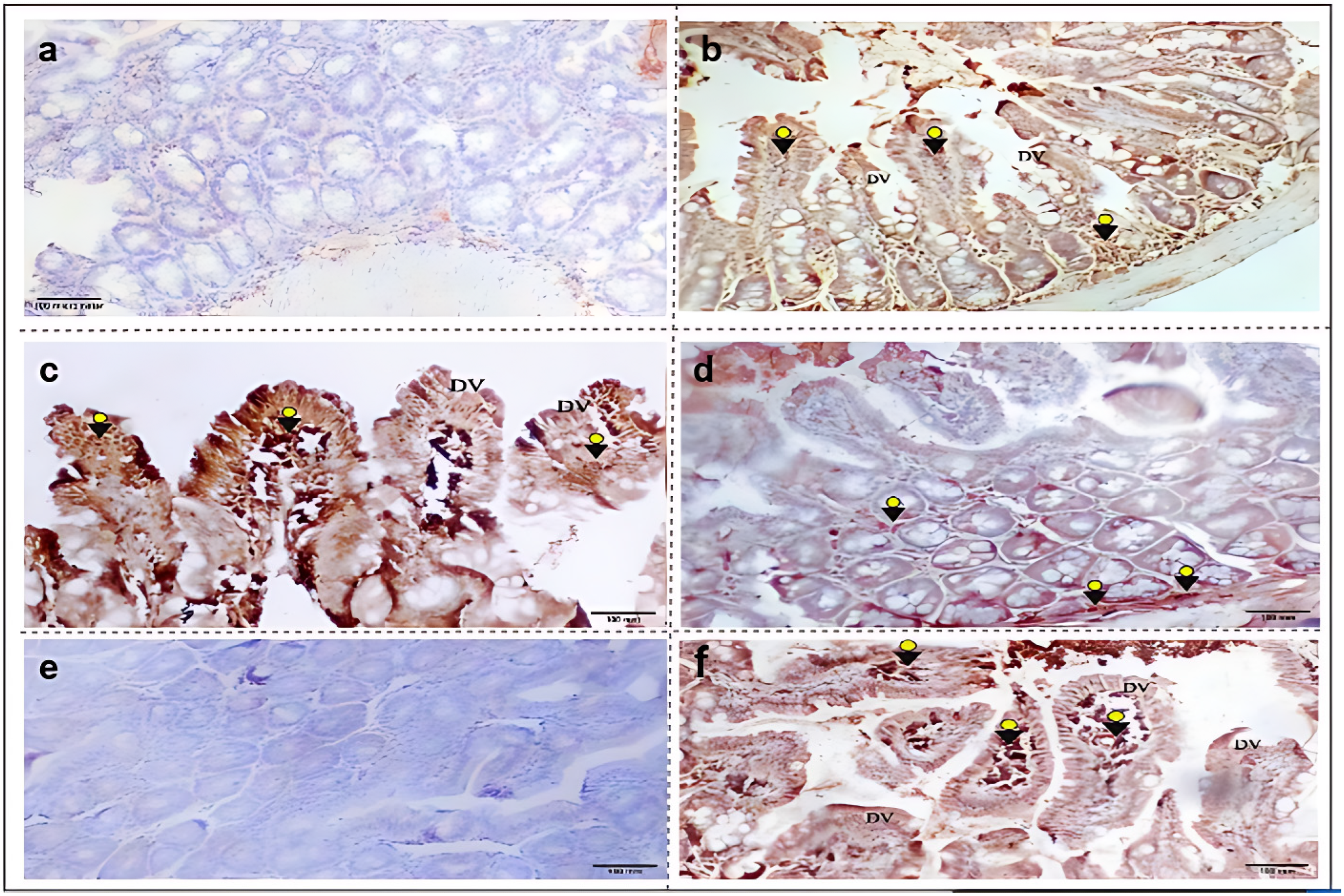
3.3.1. iNOS Immunohistochemical Expression
3.3.2. nNOS Immunohistochemical Expression
3.3.3. iNOS and nNOS Immunohistochemical Scores
3.4. Biochemical Results
4. Discussion
5. Conclusions
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T. spiralis | Trichinella spiralis |
| NBL | Newborn larvae |
| AG | Aminoguanidine |
| 7-NI | 7-Nitroindazole |
| DV | Degenerated villi |
| DMSO | Dimethyl sulfoxide |
| HCL | Hydrochloric acid |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| iNOS | Inducible nitric oxide synthase |
| nNOS | Neuronal nitric oxide synthase |
| IHC | Immunohistochemical |
| ABC | Avidin–biotin–peroxidase complex |
| PBS | Phosphate-buffered saline |
| DAB | Diaminobenzidine |
| ELISA | Enzyme linked immune-sorbent assay |
| SD | Standard deviation |
| Th1 | T helper-1 |
| Th2 | T helper-2 |
| TNF-α | Tumor Necrosis Factor-alpha |
| IFN-γ | Interferon-gamma |
| PD-1 | Programmed cell death 1 |
References
- Liu, R.D.; Wang, Z.Q.; Wang, L.; Long, S.R.; Ren, H.; Cui, J. Analysis of differentially expressed genes of Trichinella spiralis larvae activated by bile and cultured with intestinal epithelial cells using real-time PCR. Parasitol. Res. 2013, 112, 4113–4120. [Google Scholar] [CrossRef] [PubMed]
- Milton, A.A.P.; Das, S.; Ghatak, S.; Srinivas, K.; Priya, G.B.; Angappan, M.; Sen, A. Seroepidemological investigation of Toxoplasma gondii and Trichinella spp. in pigs reared by tribal communities and small-holder livestock farmers in Northeastern India. PLoS ONE 2024, 19, e0298357. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E. World distribution of Trichinella spp. infections in animals and humans. Vet. Parasitol. 2007, 149, 3–21. [Google Scholar] [CrossRef]
- Sofronic-Milosavljevic, L.; Ilic, N.; Pinelli, E.; Gruden-Movsesijan, A. Secretory products of Trichinella spiralis muscle larvae and immunomodulation: Implication for autoimmune diseases, allergies, and malignancies. J. Immunol. Res. 2015, 2015, 523875. [Google Scholar] [CrossRef]
- Gottstein, B.; Pozio, E.; Nöckler, K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef]
- Ishikawa, N.; Goyal, P.K.; Mahida, Y.R.; Li, K.F.; Wakelin, D. Early cytokine responses during intestinal parasitic infections. Immunology 1998, 93, 257–263. [Google Scholar] [CrossRef]
- Lawrence, C.E.; Paterson, J.C.; Wei, X.Q.; Liew, F.Y.; Garside, P.; Kennedy, M.W. Nitric oxide mediates intestinal pathology but not immune expulsion during Trichinella spiralis infection in mice. J. Immunol. 2000, 164, 4229–4234. [Google Scholar] [CrossRef] [PubMed]
- Bredt, D.S.; Snyder, S.H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. USA 1990, 87, 682–685. [Google Scholar] [CrossRef]
- Thomas, M.; Feron, O. Nitric oxide synthases: Which, where, how, and why? J. Clin. Investig. 1997, 100, 2146–2152. [Google Scholar] [CrossRef]
- Maloney, J.; Keselman, A.; Li, E.; Singer, S.M. Macrophages expressing arginase 1 and nitric oxide synthase 2 accumulate in the small intestine during Giardia lamblia infection. Microbes Infect. 2015, 17, 462–467. [Google Scholar] [CrossRef]
- Qu, X.W.; Wang, H.; Rozenfeld, R.A.; Huang, W.; Hsueh, W. Type I nitric oxide synthase (NOS) is the predominant NOS in rat small intestine. Regulation by platelet-activating factor. Biochim. Biophys. Acta 1999, 1451, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Palásthy, Z.; Kaszaki, J.; Lázár, G.; Nagy, S.; Boros, M. Intestinal nitric oxide synthase activity changes during experimental colon obstruction. Scand. J. Gastroenterol. 2006, 41, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.W.; Wang, H.; De Plaen, I.G.; Rozenfeld, R.A.; Hsueh, W. Neuronal nitric oxide synthase (NOS) regulates the expression of inducible NOS in rat small intestine via modulation of nuclear factor kappa B. FASEB J. 2001, 15, 439–446. [Google Scholar] [CrossRef]
- Omar, M.; Abdelal, H.O. Nitric oxide in parasitic infections: A friend or foe? J. Parasit. Dis. 2022, 46, 1147–1163. [Google Scholar] [CrossRef]
- Żeromski, J.; Boczoń, K.; Wandurska-Nowak, E.; Mozer-Lisewska, I. Effect of aminoguanidine and albendazole on inducible nitric oxide synthase (iNOS) activity in T. spiralis-infected mice muscles. Folia Histochem. Cytobiol. 2005, 43, 157–159. [Google Scholar]
- Fadl, H.O.; Amin, N.M.; Wanas, H.; El-Din, S.S.; Ibrahim, H.A.; Aboulhoda, B.E.; Bocktor, N.Z. The impact of l-arginine supplementation on the enteral phase of experimental Trichinella spiralis infection in treated and untreated mice. J. Parasit. Dis. 2020, 44, 737–747. [Google Scholar] [CrossRef]
- Giarcia, L.S.; Bruckner, D.A. Macroscopic and microscopic examination of fecal specimens. In Diagnostic Medical Parasitology, 3rd ed.; Giboda, M.N., Vokurkova, P., Kopacek, O., Eds.; ASM Press: Washington, DC, USA, 1977; pp. 608–649. [Google Scholar]
- Dunn, I.J.; Wright, K.A. Cell injury caused by Trichinella spiralis in the mucosal epithelium of B10A mice. J. Parasitol. 1985, 71, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Attia, R.A.; Mahmoud, A.E.; Farrag, H.M.; Makboul, R.; Mohamed, M.E.; Ibraheim, Z. Effect of myrrh and thyme on Trichinella spiralis enteral and parenteral phases with inducible nitric oxide expression in mice. Mem. Inst. Oswaldo Cruz. 2015, 110, 1035–1041. [Google Scholar] [CrossRef]
- Al-Bayati, M.A.; Ahmad, M.A.; Khamas, W. The potential effect of L-arginine on mice placenta. Adv. Pharmacoepidemiol. Drug Saf. 2014, 3, 1000150. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Zakaria, S.; Fahmy, A. Can Chronic Nitric Oxide Inhibition Improve Liver and Renal Dysfunction in Bile Duct Ligated Rats? Adv. Pharmacol. Sci. 2015, 2015, 298792. [Google Scholar] [CrossRef]
- Abdel Aziz, M.; Elsayed, H. Insights into the effects of inducible and neuronal nitric oxide synthase isoenzymes in experimental intestinal heterophyiasis. Parasitol. United J. 2021, 14, 86–94. [Google Scholar] [CrossRef]
- Issa, R.M.; El-Arousy, M.H.; Abd EI-Aal, A.A. Albendazole: A study of its effect on experimental Trichinella spiralis infection in rats. Egypt J. Med. Sci. 1998, 19, 281–290. [Google Scholar]
- Demoulin, J.C.; Kulbertus, H.E. Histopathological examination of concept of left hemiblock. Br. Heart J. 1972, 34, 807–814. [Google Scholar] [CrossRef]
- Saad, A.E.; Zoghroban, H.S.; Ghanem, H.B.; El-Guindy, D.M.; Younis, S.S. The effects of L-citrulline adjunctive treatment of Toxoplasma gondii RH strain infection in a mouse model. Acta Trop. 2023, 239, 106830. [Google Scholar] [CrossRef] [PubMed]
- Czarnewski, P.; Araújo, E.C.B.; Oliveira, M.C.; Mineo, T.W.P.; Silva, N.M. Recombinant TgHSP70 Immunization Protects against Toxoplasma gondii Brain Cyst Formation by Enhancing Inducible Nitric Oxide Expression. Front. Cell Infect. Microbiol. 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.P.; Pretlow, T.G.; Rao, J.S.; Pretlow, T.P. Inducible nitric oxide synthase (iNOS) is expressed similarly in multiple aberrant crypt foci and colorectal tumors from the same patients. Cancer Res. 2001, 61, 419–422. [Google Scholar]
- Chan, Y.H. Biostatistics 102: Quantitative data—Parametric & non-parametric tests. Singapore Med. J. 2003, 44, 391–396. [Google Scholar]
- Kim, H.Y. Statistical notes for clinical researchers: Post-hoc multiple comparisons. Restor. Dent. Endod. 2015, 40, 172–176. [Google Scholar] [CrossRef]
- Hosking, B.C.; Watson, T.G.; Leathwick, D.M. Multigeneric resistance to oxfendazole by nematodes in cattle. Vet. Rec. 1996, 138, 67–68. [Google Scholar] [CrossRef]
- Della Bella, C.; Benagiano, M.; De Gennaro, M.; Gomez-Morales, M.A.; Ludovisi, A.; D’Elios, S.; Bruschi, F.A. T-cell clones in human trichinellosis: Evidence for a mixed Th1/Th2 response. Parasite Immunol. 2017, 39, e12412. [Google Scholar] [CrossRef] [PubMed]
- Ashour, D.S.; Abou Rayia, D.M.; Saad, A.E.; El-Bakary, R.H. Nitazoxanide anthelmintic activity against the enteral and parenteral phases of trichinellosis in experimentally infected rats. Exp. Parasitol. 2016, 170, 28–35. [Google Scholar] [CrossRef]
- Abdeltawab, M.; Abdel-Shafi, I.; Aboulhoda, B.; Wanas, H.; Saad El-Din, S.; Amer, S.; Hamed, A. Investigating the effect of the nitric oxide donor L-arginine on albendazole efficacy in Trichinella spiralis-induced myositis and myocarditis in mice. Parasitol. United J. 2022, 15, 60–70. [Google Scholar] [CrossRef]
- Aguayo-Ortiz, R.; Méndez-Lucio, O.; Medina-Franco, J.L.; Castillo, R.; Yépez-Mulia, L.; Hernández-Luis, F.; Hernández-Campos, A. Towards the identification of the binding site of benzimidazoles to β-tubulin of Trichinella spiralis: Insights from computational and experimental data. J. Mol. Graph. Model. 2013, 41, 12–19. [Google Scholar] [CrossRef]
- Despommier, D.D. Trichinella spiralis and the concept of niche. J. Parasitol. 1993, 79, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wang, Y.Y.; Fang, Q.; Xue, Y.Q.; Shen, J.L. In vitro killing of adult Trichinella spiralis by exogenous nitric oxide. Chin. J. Parasitol. Parasit. Dis. 2012, 30, 374–377. [Google Scholar]
- Li, E.; Zhou, P.; Singer, S.M. Neuronal nitric oxide synthase is necessary for elimination of Giardia lamblia infections in mice. J. Immunol. 2006, 176, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Y.S.; Gillin, F.D.; Eckmann, L. Adaptive immunity-dependent intestinal hypermotility contributes to host defense against Giardia spp. Infect. Immun. 2006, 74, 2473–2476. [Google Scholar]
- Li, Z.; Peirasmaki, D.; Svärd, S.; Åbrink, M. Serglycin-deficiency causes reduced weight gain and changed intestinal cytokine responses in mice infected with Giardia intestinalis. Front Immunol. 2021, 12, 677722. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Du, S.; Zhang, Y.; Yu, Y.; Zhan, B.; Hao, J.; Jia, Z.; Huang, J.; Guo, Y.; et al. PD-1 deficiency impairs eosinophil recruitment to tissue during Trichinella spiralis infection. Cell Rep. 2024, 43, 114861. [Google Scholar] [CrossRef]
- Balasuriya, G.K.; Nugapitiya, S.S.; Hill-Yardin, E.L.; Bornstein, J.C. Nitric Oxide Regulates Estrus Cycle Dependent Colonic Motility in Mice. Front. Neurosci. 2021, 15, 647555. [Google Scholar] [CrossRef]
- Ricken, F.J.; Nell, J.; Grüner, B.; Schmidberger, J.; Kaltenbach, T.; Kratzer, W.; Hillenbrand, A.; Henne-Bruns, D.; Deplazes, P.; Moller, P.; et al. Albendazole increases the inflammatory response and the amount of Em2-positive small particles of Echinococcus multilocularis (spems) in human hepatic alveolar echinococcosis lesions. PLoS Negl. Trop. Dis. 2017, 11, e0005636. [Google Scholar] [CrossRef] [PubMed]
- Carbajosa, S.; Rodríguez-Angulo, H.O.; Gea, S.; Chillón-Marinas, C.; Poveda, C.; Maza, M.C.; Colombet, D.; Fresno, M.; Gironès, N. L-arginine supplementation reduces mortality and improves disease outcome in mice infected with Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2018, 12, e0006179. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej-Sobocińska, M.; Dziemian, E.; Machnicka-Rowińska, B. Inhibition of nitric oxide production by aminoguanidine influences the number of Trichinella spiralis parasites in infected “low responders” (C57BL/6) and “high responders” (BALB/c) mice. Parasitol. Res. 2006, 99, 194–196. [Google Scholar] [CrossRef]
- Frydas, S.; Papaioannou, N.; Hatzistilianou, M.; Merlitti, D.; Di Gioacchino, M.; Castellani, M.L.; Conti, P. Generation of TNFα, IFNγ, IL-4, IL-6 and IL-10 in Trichinella spiralis infected mice: Effect of the anti-inflammatory compound mimosine. Inflammopharmacology 2001, 9, 389–400. [Google Scholar] [CrossRef]




| Groups | Mean ± SD | Range | Change% | F-Test | p-Value | Pairwise Comparisons |
|---|---|---|---|---|---|---|
| G2: Infected untreated group | 54.38 ± 3.16 | 50–60 | -------- | 1151.878 | <0.001 ** | p1: <0.001 ** p2: <0.001 ** p3: 0.19 (NS) p4: 0.92 (NS) p5: <0.001 ** |
| G3: Infected–Albendazole-treated group | 8.38 ± 1.69 | 5–10 | 84.6% reduction | |||
| G4: Infected–L-arginine-treated group | 11.38 ±1.41 | 9–13 | 79.1% reduction | |||
| G5: Infected–Aminoguanidine-treated group | 55.50 ± 3.34 | 52–62 | 2.1% increase | |||
| G6: Infected–7-Nitroindazole-treated group | 83.88 ± 3.18 | 80–90 | 54.2% increase |
| Groups | Inflammatory Response Score | χ2 | p-Value | Pairwise Comparisons | |||
|---|---|---|---|---|---|---|---|
| Mild | Moderate | Large | Extensive | ||||
| G2: Infected untreated group | 0% | 12.5% | 25% | 62.5% | 22.70 | 0.030 * | p1: 0.58 (NS) p2: 0.60 (NS) p3: 0.014 * p4: 0.062 (NS) |
| G3: Infected–Albendazole treated group | 0% | 0% | 25% | 75% | |||
| G4: Infected–L-arginine treated group | 12.5% | 25% | 25% | 37.5% | |||
| G5: Infected–Aminoguanidine treated group | 62.5% | 25% | 12.5% | 0% | |||
| G6: Infected–7-Nitroindazole treated group | 50% | 25% | 12.5% | 12.5% | |||
| Groups | iNOS Immunohistochemical Score | nNOS Immunohistochemical Score | ||||
|---|---|---|---|---|---|---|
| Negative/Low | Moderate | High | Negative/Low | Moderate | High | |
| G1: Control negative group | 100% | 0% | 0% | 75% | 25% | 0% |
| G2: Infected untreated group | 12.5% | 37.5% | 50% | 0% | 37.5% | 62.5% |
| G3: Infected–Albendazole treated group | 0% | 25% | 75% | 12.5% | 37.5% | 50% |
| G4: Infected–L-arginine treated group | 50% | 37.5% | 12.5% | 0% | 12.5% | 87.5% |
| G5: Infected–Aminoguanidine treated group | 100% | 0% | 0% | 12.5% | 25% | 62.5% |
| G6: Infected–7-Nitroindazole treated group | 0% | 50% | 50% | 100% | 0% | 0% |
| χ2 p-value Pairwise comparisons | 40.029 <0.001 ** p1: 0.002 * p2: 0.45 (NS) p3: 0.17 (NS) p4: 0.002 * p5: 0.6 (NS) | 37.83 <0.001 ** p1: 0.004 * p2: 0.6 (NS) p3: 0.25 (NS) p4: 0.46(NS) p5: <0.001 ** | ||||
| Groups | Serum Values (Mean ± SD) | ||
| NO (Umol/L) | TNF-α (Pg/mL) | (IFN-γ) (Pg/mL) | |
| G1: Control negative group | 13.1 ± 2.17 | 72.88 ± 3.49 | 1.59 ± 0.34 |
| G2: Infected untreated group | 30.53 ± 0.38 | 213.85 ± 24.22 | 117.94 ± 1.61 |
| G3: Infected–Albendazole treated group | 32.45 ± 0.33 | 397.28 ± 53.14 | 223.58 ± 19.81 |
| G4: Infected–L-arginine treated group | 34.51 ± 0.35 | 540.23 ± 39.59 | 316.04 ± 16.18 |
| G5: Infected–Aminoguanidine treated group | 30.41 ± 1.24 | 211.54 ± 22.81 | 109.15 ± 7.49 |
| G6: Infected–7-Nitroindazole treated group | 18.55 ± 0.37 | 80.90 ± 0.62 | 68.13 ± 2.55 |
| F-test | 528.7221 | 293.7443 | 850.2002 |
| p-value | <0.001 ** | <0.001 ** | <0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, M.; Fathy, G.; Mohammed, S.; El-taib, A. Investigating the Dichotomous Nature of Nitric Oxide During the Enteral Phase of Trichinella spiralis Infection in Mice: An Experimental Study. Immuno 2025, 5, 59. https://doi.org/10.3390/immuno5040059
Omar M, Fathy G, Mohammed S, El-taib A. Investigating the Dichotomous Nature of Nitric Oxide During the Enteral Phase of Trichinella spiralis Infection in Mice: An Experimental Study. Immuno. 2025; 5(4):59. https://doi.org/10.3390/immuno5040059
Chicago/Turabian StyleOmar, Marwa, Ghada Fathy, Samira Mohammed, and Asmaa El-taib. 2025. "Investigating the Dichotomous Nature of Nitric Oxide During the Enteral Phase of Trichinella spiralis Infection in Mice: An Experimental Study" Immuno 5, no. 4: 59. https://doi.org/10.3390/immuno5040059
APA StyleOmar, M., Fathy, G., Mohammed, S., & El-taib, A. (2025). Investigating the Dichotomous Nature of Nitric Oxide During the Enteral Phase of Trichinella spiralis Infection in Mice: An Experimental Study. Immuno, 5(4), 59. https://doi.org/10.3390/immuno5040059







