PD-1 Expression in Endometriosis
Abstract
1. Background
2. Methods
2.1. Study Design and Subjects
2.2. Clinical and Demographic Information
2.3. Sample Collection
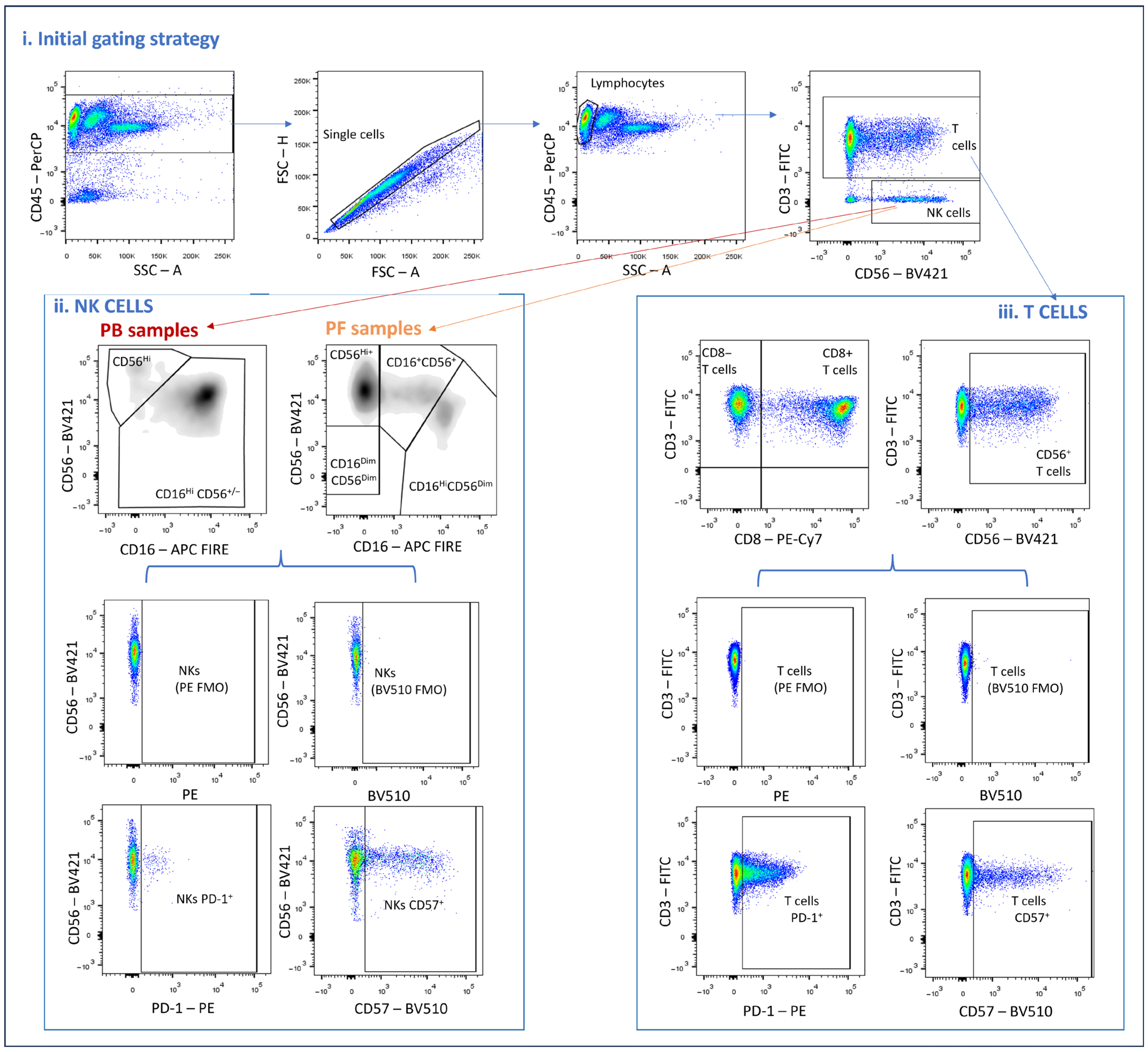
2.4. Analysis of Immunophenotyping Using Flow Cytometry
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Patients
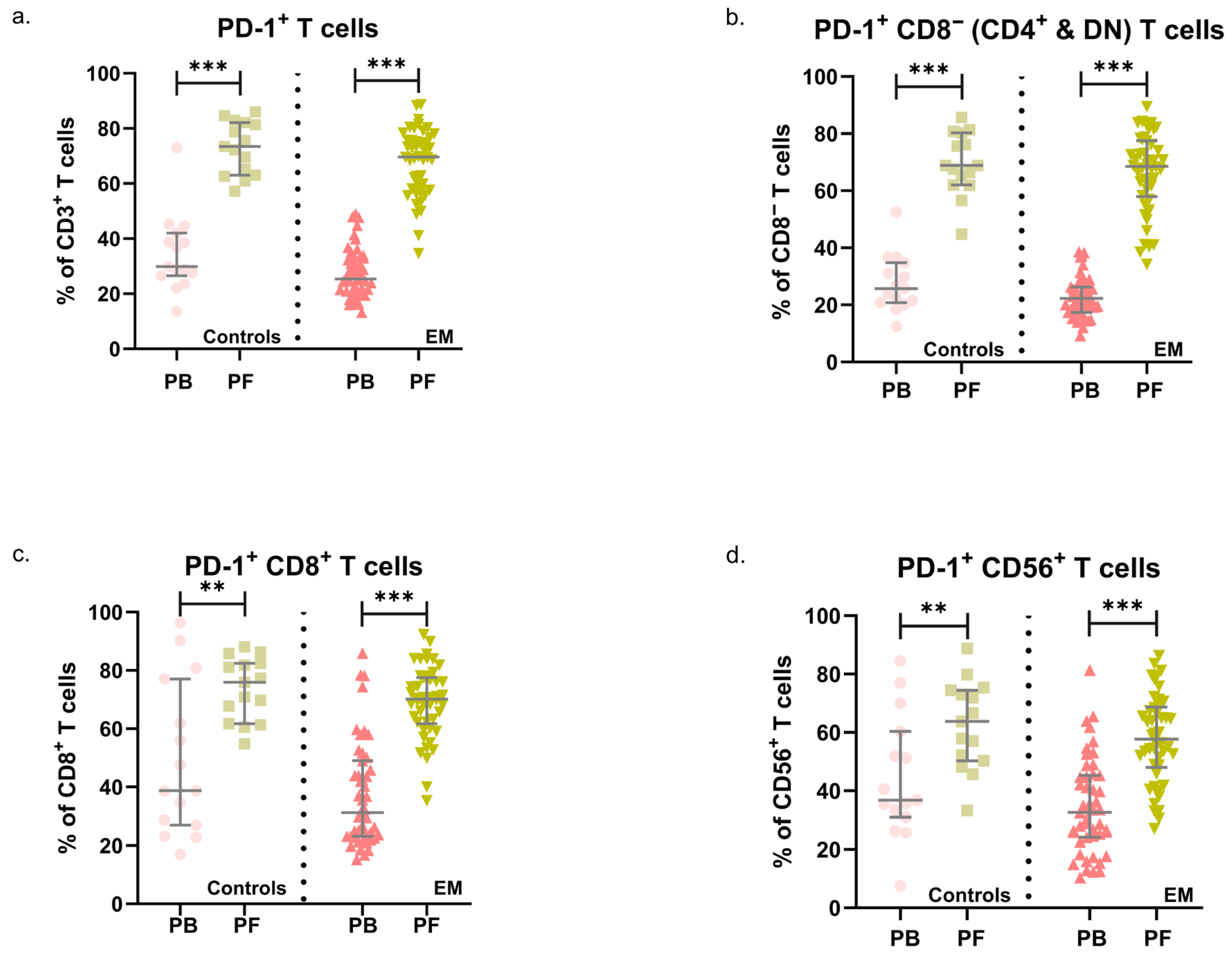
3.2. PD-1 in Endometriosis: Expression Patterns in PB and PF
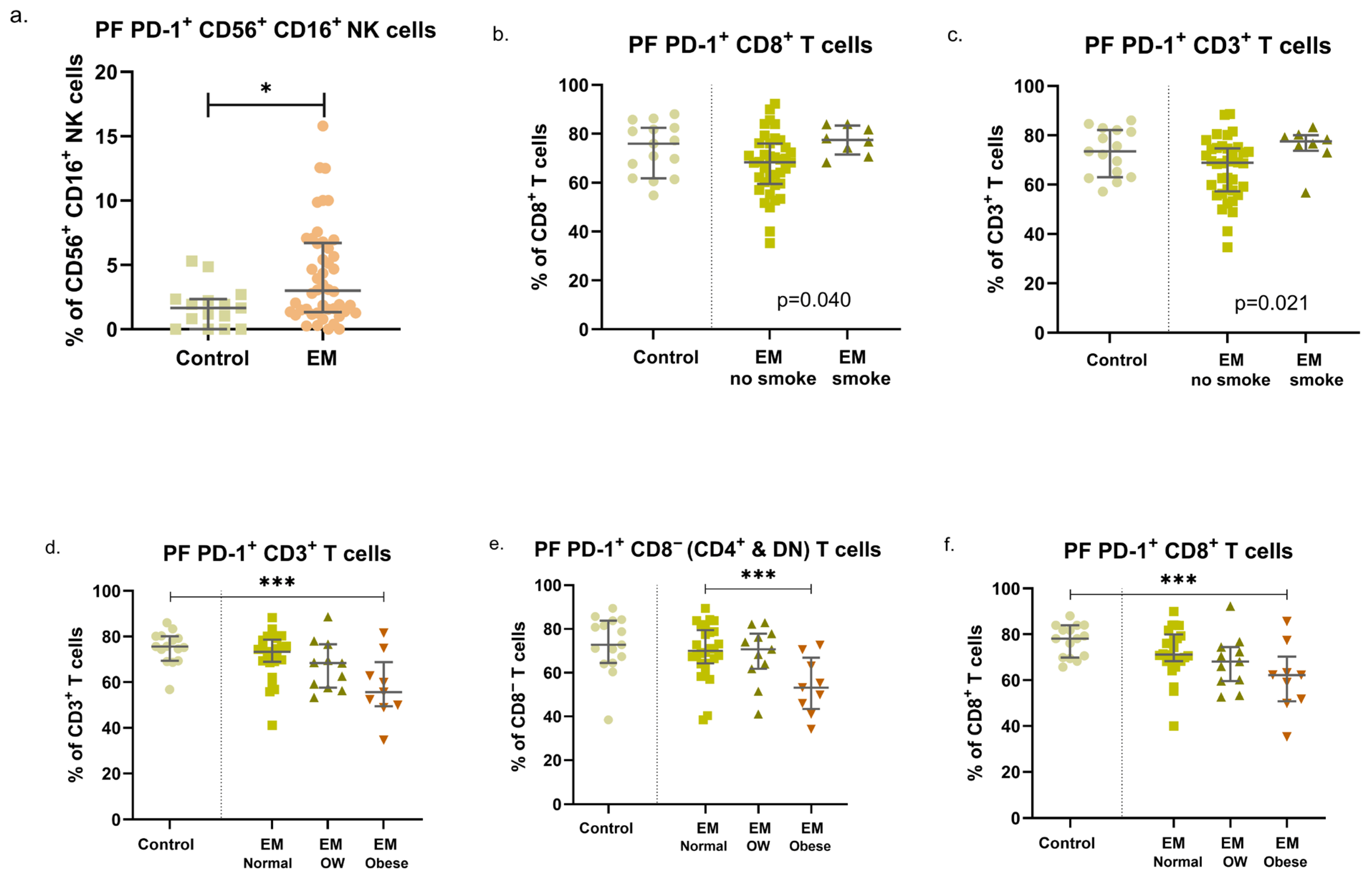
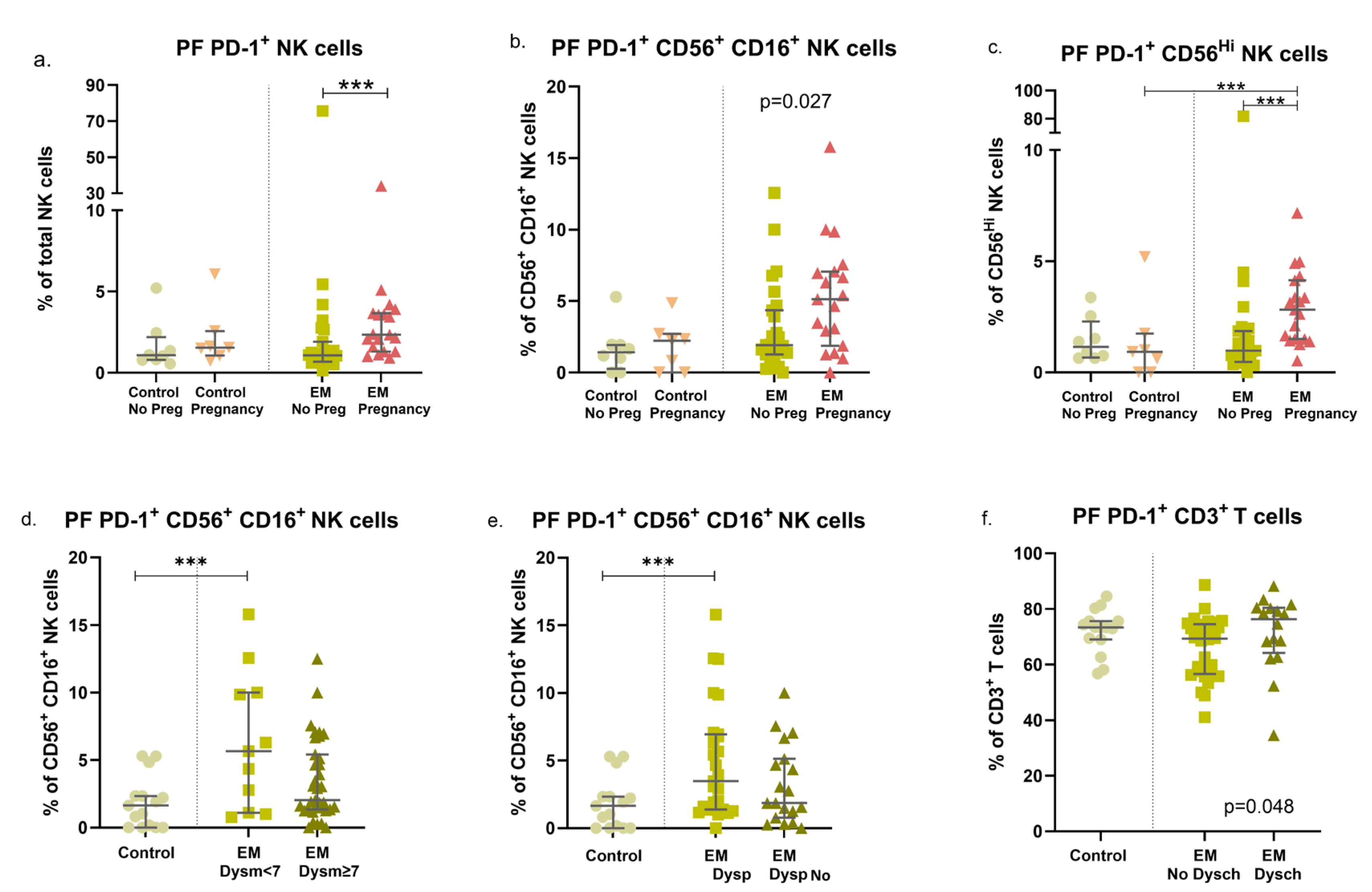
3.3. Endometriosis, Previous Pregnancies, and Fertility Status: Modulation of PD-1 Expression in Circulating and Peritoneal Immune Cells
3.4. EM Symptoms Are Associated with Different Expression Patterns of PD-1 in NK and T-Cell Subsets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukui, A.; Mai, C.; Saeki, S.; Yamamoto, M.; Takeyama, R.; Kato, T.; Ukita, Y.; Wakimoto, Y.; Yamaya, A.; Shibahara, H. Pelvic endometriosis and natural killer cell immunity. Am. J. Reprod. Immunol. 2021, 85, e13342. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef]
- Capezzuoli, T.; Rossi, M.; La Torre, F.; Vannuccini, S.; Petraglia, F. Hormonal drugs for the treatment of endometriosis. Curr. Opin. Pharmacol. 2022, 67, 102311. [Google Scholar] [CrossRef]
- Tanaka, E.; Sendo, F.; Kawagoe, S.; Hiroi, M. Decreased natural killer cell activity in women with endometriosis. Gynecol. Obstet. Investig. 1992, 34, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, D.; Dufour, P.; Oosterlynck, D. Immunological aspects of endometriosis. Hum. Reprod. Update 1996, 2, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.L.; Rosa, N.N.; Angelo-Dias, M.; Martins, C.; Borrego, L.M.; Lima, J. Natural Killer Cell Receptors and Endometriosis: A Systematic Review. Int. J. Mol. Sci. 2022, 24, 331. [Google Scholar] [CrossRef]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immunol. 2017, 139, 335–346.e333. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Concha-Benavente, F.; Kansy, B.; Moskovitz, J.; Moy, J.; Chandran, U.; Ferris, R.L. PD-L1 Mediates Dysfunction in Activated PD-1+ NK Cells in Head and Neck Cancer Patients. Cancer Immunol. Res. 2018, 6, 1548–1560. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.; Zhang, Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front. Pharmacol. 2021, 12, 731798. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef]
- Makuku, R.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. Current and Future Perspectives of PD-1/PDL-1 Blockade in Cancer Immunotherapy. J. Immunol. Res. 2021, 2021, 6661406. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Andrews, W.C.; Buttram, V.C., Jr.; Weed, J.C.; Hammond, C.B.; Thomas, H.H.; Behrman, S.J. Revised American Fertility Society classification of endometriosis: 1985. Fertil. Steril. 1985, 43, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Hensley, T.R.; Easter, A.B.; Gerdts, S.E.; De Rosa, S.C.; Heit, A.; McElrath, M.J.; Andersen-Nissen, E. Enumeration of major peripheral blood leukocyte populations for multicenter clinical trials using a whole blood phenotyping assay. J. Vis. Exp. 2012, 67, e4302. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kurashina, K.; Saito, S.; Kanamaru, R.; Ohzawa, H.; Yamaguchi, H.; Miyato, H.; Hosoya, Y.; Lefor, A.K.; Sata, N.; et al. Flow cytometry-based analysis of tumor-leukocyte ratios in peritoneal fluid from patients with advanced gastric cancer. Cytometry B Clin. Cytom. 2021, 100, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Bernson, E.; Huhn, O.; Karlsson, V.; Hawkes, D.; Lycke, M.; Cazzetta, V.; Mikulak, J.; Hall, J.; Piskorz, A.M.; Portuesi, R.; et al. Identification of Tissue-Resident Natural Killer and T Lymphocytes with Anti-Tumor Properties in Ascites of Ovarian Cancer Patients. Cancers 2023, 15, 3362. [Google Scholar] [CrossRef]
- Ustiuzhanina, M.O.; Vavilova, J.D.; Boyko, A.A.; Streltsova, M.A.; Kust, S.A.; Kanevskiy, L.M.; Sapozhnikov, A.M.; Iskhakov, R.N.; Gubernatorova, E.O.; Drutskaya, M.S.; et al. Coordinated Loss and Acquisition of NK Cell Surface Markers Accompanied by Generalized Cytokine Dysregulation in COVID-19. Int. J. Mol. Sci. 2023, 24, 1996. [Google Scholar] [CrossRef]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 2006, 214, 73–91. [Google Scholar] [CrossRef]
- Moretta, L.; Montaldo, E.; Vacca, P.; Del Zotto, G.; Moretta, F.; Merli, P.; Locatelli, F.; Mingari, M.C. Human natural killer cells: Origin, receptors, function, and clinical applications. Int. Arch. Allergy Immunol. 2014, 164, 253–264. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Perussia, B. Fc receptors on natural killer cells. Curr. Top. Microbiol. Immunol. 1998, 230, 63–88. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Capsomidis, A.; Smits, E.L.; Van Tendeloo, V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892. [Google Scholar] [CrossRef]
- Ilavská, S.; Horváthová, M.; Szabová, M.; Nemessányi, T.; Jahnová, E.; Tulinská, J.; Líšková, A.; Wsolová, L.; Staruchová, M.; Volkovová, K. Association between the human immune response and body mass index. Hum. Immunol. 2012, 73, 480–485. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Sobstyl, M.; Mertowska, P.; Mertowski, S.; Zaborek-Łyczba, M.; Dudziński, D.; Polak, G.; Grywalska, E. The PD-1/PD-L1 Gateway: Peripheral Immune Regulation in the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2024, 25, 6775. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, R.; Moini, A.; Hosseini, R.; Fatehnejad, M.; Yekaninejad, M.S.; Javidan, M.; Changaei, M.; Feizisani, F.; Rajaei, S. A higher number of exhausted local PD1+, but not TIM3+, NK cells in advanced endometriosis. Heliyon 2024, 10, e23294. [Google Scholar] [CrossRef]
- Wu, L.; Lv, C.; Su, Y.; Li, C.; Zhang, H.; Zhao, X.; Li, M. Expression of programmed death-1 (PD-1) and its ligand PD-L1 is upregulated in endometriosis and promoted by 17beta-estradiol. Gynecol. Endocrinol. 2019, 35, 251–256. [Google Scholar] [CrossRef]
- Walankiewicz, M.; Grywalska, E.; Polak, G.; Korona-Glowniak, I.; Witt, E.; Surdacka, A.; Kotarski, J.; Rolinski, J. The Increase of Circulating PD-1- and PD-L1-Expressing Lymphocytes in Endometriosis: Correlation with Clinical and Laboratory Parameters. Mediat. Inflamm. 2018, 2018, 7041342. [Google Scholar] [CrossRef]
- Suszczyk, D.; Skiba, W.; Zardzewiały, W.; Pawłowska, A.; Włodarczyk, K.; Polak, G.; Tarkowski, R.; Wertel, I. Clinical Value of the PD-1/PD-L1/PD-L2 Pathway in Patients Suffering from Endometriosis. Int. J. Mol. Sci. 2022, 23, 11607. [Google Scholar] [CrossRef] [PubMed]
- Okşaşoğlu, B.; Hepokur, C.; Misir, S.; Yildiz, Ç.; Sönmez, G.; Yanik, A. Determination of PD-1 expression in peripheral blood cells in patients with endometriosis. Gynecol. Endocrinol. 2021, 37, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Nero, C.; Romito, I.; Spadola, S.; Romito, A.; Turco, L.C.; Cosentino, F.; De Ninno, M.; Catena, U.; De Cicco Nardone, A.; Moroni, R.; et al. Infiltrating T lymphocytes and programmed cell death protein-1/programmed death-ligand 1 expression in endometriosis-associated ovarian cancer. Fertil. Steril. 2022, 117, 160–168. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Control (n = 15) | Endometriosis (n = 47) | p-Value |
|---|---|---|---|
| Age, mean ± SD (min–max), yr | 38.5 ± 9.20 (20–55) | 36.2 ± 6.3 (31–42) | 0.434 a |
| Race, n (%) | 0.113 b | ||
| White | 15 (100) | 40 (85.1) | |
| Black | 0 (0) | 7 (14.9) | |
| BMI, mean ± SD (min–max), kg/m2 | 23.2 ± 3.0 (17–28) | 25.2 ± 5.6 (20.8–28.0) | 0.430 a |
| Fertile, n (%) | 0.003 b | ||
| Yes | 14 (93.3) | 24 (51.1) | |
| No | 1 (6.7) | 23 (48.9) | |
| Any Pregnancy, n (%) | 0.087 b | ||
| Yes | 7 (46.7) | 19 (40.4) | |
| No | 8 (53.3) | 28 (59.6) | |
| Smoker, n (%) | 0.087 b | ||
| Yes | 0 (0) | 8 (17.0) | |
| No | 15 (100) | 39 (83.0) | |
| Hormonal therapy, n (%) | 0.090 b | ||
| Yes | 5 (33.3) | 31 (66.0) | |
| No | 10 (66.7) | 16 (34.0) |
| Characteristics | Endometriosis (n = 47) |
|---|---|
| Endometriosis diagnosis, mean ± SD (min–max), yr | 2.8 ± 2.5 (1–14) |
| Endometriosis severity, n (%) | |
| Stage I/II | 10 (21.3) |
| Stage III/IV | 37 (78.7) |
| Dysmenorrhea complaints, n (%) | 44 (93.6) |
| Dysmenorrhea severity, n (%) | |
| None/Mild/Moderate (<7/10) | 11 (23.4) |
| Severe (≥7/10) | 36 (76.6) |
| Dyspareunia complaints, n (%) | 28 (59.6) |
| Chronic pelvic pain, n (%) | 19 (40.4) |
| Dyschesia complaints, n (%) | 16 (34.0) |
| Bowel symptoms, n (%) | 15 (31.9) |
| Urinary tract symptoms, n (%) | 5 (10.6) |
| Rectorrhagia. n (%) | 4 (8.5) |
| Extra-pelvic endometriosis, n (%) | 2 (4.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, J.L.; Martins, C.; Ângelo-Dias, M.; Rosa, N.N.; Borrego, L.M.; Lima, J. PD-1 Expression in Endometriosis. Immuno 2025, 5, 49. https://doi.org/10.3390/immuno5040049
Reis JL, Martins C, Ângelo-Dias M, Rosa NN, Borrego LM, Lima J. PD-1 Expression in Endometriosis. Immuno. 2025; 5(4):49. https://doi.org/10.3390/immuno5040049
Chicago/Turabian StyleReis, José Lourenço, Catarina Martins, Miguel Ângelo-Dias, Natacha Nurdine Rosa, Luís Miguel Borrego, and Jorge Lima. 2025. "PD-1 Expression in Endometriosis" Immuno 5, no. 4: 49. https://doi.org/10.3390/immuno5040049
APA StyleReis, J. L., Martins, C., Ângelo-Dias, M., Rosa, N. N., Borrego, L. M., & Lima, J. (2025). PD-1 Expression in Endometriosis. Immuno, 5(4), 49. https://doi.org/10.3390/immuno5040049






